Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
The Association Between the Imbalance of Single-Carbon Nutrients in Early Pregnancy and Gestational Diabetes Mellitus Risk is Influenced by Serum Selenium Status: A Cohort Study
Authors Liu PJ , Ma L , Li R, Liu Y
Received 30 June 2023
Accepted for publication 10 October 2023
Published 20 October 2023 Volume 2023:16 Pages 3275—3283
DOI https://doi.org/10.2147/DMSO.S428286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Peng Ju Liu,1 Liangkun Ma,2 Rui Li,1 Yanping Liu1
1Department of Clinical Nutrition, Peking Union Medical College Hospital, China Academic Medical Science and Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Gynaecology and Obstetrics, Peking Union Medical College Hospital, China Academic Medical Science and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Yanping Liu, Tel/Fax +86-10-69155535, Email [email protected]
Purpose: The role of imbalanced one-carbon nutrients in gestational diabetes mellitus (GDM) risk has garnered significant interest, yet existing studies yield inconsistent results. Our objective was to assess whether the association between an unbalanced ratio of folate to vitamin B12 and GDM risk is influenced by the status of other micronutrients.
Methods: This cohort study included 366 singleton-pregnancy Han women enrolled at the Shunyi District Maternal and Child Health Hospital in Beijing, China. During the first trimester of pregnancy, we measured red blood cell (RBC) folate, serum levels of vitamin B12, vitamin D, and selenium. We examined the association between the imbalanced status of RBC folate and vitamin B12 and GDM risk using logistic regression, stratified by serum selenium or vitamin D status.
Results: Among the 366 women, 67 (18.3%) were diagnosed with GDM, 201 (54.9%) had vitamin D deficiency, and 245 (66.9%) had selenium deficiency. Overall, women with higher RBC folate/vitamin B12 ratios did not exhibit a significantly higher risk of GDM compared to those in reference tertile (all P > 0.05). Subsequently, we divided women into deficient and non-deficient groups based on serum selenium or vitamin D levels. In women with selenium deficiency, those in the highest tertile of the RBC folate/vitamin B12 ratio had the highest odds of GDM [OR: 3.40 (1.16– 9.97), P=0.026] after adjusting for covariates. However, similar findings were not observed in pregnancies with normal selenium status. Irrespective of vitamin D status, women with higher RBC folate/vitamin B12 ratios did not exhibit a significantly increased risk of GDM.
Conclusion: Micronutrient deficiencies are common in early pregnancy. Women with a higher folate/vitamin B12 ratio coupled with selenium deficiency in early pregnancy have a higher GDM risk. These findings underscore the importance of micronutrient assessment in early pregnancy and subsequent interventions for micronutrient deficiencies.
Keywords: gestational diabetes mellitus, folate, vitamin B12, early pregnancy, selenium deficiency
Introduction
Gestational diabetes mellitus (GDM), defined as any degree of glucose intolerance with onset or first recognition during pregnancy,1 has emerged as an epidemic, imposing significant health and economic burdens in China.2 A recent meta-analysis examining GDM prevalence in Eastern and Southeast Asia reported an incidence of 11.9% in China, notably higher than in Japan, Korea, and Thailand.3 GDM carries multiple adverse implications for expectant mothers and their offspring, including elevated rates of cesarean deliveries, preeclampsia, birth trauma, fetal macrosomia, neonatal hypoglycemia, and an increased risk of obesity and type 2 diabetes mellitus in later life for offspring born to mothers with GDM.4–6 Identifying high-risk individuals early and implementing corresponding preventive and intervention measures is crucial to mitigate the risk of GDM and its associated perinatal complications.2
It is now widely acknowledged that environmental factors, such as poor or unbalanced nutrition, sedentary lifestyles, tobacco use, alcohol consumption, exposure to environmental pollutants, and psychological stress, contribute to an individual’s risk of metabolic diseases over their lifetime.7 Regarding GDM, several risk factors, both unmodifiable and modifiable, have been extensively documented. Unmodifiable factors include advanced maternal age, family history of diabetes, and previous GDM, while modifiable factors encompass pre-pregnancy overweight or obesity, excessive gestational weight gain, and dietary patterns.2 In recent decades, alongside macronutrients, accumulating evidence suggests that deficiencies, excesses or imbalances in certain micronutrients may be linked to GDM risk. Examples include elevated folate levels or elevated folate levels coupled with low vitamin B12 levels (folate and vitamin B12 imbalance),8–11 high doses or prolonged durations of folic acid (FA) supplementation,12 vitamin D deficiency,13 and low serum selenium levels.14,15 The role of folate and vitamin B12 in the development of GDM has garnered particular interest.
