Back to Journals » Clinical Epidemiology » Volume 14
The Association Between Statin Use and Risk of Chronic Kidney Disease in Community-Dwelling Older People in Shanghai, China
Authors Zhao M, Ren L , Zhou Z, Wang T , Li J
Received 31 January 2022
Accepted for publication 9 June 2022
Published 25 June 2022 Volume 2022:14 Pages 779—788
DOI https://doi.org/10.2147/CLEP.S360395
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eyal Cohen
Miaomiao Zhao,1,2 Longbing Ren,3 Zhitong Zhou,3 Tao Wang,3 Jue Li2
1School of Clinical Medicine, Shanghai University of Medicine and Health Sciences, Shanghai, People’s Republic of China; 2Department of Epidemiology, Tongji Hospital Affiliated to Tongji University School of Medicine, Shanghai, People’s Republic of China; 3Institute of Clinical Epidemiology and Evidence-Based Medicine, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Jue Li, Department of Epidemiology, Tongji Hospital Affiliated to Tongji University School of Medicine, 389 Xincun Road, Shanghai, 200442, People’s Republic of China, Tel +86-21-65986735, Fax +86-21-65980448, Email [email protected]
Purpose: The effects of statins on renal outcomes have already been studied in patients with chronic kidney disease (CKD); however, data on the general population are limited. We evaluated the association between statin use and risk of CKD in community-dwelling older people in Shanghai, China.
Patients and Methods: This registry-based cohort study was conducted in four communities in four districts in Shanghai. Participants with an estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2 in 2016 were eligible for the study, and new-onset CKD in 2017, 2018, and 2019 was recorded. Poisson generalized linear models were conducted to examine the relationships among statin therapy, dyslipidemia, and CKD; linear mixed-effects models were conducted to examine the relationships between statin therapy and changes in eGFR. All analyses were performed with both conventional adjustment and propensity score-matching methods.
Results: Of the study cohort of 2455 participants (41.1% men; average age, 68.06 years), 624 (25.4%) were treated with stains. Two propensity score-matched cohorts of 604 participants each were analyzed (statin users and nonusers). Statin use was significantly associated with a decreased risk of new-onset CKD with hazard ratios (HRs) and 95% confidence intervals (CIs) of 0.73 (0.59 to 0.91) (p< 0.01) in the unmatched cohort and 0.75 (0.59 to 0.97) (p=0.02) in the matched cohort. There were significant differences in the eGFR decline between statin users and nonusers from baseline to 3 years in the unmatched and matched cohorts (both p< 0.05). In addition, both statin users and nonusers with dyslipidemia experienced more new-onset CKD (both p< 0.05).
Conclusion: Statin use was significantly associated with a decreased risk of new-onset CKD and a slower decline in eGFR in community-dwelling older people. Meanwhile, dyslipidemia was a risk factor for CKD progression among both statin users and nonusers.
Keywords: chronic kidney disease, statins, dyslipidemia, community-dwelling older people
Introduction
Chronic kidney disease (CKD) is a major health problem associated with increased risk of all-cause mortality, cardiovascular disease, and end-stage renal disease.1–5 When CKD is defined as an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2, its prevalence is 5.2% in England6 and between 2.5% and 11.2% in Europe, North America, Asia, and Australia.7 In 2012, the prevalence of CKD was 10.8% in China, suggesting that nearly 120 million adults had the disease.8 The global incidence and mortality of CKD is continually rising, and its high medical costs impose a considerable burden on patients, their families, and the world.
Statins are the mainstay of the primary and secondary prevention of cardiovascular disease in the general population.9,10 Statins also have beneficial effects in the treatment of diabetic nephropathy.11–15 Growing numbers of studies have evaluated the renal protective effects of statins. The Assessment of Lescol in Renal Transplant (ALERT) trial and the Scandinavian Simvastatin Survival Study (4S) demonstrated that statins slowed CKD progression.16,17 However, the large Study of Heart and Renal Protection (SHARP), which included 6245 participants with advanced CKD, found that statin administration did not reduce the risk of kidney failure or rate of change in eGFR.18 A previous systematic review and meta-analysis including 57 studies suggested that statin therapy does not reduce the risk of kidney failure events in adults not receiving dialysis but may modestly reduce proteinuria and the rate of eGFR decline.19 Most of these studies were performed using patients with kidney disease. To our knowledge, no research has thus far been conducted into the associations between statins and renal outcomes in a community-dwelling population.
An abnormal lipid metabolism is common in patients with kidney disease.20,21 Post hoc analyses of several large trials have demonstrated that dyslipidemia is significantly associated with increased risk of a reduced kidney function or faster eGFR decline in a general population without kidney disease.22,23 Statins can significantly improve lipid profiles and the use of statins to lower lipid levels has renoprotective effects.24,25 However, few studies have evaluated whether dyslipidemia has a differential impact on renal outcomes between statin users and nonusers.
In the present study, we investigated the association of statin use with the risk of CKD and changes in eGFR in community-dwelling older people in Shanghai, China. In addition, we evaluated the impact of dyslipidemia on CKD among statin users and nonusers.
Materials and Methods
Study Design and Participants
Data of this investigation were obtained from the “Research on Community Elderly Population of Tongji University” (RECEPT) study. The purpose of the RECEPT study was to describe the prevalence, incidence, and natural history of cardiovascular risk factors in community-dwelling older people in Shanghai, China. We adopted a multi-stage, stratified, random cluster sampling scheme. In the first stage, two districts were selected from each of the eight urban districts and eight suburbs of Shanghai; in the second stage, one community was randomly selected from each district. Free medical physical examination services are provided annually to community-dwelling people aged ≥ 60 years in Shanghai, China. We obtained the electronic medical records of health examinations from the Physical Examination System in these four districts in 2016, 2017, 2018, and 2019.
Given the focus of this investigation, we assembled a cohort, with participants with an eGFR≥60 mL/min/1.73 m2 in 2016 eligible for the baseline study. Follow-up was conducted in 2017, 2018, and 2019 and new-onset CKD during follow-up was recorded. Therefore, using the physical examination data from 2016 to 2019, we analyzed the relationship of statin prescription with new-onset CKD and changes in eGFR and that of dyslipidemia with new-onset CKD in both statin users and nonusers. The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Tongji University. All procedures were performed in accordance with ethical standards. Written consent was obtained from each participant after they had been informed of the objectives, benefits, medical items, and confidentiality of personal information.
Participants were eligible for the study if they (1) were≥60 years old, (2) had a Shanghai household registration or were resident in this community for more than three years, (3) had an eGFR ≥ 60 mL/min/1.73 m2 in 2016, and (4) had complete baseline data in 2016, and serum creatinine (Scr) measurements in 2017, 2018, and 2019. Exclusion criteria included (1) an eGFR < 60 mL/min/1.73 m2 in 2016, (2) incomplete baseline data provided in 2016, (3) presence of severe mental illness, cognitive impairment or malignant tumors in 2016, and (4) incomplete Scr measurements provided in 2017, 2018, or 2019 (Figure 1).
 |
Figure 1 Flowchart of study inclusion. |
Data Collection and Outcome Measures
For each patient, comprehensive data were extracted from electronic medical records. This included participant demographics (age and sex), body mass index (BMI), waist-to-hip ratio (WHR), systolic blood pressure (SBP) and diastolic blood pressure (DBP). We also collected information on participant clinical data (including the prescription of angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers [ACEIs/ARBs], calcium channel blockers [CCBs], beta-blockers, diuretics, and antidiabetic drugs, and medical history of hypertension and diabetes mellitus) and laboratory data (including glycated hemoglobin [HbA1c], fasting plasma glucose [FPG], total cholesterol [TC], triglyceride [TG], high-density lipoprotein [HDL], low-density lipoprotein [LDL], aspartate transaminase [AST], alanine transaminase [ALT], blood urea nitrogen [BUN], total bilirubin [TBIL], uric acid [UA] and Scr).