Folate and vitamin B12 are essential micronutrients in one-carbon metabolism, serving as cofactors in the conversion of homocysteine to methionine.16 Global recommendations endorse FA supplementation before and during pregnancy to prevent neural tube defects.17 In China, a nationwide program launched in 2009 aimed to increase folic acid intake among women to prevent neural tube defects (NTDs). This program provides free folic acid supplements (0.4 mg per tablet) to women planning pregnancy.18 However, the current range of FA supplement dosages (200–5000 μg/day) and durations during early pregnancy is relatively wide. With the widespread use of FA supplements among pregnant women, concerns have arisen about potential adverse effects of FA supplementation or elevated folate levels in mothers on insulin resistance in their offspring.19,20 Additionally, high FA intake can mask vitamin B12 deficiency,21 which has been linked to metabolic abnormalities in pregnant women, including insulin resistance and GDM.22 Although the precise mechanisms remain unclear, some studies have suggested that folic acid may play a role in diabetes development through natural killer cell dysfunction,23 and vitamin B12 deficiency during pregnancy may alter adipose-derived circulating microRNAs, potentially leading to adipogenesis and insulin resistance.24
Several studies from Asian countries have reported associations between higher maternal folate levels coupled with lower vitamin B12 concentrations or higher ratios of folate to vitamin B12 and an elevated risk of GDM.9,11,25 These associations appear to be more pronounced in women of advanced maternal age or with higher pre-pregnancy body mass index (BMI).11 Conversely, a recent cohort study in the UK revealed that elevated folate levels and vitamin B12 deficiency were not only prevalent in early pregnancy but also linked to GDM risk.10 Conversely, a Canadian study found that maternal one-carbon nutrient levels were not associated with GDM risk,26 while another Chinese cohort study reported significant associations between higher maternal red blood cell (RBC) folate and vitamin B12 levels and GDM risk, but no association with higher RBC folate-to-vitamin B12 ratios.27
The above findings are inconsistent, underscoring the equivocal nature of the associations between high folate levels, low vitamin B12 levels, high folate coupled with low vitamin B12, and GDM risk. Moreover, these inconsistencies suggest that the relationship between folate and vitamin B12 imbalance and GDM risk may be further influenced by additional maternal risk factors.11 Given that vitamin D deficiency and low selenium status have been closely linked to GDM,13–15,28 and that deficiencies in these micronutrients are prevalent among Chinese pregnant women,29,30 we hypothesized that the association between higher folate-to-vitamin B12 ratios and GDM risk may be influenced by vitamin D deficiency or low selenium status in early pregnancy.
Methods
Ethical Statement
The study protocol received approval from the Ethics Committee of Peking Union Medical College Hospital, Chinese Academy of Medical Science (Unique Protocol ID: hs-1646), and was registered on www.ClinicalTrials.gov (registration ID: NCT03651934). All participants voluntarily consented to take part in the study and provided written informed consent. This study adhered to the standards of the International Committee on Harmonization of Good Clinical Practice and the revised version of the Declaration of Helsinki.
The Study Participants
Participants in this study were recruited during early pregnancy as part of a cohort study conducted at the Shunyi Women’s and Children’s Hospital of Beijing Children’s Hospital, Beijing, PR China, between October and December 2018. Detailed participant information can be found in our recent publications.30–33 In summary, the primary objective of the previous cohort study was to investigate the relationship between micronutrient status in early pregnancy and the subsequent risk of GDM in Chinese pregnant women. The current study focuses on whether the association between unbalanced RBC folate/vitamin B12 ratios and GDM risk can be influenced by the status of other micronutrients in secondary analysis. Participants in this cohort study were Han Chinese residents who had established prenatal records before recruitment and planned to deliver at this hospital. All participants were over 18 years old and received routine pregnancy examinations at the same hospital throughout their pregnancy. An oral glucose tolerance test (OGTT) was administered to all women between 24 and 28 weeks of gestation.
Only singleton-pregnancy women with complete records of RBC folate, serum vitamin B12, serum selenium, and serum 25-hydroxy vitamin D [25(OH)D] were included in this study, as described elsewhere.30,31 Women were excluded if they (1) were not of Han ethnicity, (2) had a diabetes diagnosis or had lab-tested fasting glucose ≥6.1 mmol/L or HbA1c > 6.5% before pregnancy, (3) had incomplete measurements of RBC folate, selenium, 25(OH)D, and serum vitamin B12, (4) had a history of autoimmune diseases or were currently using corticosteroids, (5) had definite hyperthyroidism or hypothyroidism, (6) had a history of liver or renal insufficiency, or (7) had experienced a miscarriage prior to the 75 g OGTT.
Trained researchers used a standard questionnaire to collect clinicodemographic information, including age, education level, lifestyle factors (drinking and smoking habits, and physical activity), medical and family history, parity, and use of multi-nutrient supplements. Participants were followed up until delivery. The diagnostic criteria for GDM were based on the International Association of Diabetes and Pregnancy Study Groups (LADPSG) criteria, which included fasting plasma glucose (FPG) ≥ 5.1 mmol/L, 1-hour plasma glucose (PG) ≥ 10.0 mmol/L, and 2-hour PG ≥ 8.5 mmol/L.
Anthropometric and Blood Sample Measurements
Methods for anthropometric and blood sample measurements were previously described in detail in our previous papers.30–33 After an overnight fast of at least 8 hours, blood samples were collected from all participants in the morning during their initial visit (before 12 weeks of gestation). In the current study, the following parameters were retrieved from the records of all participants: hemoglobin, ferritin, serum folate, homocysteine (Hcy), fasting plasma glucose (FPG), selenium, 25-hydroxy vitamin D [25(OH)D], serum vitamin B12, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and RBC folate. The specific determination methods were described in our recent publications.30–33 In brief, plasma folate, RBC folate, and vitamin B12 concentrations were quantified through chemiluminescence assay using a Beckman Coulter DxI 800 chemistry analyzer (Beckman Coulter Inc., Brea, CA, USA). The intra- and total coefficients of variation (%CV) for vitamin B12 were 4.8% and 6.6%, respectively. For serum folate, the mean intra/inter and total %CV were 3.0%/7.0% and 7.6%, respectively. The intra/inter and total %CV for RBC folate were 1.4%/1.6% and 2.1%, respectively. The limit of quantitation (LOQ) for folate was 0.18 ng/mL. Serum 25(OH)D concentrations were measured using rapid liquid chromatography—tandem mass spectrometry (LC-MS/MS) with a Waters ACQUITY UPLC system (Waters Corporation, Milford, MA, USA) and an AB Sciex 4000 QTrap system (Sciex Applied Biosystems, Foster City, CA, USA). The inter- and total %CV for 25OHD2 were 3.1%-4.1% and 4.2%-5.2%, respectively. For 25OHD3, the inter- and total %CV were 2.5%-2.8% and 3.8%-4.4%, respectively. The LOQ for 25OHD2 and 25OHD3 were 0.25 ng/mL and 0.22 ng/mL, respectively. Serum selenium levels were measured using inductively coupled plasma mass spectrometry (ICP-MS) (YINGSHENG BIOLOGY, YS EXT8600MD). The inter- and total %CV for selenium were 1.8%-2.1% and 4.7%-5.7%, respectively. The limit of detection (LOD) for selenium was 4.3 µg/L. CRP was measured using a Beckman Coulter AU5800 chemistry analyzer (Beckman Coulter Inc., Brea, CA, USA) with its supporting reagent.