The eGFR was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula: for males, eGFR = 141 × (Scr/0.9)−0.411 × 0.993age if Scr ≤ 80 mmol/L and eGFR = 141 × (Scr/0.9)−1.209 × 0.993age if Scr > 80 mmol/L; for females, eGFR = 144 × (Scr/0.7)−0.329 × 0.993age if Scr ≤ 62 mmol/L and eGFR = 144 × (Scr/0.7)−1.209 × 0.993age if Scr > 62 mmol/L.26 New-onset CKD was defined as the occurrence of eGFR < 60 mL/min/1.73 m2 during follow-up. Dyslipidemia was defined as TC ≥ 6.2 mmol/L, LDL ≥ 4.1 mmol/L, HDL < 1.0 mmol/L, or TG ≥ 2.3 mmol/L.27
Statistical Analysis
To reduce potential confounding and selection bias, we used the propensity score-matching method. Propensity scores were calculated through a logistic regression model, with statin users versus nonusers as the dependent variable and all covariates measured at baseline as independent variables (Table 1). Matched pairs were obtained using a greedy nearest-neighbor matching algorithm (1:1 ratio, without replacement) and with a caliper width equal to 0.2 of the SD of the logit of the propensity score.28 A standardized difference of less than 0.1 was used to indicate a negligible difference in covariates between the groups.
 |
Table 1 Baseline Characteristics of Statin Users and Nonusers Before and After Propensity Score Matching |
Poisson generalized linear models were conducted to examine the association between statin use and new-onset CKD rates. The covariates listed in Table 1 were adjusted in the unmatched cohort analyses. Linear mixed-effects models were conducted to examine the association between statin use and changes in eGFR. Participant ID was included as a random effect, with time (baseline, 1, 2, and 3 years), group (statin users versus nonusers), and time-group interactions included as fixed effects. The covariates listed in Table 1 were included as fixed effects in the unmatched cohort analyses.
Meanwhile, the impact of dyslipidemia on new-onset CKD was assessed among both statin users and nonusers. We repeated the propensity score matching (Supplementary Tables 1 and 2) and compared the event rates between participants with normal blood lipids and those with dyslipidemia. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA) and R 4.0.2. Two-sided p values less than 0.05 indicated statistical significance.
Results
The study cohort comprised 2455 older Chinese people. Their average age was (68.06 ± 2.65) years old, and 1008 (41.1%) were men. Statins were prescribed to 25.4% (624 of 2455) of the participants. Compared with the nonstatin group, the statin group had a higher prevalence of female participants, nonsmokers, antihypertensive drugs and antidiabetic drugs, and higher levels of DBP, HbA1c, TC, HDL, TG, AST, and ALT. Two propensity score-matched cohorts of 604 participants were defined according to statin use and analyzed. After propensity score matching, no significant differences in baseline characteristics were found between statin and nonstatin groups (Table 1).
Participants were followed up for 3 years. The incidences of new-onset CKD for one, two, and three times were 10.6% (66 of 624), 3.0% (19 of 624), and 0.5% (3 of 624) in the unmatched cohort for statin users, and 14.6% (267 of 1831), 3.6% (66 of 1831), and 1.0% (19 of 1831) for nonusers (p=0.03) (Figure 2A). After propensity score matching, the incidences were 10.4% (63 of 604), 3.1% (19 of 604), and 0.5% (3 of 604) for statin users and 13.9% (84 of 604), 3.6% (22 of 604), and 1.0% (6 of 604) for nonusers (p=0.18) (Figure 2B). Poisson generalized linear analyses showed that statin use was significantly associated with a decreased risk of new-onset CKD, with hazard ratios (HRs) and 95% confidence intervals (CIs) of 0.73 (0.59 to 0.91) (p<0.01) after adjustment for the covariates in the unmatched cohort and 0.75 (0.59 to 0.97) (p=0.02) in the matched cohort (Table 2).
 |
Table 2 Association of Statin Use with Risk of CKD Progression and Changes in eGFR |
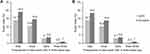 |
Figure 2 Frequencies of new-onset CKD in three follow-up visits among statin users and nonusers in the unmatched cohort (A) and matched cohort (B). |
The changes in eGFR from baseline to 1, 2, and 3 years in statin users and nonusers are demonstrated in Table 2. The declines in eGFR were −7.11 mL/min/1.73 m2 (95% CI, −8.10 to −6.11) and −8.54 mL/min/1.73 m2 (95% CI, −9.16 to −7.92) in the statin and nonstatin groups from baseline to 3 years in the unmatched cohort (p=0.02). These declines were −7.00 mL/min/1.73 m2 (95% CI, −7.93 to −5.99) and −8.30 mL/min/1.73 m2 (95% CI, −9.33 to −7.27) in the matched cohort (p=0.04).