Selenium deficiency was diagnosed if serum selenium levels were < 70 µg/L, and normal selenium status was defined as a level of ≥70 µg/L.31 Vitamin D deficiency was defined as a serum 25(OH)D concentration < 20 nmol/L, and non-vitamin D deficiency as a concentration of ≥20 nmol/L.34
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Science (version 16.0; SPSS, Chicago, IL, USA). Normally distributed variables were presented as means (SDs), while non-normally distributed variables were described as medians (interquartile range, 25%–75%). The independent-sample t-test was used to compare continuous variables between women with and without GDM, while the chi-square test was used for categorical variables. The ratio of RBC folate to vitamin B12 concentrations (RBC folate/vitamin B12 ratio) was calculated by dividing RBC folate concentrations (ng/mL) by vitamin B12 concentrations (pg/mL) using their raw data.11,27 The RBC folate/vitamin B12 ratios were divided into tertiles (T1, 0.47–1.79; T2, 1.80–2.65; T3, ≥ 2.66) based on their distribution in the overall study population. The association between RBC folate and vitamin B12 imbalance status and GDM risk was examined using logistic regression, stratified by serum selenium or vitamin D status. Results with P < 0.05 were considered statistically significant.
Results
Basic Characteristics of the Participants
A total of 366 eligible women with complete data were finally enrolled in this study. The participant flowchart is presented in Figure 1.
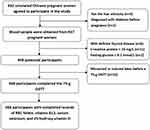 |
Figure 1 Participants flowchart. |
In this study population, 18.3% (n=67) were diagnosed with GDM, 66.9% (n=245) had selenium deficiency, and 54.9% (n=201) were determined to be vitamin D deficient. We did not report the participants’ smoking and drinking habits because no one had smoked or consumed alcohol for at least 3 months before recruitment.
Participants in the GDM group had significantly higher age, weight, BMI, CRP concentration, FPG, TG, and LDL-c (all P < 0.05). They also tended to have higher RBC folate concentrations (P = 0.069), serum selenium levels (P = 0.083), and 25(OH)D concentrations (P = 0.067). Furthermore, they were more likely to have a family history of diabetes (P < 0.01) but engaged in less exercise per week (P = 0.01) compared to those in the non-GDM group. No significant differences in height, parity status, FA supplement use (at enrollment), hemoglobin concentrations, ferritin levels, serum folate, Hcy, vitamin B12 concentrations, TC, and RBC folate to vitamin B12 ratios were observed between the two groups (all P > 0.05) (Table 1).
 |
Table 1 Maternal Characteristics in the GDM and Non-GDM Groups |
Associations of RBC Folate/Vitamin B12 Ratios with GDM Risk
RBC folate/vitamin B12 ratios were divided into tertiles according to the cutoff points of the distribution for the study population. In the overall study population, when using the lowest tertile as a reference, binary logistic analyses showed that women in the upper two tertiles of RBC folate/vitamin B12 ratio did not have a significantly higher risk of GDM, with or without adjustment for covariates (all P > 0.05) (Table 2). These covariates included age, BMI (at enrollment), education level, physical activity, parity status, family history of diabetes, FA supplement use, lipid profiles, CRP, ferritin, Hcy, hemoglobin, vitamin D status, and serum selenium.
 |
Table 2 Associations of GDM Risk with RBC Folate/Vitamin B12 Ratios in Overall Study Population |
Further, associations of RBC folate/vitamin B12 ratios with GDM risk were examined with logistic regression stratified by vitamin D status [deficiency, 25(OH)D < 20 nmol/L and non-deficiency, 25(OH)D ≥ 20 nmol/L] or serum selenium levels (deficiency, < 70 μg/L and normal, ≥ 70 μg/L).