We repeated the propensity score matching for statin users and nonusers with dyslipidemia and normal blood lipids (Supplementary Tables 1 and 2). Poisson generalized linear analyses showed that compared with individuals with normal blood lipids, those with dyslipidemia experienced more new-onset CKD, both statin users (HR: 1.61, 95% CI: 1.10 to 2.36, p=0.01) and nonusers (HR: 1.49, 95% CI: 1.22 to 1.81, p<0.01), after adjustment for the covariates in the unmatched cohort. In addition, the HRs (95% CIs) were 1.54 (1.01 to 2.36) (p=0.04) and 1.32 (1.05 to 1.68) (p=0.02) in the matched cohort, respectively (Table 3).
 |
Table 3 Association Between Dyslipidemia and Risk of CKD Progression in Statin Users and Nonusers |
Discussion
In the present study, we used both a conventional adjustment method and propensity score-matching method to evaluate the association between statin prescription and kidney function in community-dwelling older people. Statin use was significantly associated with a decreased risk of new-onset CKD with HRs (95% CIs) of 0.73 (0.59 to 0.91) (p<0.01) in the unmatched cohort and 0.75 (0.59 to 0.97) (p=0.02) in the matched cohort. There were significant differences in the eGFR decline between statin users and nonusers in the unmatched and matched cohorts from baseline to 3 years. Additionally, both statin users and nonusers with dyslipidemia experienced more new-onset CKD.
CKD is a global health concern.29 Individuals with CKD have a higher risk of premature mortality related to cardiovascular disease, even during the early stages of the disease; this represents a significant challenge for physicians.30 Epidemiological studies have determined an estimated 8% to 16% prevalence of CKD worldwide,31 as well as 10.2% prevalence in adults in the United States.32 With ever increasing global population aging, the disease burden of CKD is continually increasing. The effects of statins on kidney disease progression remain subject to debate. Although some trials evaluating the effects of statins on kidney disease outcomes have shown benefits of statins,16,17,33 others have shown no effect.18,34,35 However, most of these studies analyzed patients with kidney disease. In the present study, the cohort comprised individuals without kidney disease. To our knowledge, our study is the first to investigate the associations between statins and renal outcomes in community-dwelling older people. We found that statin use was associated with decreased new-onset CKD rates in the 3-year follow-up. Accumulating evidence shows the favorable efficacy and safety profiles of statin therapy in community-dwelling older populations.36,37 Therefore, it can be expected that the prevalence of statin use in the elderly population will continue growing. The additional benefits of statins in preventing incident CKD may be of great clinical importance.
Statins, which are inhibitors of the hydroxymethylglutaryl-CoA (HMG-CoA) reductase enzyme, are powerful cholesterol-lowering medications. Statins limit cholesterol biosynthesis by competitively inhibiting HMG-CoA reductase activity38 and further upregulating the expression of LDL receptors in liver cell membranes, increasing the clearance of circulating LDL-cholesterol from the blood.39 They then improve renal function by ameliorating the damage to glomerular and tubular caused by cell lipids.40 Beyond lipid lowering, statins slow the decline of the GFR and may also reduce proteinuria.41,42 The mechanisms involved may include effects on renal nitric oxide (NO), endothelin-1 bioactivity and reduction of prenylation.41,43,44 Statins have other therapeutic benefits, such as anti-inflammatory and antioxidant properties, apoptosis induction, inhibition of vascular smooth muscle cell proliferation, platelet activation and aggregation reduction, and increased atherosclerotic plaque stability.15,29,45 Many of these effects potentially arise from small G-protein disruption. Because of the resultant biological and genetic stress, activation of G-protein signaling is pivotal in renal pathologies.46 Effects on endothelial function may also explain the apparent renal benefits of statin therapy, which may improve renal perfusion while reducing abnormal permeability to plasma proteins.47,48 Accordingly, the mechanisms by which statin therapy reduces CKD risk remain controversial.