When the analyses were stratified by serum selenium levels, in women with selenium deficiency, we found that women with RBC folate/vitamin B12 ratios in the highest tertile had a significantly higher risk of GDM [adjusted OR=3.398, 95% CI= 1.158–9.974, P = 0.026] than those in the lowest tertile of the ratios (Table 3). This result held after adjusting for age, BMI (at enrollment), education level, physical activity, parity status, family history of diabetes, FA supplement use, lipid profiles, CRP, ferritin, Hcy, hemoglobin, and vitamin D status. In contrast, similar associations were not observed in women with normal serum selenium.
 |
Table 3 Associations Between the RBC Folate/Vitamin B12 Ratios and GDM in Women with Normal Serum Selenium or Selenium Deficiency |
When the analyses were stratified by serum 25(OH)D levels, the results showed that women with RBC folate/vitamin B12 ratios in the upper two tertiles did not exhibit a significantly higher risk of GDM than those in the lowest tertile, in women with or without vitamin D deficiency (Table 4). This was after adjusting for age, BMI (at enrollment), education level, physical activity, parity status, family history of diabetes, FA supplement use, lipid profiles, CRP, ferritin, Hcy, hemoglobin, and serum selenium levels.
 |
Table 4 Associations Between the RBC Folate/Vitamin B12 Ratios and GDM in Women with or without Serum Vitamin D Deficiency |
Discussion
The data for this study were derived from a re-analysis of our previous cohort study, the primary aim of which was to investigate the effects of nutrients or metabolic status in early pregnancy on perinatal outcomes.30–33 Given the inconsistency observed in previous studies regarding the association between folate and/or vitamin B12 status and GDM, we hypothesized that the nutritional status of selenium or vitamin D might have an additional impact on this relationship. To our knowledge, this study is the first to explore whether selenium or vitamin D status affects the association between folate and/or vitamin B12 and the risk of GDM. The results of our secondary analysis did not reveal a significant difference in GDM risk between women with higher and lower levels of the RBC folate/vitamin B12 ratio. However, we did observe that the association between the unbalanced status of RBC folate and vitamin B12 and GDM risk could be influenced by serum selenium status, rather than vitamin D status.
Several previous studies have explored the relationship between the folate-to-vitamin B12 ratio and GDM risk.11,27 Li et al11 found a strong association between a higher folate-to-vitamin B12 ratio and an increased risk of GDM, whereas Chen et al27 reported no significant association between higher ratios of RBC folate to vitamin B12 and GDM. Although the reason for this discrepancy remains unknown, Chen and colleagues suggested that differences in the timing of vitamin B12 determinations during gestation might explain it.27 However, two recent studies, in which vitamin levels were measured early in pregnancy, reported differing results regarding the relationship between folate and vitamin B12 imbalances and GDM risk.10,27 In our study, we divided RBC folate/vitamin B12 ratios into tertiles, with the highest tertile representing an imbalance in folate and vitamin B12 status. We then conducted binary logistic analyses in the overall study population and found that women in the upper two tertiles of RBC folate/vitamin B12 ratios did not exhibit a significantly higher risk of GDM, with or without adjusting for covariates. Together with previous findings,10,27 these inconsistent results suggest that the association between nutrient imbalances and GDM risk may be influenced by other maternal factors.11,35
In our study, we observed that selenium and vitamin D deficiencies were common in early pregnancy. Some meta-analyses and reviews have reported a slight increase in the odds of GDM among women with low serum vitamin D levels.13,36 However, we found no significant relationship between vitamin D deficiency and GDM risk in our study (data not shown), nor did vitamin D status affect the relationship between the RBC folate-to-vitamin B12 ratio and GDM. On the other hand, selenium is an essential trace element with insulin-like properties that may play a role in maintaining normal glucose uptake, regulating cellular glucose utilization, and reducing the severity of insulin resistance.37,38 Therefore, selenium is expected to have a protective effect against GDM, and recent meta-analyses have shown that women with GDM have lower serum selenium levels compared to healthy pregnant women.14,15 Consistent with our hypothesis, we found that, in women with selenium deficiency, those with the highest tertile of RBC folate/vitamin B12 ratios in early pregnancy had a significantly higher risk of GDM than those with the lowest tertile of these ratios. In contrast, a similar association between high folate/low vitamin B12 and GDM was not observed in women with normal selenium levels. Conversely, when the analyses were stratified by vitamin D status, women with RBC folate/vitamin B12 ratios in the upper two tertiles did not exhibit a significantly higher risk of GDM, regardless of vitamin D status. Our findings suggest that an interaction between a high folate/low vitamin B12 status and selenium deficiency, rather than vitamin D deficiency, in early pregnancy may increase the risk of GDM. Although we cannot provide an explanation for these findings due to the study’s design, high folate status and/or low vitamin B12 may be related to immune dysfunction or adipogenic changes leading to insulin resistance.23,24 To our knowledge, this is the first study to examine whether a high folate/low vitamin B12 status, combined with other micronutrient deficiencies, can synergistically increase the risk of GDM in early pregnancy. Given the prevalence of high folate/low vitamin B12 status10 and selenium deficiency30 in early pregnancy, further research in this area is warranted.
The strengths of our study include its novel analysis of the associations between GDM risk and a high folate/low vitamin B12 status, in conjunction with other nutrient deficiencies, during the first trimester of pregnancy. Additionally, obstetric outcomes were carefully recorded by researchers who were blinded to blood measurements. However, our study has several limitations. First, the sample size was relatively small. Second, we did not quantitatively assess dietary folate and selenium intake or the duration of FA supplementation. Third, we only measured RBC folate concentration and serum selenium levels once during early pregnancy. Fourth, we cannot explain the underlying mechanisms behind our findings.
Conclusion
Our study suggests that women with a high maternal folate/vitamin B12 ratio, coupled with selenium deficiency in early pregnancy, have a higher risk of GDM. This finding underscores the importance of assessing micronutrient status in early pregnancy and implementing interventions for micronutrient deficiencies.
Abbreviations
GDM, gestational diabetes mellitus; 25(OH)D, 25-hydroxy vitamin D; FPG, fasting plasma glucose; IGT, impaired glucose tolerance; TC, total cholesterol; TG, triglycerides; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; CRP, C-reactive protein; OGTT, oral glucose tolerance test; RBC, red blood cell; Hcy, homocysteine; FA, folic acid.
Acknowledgment
We thank Shanghai GeneX Biotech Co., Ltd and Beijing Malt Health Management Co. Ltd for their free technical support. We also acknowledge Li ShanShan, Wang BingXin, Li BaoLei, Li Rui, Luo Haoze, Zhou Zikun, and Bao Yuanyuan, who helped with collecting anonymous data from routinely collected maternity records.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors.
Disclosure
The authors declare no conflict of interest.
References
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi:10.2337/dc14-S081
2. Juan J, Yang H. Prevalence, Prevention, and Lifestyle Intervention of Gestational Diabetes Mellitus in China. Int J Environ Res Public Health. 2020;17(24):9517. doi:10.3390/ijerph17249517
3. Nguyen CL, Pham NM, Binns CW, Duong DV, Lee AH. Prevalence of Gestational Diabetes Mellitus in Eastern and SouthEastern Asia: a Systematic Review and Meta-Analysis. J Diabetes Res. 2018;2018:6536974. doi:10.1155/2018/6536974
4. Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(Suppl 3):S173–S211. doi:10.1016/S0020-7292(15)30007-2
5. Lowe WL, Scholtens DM, Kuang A, et al. HAPO Follow-up Study Cooperative Research Group (2019) Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care. 2019;42(3):372–380. doi:10.2337/dc18-1646
6. Di Cianni G, Lacaria E, Lencioni C, Resi V. Preventing type 2 diabetes and cardiovascular disease in women with gestational diabetes—the evidence and potential strategies. Diabetes Res Clin Pract. 2018;145:184–192. doi:10.1016/j.diabres.2018.04.021
7. Franzago M, Fraticelli F, Stuppia L, Vitacolonna E. Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child. Epigenetics. 2019;14:215–235.
8. Zhu B, Ge X, Huang K, et al. Folic Acid Supplement Intake in Early Pregnancy Increases Risk of Gestational Diabetes Mellitus: evidence From a Prospective Cohort Study. Diabetes Care. 2016;39(3):e36–7. doi:10.2337/dc15-2389
9. Lai JS, Pang WW, Cai S, et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr. 2018;37:940–947. doi:10.1016/j.clnu.2017.03.022
10. Saravanan P, Sukumar N, Adaikalakoteswari A, et al. Association of maternal vitamin B12 and folate levels in early pregnancy with gestational diabetes: a prospective UK cohort study (PRiDE study). Diabetologia. 2021;64(10):2170–2182. doi:10.1007/s00125-021-05510-7
11. Li S, Hou Y, Yan X, et al. Joint effects of folate and vitamin B 12 imbalance with maternal characteristics on gestational diabetes mellitus. J Diabetes. 2019;11(9):744–751. doi:10.1111/1753-0407.12899
12. Li Q, Zhang Y, Huang L, et al. High-dose folic acid supplement use from prepregnancy through midpregnancy is associated with increased risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2019;42(7):e113–e115. doi:10.2337/dc18-2572
13. Milajerdi A, Abbasi F, Mousavi SM, Esmaillzadeh A. Maternal vitamin D status and risk of gestational diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Clin Nutr. 2021;40(5):2576–2586. doi:10.1016/j.clnu.2021.03.037
14. Kong FJ, Ma LL, Chen SP, Li G, Zhou JQ. Serum selenium level and gestational diabetes mellitus: a systematic review and meta-analysis. Nutr J. 2016;15(1):94. doi:10.1186/s12937-016-0211-8
15. Xu W, Tang Y, Ji Y, et al. The association between serum selenium level and gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2022;38(4):e3522. doi:10.1002/dmrr.3522
16. Paul L, Selhub J. Interaction between excess folate and low vitamin B12 status. Mol Aspects Med. 2017;53:43–47. doi:10.1016/j.mam.2016.11.004
17. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:183–189. doi:10.1001/jama.2016.19438
18. Policy and Research Team, Save the Children China Programme. Laws and Policies for Maternal and Young Child Health Care in China. Available from: http://resourcecentre.savethechildren.se/sites/default/files/documents/3378.pdf.
19. Yajnik CS, Deshpande SS, Jackson AA, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51(1):29–38. doi:10.1007/s00125-007-0793-y
20. Huang Y, He Y, Sun X, He Y, Li Y, Sun C. Maternal High Folic Acid Supplement Promotes Glucose Intolerance and Insulin Resistance in Male Mouse OffspringFed a High-Fat Diet. Int J Mol Sci. 2014;15(4):6298–6313. doi:10.3390/ijms15046298
21. Patel KR, Sobczyńska-Malefora A. The adverse effects of an excessive folic acid intake. Eur J Clin Nutr. 2017;71(2):159–163. doi:10.1038/ejcn.2016.194
22. Kouroglou E, Anagnostis P, Daponte A, Bargiota A. Vitamin B12 insufficiency is associated with increased risk of gestational diabetes mellitus: a systematic review and meta-analysis. Endocrine. 2019;66(2):149–156. doi:10.1007/s12020-019-02053-1
23. Bayer AL, Fraker CA. The Folate Cycle As a Cause of Natural Killer Cell Dysfunction and Viral Etiology in Type 1 Diabetes. Front Endocrinol (Lausanne). 2017;8:315. doi:10.3389/fendo.2017.00315
24. Adaikalakoteswari A, Vatish M, Alam MT, Ott S, Kumar S, Saravanan P. Low Vitamin B12 in Pregnancy Is Associated With Adipose-Derived Circulating miRs Targeting PPARγ and Insulin Resistance. J Clin Endocrinol Metab. 2017;102(11):4200–4209. doi:10.1210/jc.2017-01155
25. Krishnaveni GV, Hill JC, Veena SR, et al. Low plasma vitamin B12 in pregnancy is associated with gestational “diabesity” and later diabetes. Diabetologia. 2009;52:2350–2358. doi:10.1007/s00125-009-1499-0
26. Barzilay E, Moon A, Plumptre L, et al. Fetal one-carbon nutrient concentrations may be affected by gestational diabetes. Nutr Res. 2018;55:57–64. doi:10.1016/j.nutres.2018.04.010
27. Chen X, Zhang Y, Chen H, et al. Association of Maternal Folate and Vitamin B12 in Early Pregnancy With Gestational Diabetes Mellitus: a Prospective Cohort Study. Diabetes Care. 2021;44(1):217–223. doi:10.2337/dc20-1607
28. Zhang Y, Gong Y, Xue H, Xiong J, Cheng G. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG. 2018;125(7):784–793. doi:10.1111/1471-0528.15060
29. Yang L, Song L, Xu X, Liu Y, Li H, Tang L. Prevalence of Vitamin D Deficiency during Second Trimester of Pregnancy in Shanghai China, Risk Factors and Effects on Pregnancy Outcomes. Iran J Public Health. 2018;47(8):1145–1150.
30. Liu PJ, Yao A, Ma L, et al. Associations of Serum Selenium Levels in the First Trimester of Pregnancy with the Risk of Gestational Diabetes Mellitus and Preterm Birth: a Preliminary Cohort Study. Biol Trace Elem Res. 2021;199(2):527–534. doi:10.1007/s12011-020-02191-y
31. Liu PJ, Liu Y, Ma L, et al. Associations Between Gestational Diabetes Mellitus Risk and Folate Status in Early Pregnancy and MTHFR C677T Polymorphisms in Chinese Women. Diabetes Metab Syndr Obes. 2020;13:1499–1507. doi:10.2147/DMSO.S250279
32. Liu PJ, Yao A, Chen XY, Liu Y, Ma L, Hou YX. Associations of TMPRSS6 Polymorphisms with Gestational Diabetes Mellitus in Chinese Han Pregnant Women: a Preliminary Cohort Study. Biol Trace Elem Res. 2021;199(2):473–481. doi:10.1007/s12011-020-02169-w
33. Liu PJ, Liu Y, Ma L, et al. The Predictive Ability of Two Triglyceride-Associated Indices for Gestational Diabetes Mellitus and Large for Gestational Age Infant Among Chinese Pregnancies: a Preliminary Cohort Study. Diabetes Metab Syndr Obes. 2020;13:2025–2035. doi:10.2147/DMSO.S251846
34. Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr. 2007;46(1):42–44. doi:10.1177/0009922806289311
35. Maher A, Sobczyńska-Malefora A. The Relationship Between Folate, Vitamin B12 and Gestational Diabetes Mellitus With Proposed Mechanisms and Foetal Implications. J Family Reprod Health. 2021;15:141–149. doi:10.18502/jfrh.v15i3.7131
36. Sadeghian M, Asadi M, Rahmani S, et al. Circulating vitamin D and the risk of gestational diabetes: a systematic review and dose-response meta-analysis. Endocrine. 2020;70(1):36–47. doi:10.1007/s12020-020-02360-y
37. Ezaki O. The insulin-like effects of selenate in rat adipocytes. J Biol Chem. 1990;265:1124–1128. doi:10.1016/S0021-9258(19)40166-X
38. Stapleton SR. Selenium: an insulin-mimetic. Cell Mol Life Sci. 2000;57(13):1874–1879. doi:10.1007/PL00000669
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