One of the strengths of our study is the application of robust statistical methods, including propensity score matching, to real-world patients. The propensity score matching was based on numerous covariates and allowed us to reduce the impact of selection biases and compare two matched groups. The participants in our study were enrolled from multiple medical centers in Shanghai, and a multi-stage, stratified, random cluster sampling scheme was adopted to verify data accuracy. In addition, we not only evaluated the relationship of statin therapy with new-onset CKD and changes in eGFR in community residents, but also analyzed the impact of dyslipidemia on kidney function in both statin users and nonusers. At the same time, we examined the frequency of new-onset CKD in three follow-up visits, not just whether it occurred, which is an additional strength of our study.
In light of these results, several limitations should also be considered. First, we could not conclude whether the association depends on statin type, dosage, or treatment duration, thus, further research is needed. Second, this was a retrospective observational study, which is not a perfect substitute for a randomized trial. Third, we did not collect data regarding the presence of albuminuria, which limited the interpretation of the results. Finally, our findings were obtained from random sampling in Shanghai and are therefore inadequate for generalization to China as a whole. Thus, further validation of studies incorporating other regions would better depict the results of a nationwide population.
Conclusions
The results of this study suggest that statin use was significantly associated with a decreased risk of new-onset CKD and a slower decline in eGFR in community-dwelling older people in Shanghai, China. Meanwhile, dyslipidemia was a risk factor for CKD progression among both statin users and nonusers. Further randomized controlled studies are needed to confirm the renoprotective efficacy of statins among community-dwelling older people.
Abbreviations
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; Scr, serum creatinine; BMI, body mass index; WHR, waist-to-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEIs/ARBs, angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers; CCBs, calcium channel blockers; HbA1c, glycated hemoglobin; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate transaminase; ALT, alanine transaminase; BUN, blood urea nitrogen; TBIL, total bilirubin; UA, uric acid; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; HR, hazard ratio; CI, confidence interval; HMG-CoA, hydroxymethylglutaryl-CoA.
Data Sharing Statement
All the data supporting the study findings are within the manuscript. Additional detailed information and raw data are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Tongji University. Written consent was obtained from each participant after they had been informed of the objectives, benefits, medical items, and confidentiality of personal information.
Acknowledgments
We thank all participants for their dedication to the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research was funded by National Natural Science Foundation of China (82003540), Three-Year ActionProgram of Shanghai Municipality for Strengthening the Construction of Public Health System (GWV-10.2-YQ39), Special Project for Clinical Research in Health Industry of Shanghai Municipal Health Commission (20194Y0116), and Academic Mentorship for Scientific Research Cadre Project of Shanghai University of Medicine and Health Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80(1):17–28. doi:10.1038/ki.2010.483
2. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi:10.1016/S0140-6736(10)60674-5
3. van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi:10.1038/ki.2010.536
4. Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi:10.1038/ki.2010.531
5. Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi:10.1038/ki.2010.550
6. Fraser SD, Aitken G, Taal MW, et al. Exploration of chronic kidney disease prevalence estimates using new measures of kidney function in the health survey for England. PLoS One. 2015;10(2):e0118676. doi:10.1371/journal.pone.0118676
7. James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375(9722):1296–1309. doi:10.1016/S0140-6736(09)62004-3
8. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi:10.1016/S0140-6736(12)60033-6
9. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71(1):85–94. doi:10.1016/j.jacc.2017.10.080
10. Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757–769. doi:10.1038/s41569-018-0098-5
11. Takazakura A, Sakurai M, Bando Y, et al. Renoprotective effects of atorvastatin compared with pravastatin on progression of early diabetic nephropathy. J Diabetes Investig. 2015;6(3):346–353. doi:10.1111/jdi.12296
12. Shen X, Zhang Z, Zhang X, et al. Efficacy of statins in patients with diabetic nephropathy: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2016;15(1):179. doi:10.1186/s12944-016-0350-0
13. Lv J, Ren C, Hu Q. Effect of statins on the treatment of early diabetic nephropathy: a systematic review and meta-analysis of nine randomized controlled trials. Ann Palliat Med. 2021;10(11):11548–11557. doi:10.21037/apm-21-2673
14. Abe M, Maruyama N, Okada K, Matsumoto S, Matsumoto K, Soma M. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy. J Atheroscler Thromb. 2011;18(11):1018–1028. doi:10.5551/jat.9084
15. de Zeeuw D, Anzalone DA, Cain VA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol. 2015;3(3):181–190. doi:10.1016/S2213-8587(14)70246-3
16. Fellström B, Holdaas H, Jardine AG, et al. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney Int. 2004;66(4):1549–1555. doi:10.1111/j.1523-1755.2004.00919.x
17. Huskey J, Lindenfeld J, Cook T, et al. Effect of simvastatin on kidney function loss in patients with coronary heart disease: findings from the Scandinavian simvastatin survival study (4S). Atherosclerosis. 2009;205(1):202–206. doi:10.1016/j.atherosclerosis.2008.11.010
18. Haynes R, Lewis D, Emberson J, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 2014;25(8):1825–1833. doi:10.1681/ASN.2013090965
19. Su X, Zhang L, Lv J, et al. Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am J Kidney Dis. 2016;67(6):881–892. doi:10.1053/j.ajkd.2016.01.016
20. Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166(17):1884–1891. doi:10.1001/archinte.166.17.1884
21. Noels H, Lehrke M, Vanholder R, Jankowski J. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol. 2021;17(8):528–542. doi:10.1038/s41581-021-00423-5
22. Schaeffner ES, Kurth T, Curhan GC, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14(8):2084–2091. doi:10.1681/ASN.V1482084
23. Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293–301. doi:10.1046/j.1523-1755.2000.00165.x
24. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008–2024. doi:10.1001/jama.2015.15629
25. Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z. Lipid accumulation and chronic kidney disease. Nutrients. 2019;11(4):722. doi:10.3390/nu11040722
26. Multi-Disciplinary Expert Task Force on Hyperuricemia and Its Related Diseases. Chinese multidisciplinary expert consensus on the diagnosis and treatment of hyperuricemia and related diseases. Chin Med J. 2017;130(20):2473–2488. doi:10.4103/0366-6999.216416
27. Joint committee for guideline revision 2016. Chinese guideline for the management of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44(10):833–853. doi:10.3760/cma.j.issn.0253-3758.2016.10.005
28. Jiang S, Huang X, Zhang L, et al. Estimated survival and major comorbidities of very preterm infants discharged against medical advice vs treated with intensive care in China. JAMA Netw Open. 2021;4(6):e2113197. doi:10.1001/jamanetworkopen.2021.13197
29. Hu PJ, Wu MY, Lin TC, et al. Effect of statins on renal function in chronic kidney disease patients. Sci Rep. 2018;8(1):16276. doi:10.1038/s41598-018-34632-z
30. Cho EY, Myoung C, Park HS, et al. Efficacy of statin treatment in early-stage chronic kidney disease. PLoS One. 2017;12(1):e0170017. doi:10.1371/journal.pone.0170017
31. Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi:10.1016/S0140-6736(13)60687-X
32. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi:10.1001/jama.298.17.2038
33. Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112(2):171–178. doi:10.1161/CIRCULATIONAHA.104.517565
34. Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the air force/texas coronary atherosclerosis prevention study. Am J Kidney Dis. 2010;55(1):42–49. doi:10.1053/j.ajkd.2009.09.020
35. Rahman M, Baimbridge C, Davis BR, et al. Progression of kidney disease in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Am J Kidney Dis. 2008;52(3):412–424. doi:10.1053/j.ajkd.2008.05.027
36. Zhou Z, Ofori-Asenso R, Curtis AJ, et al. Association of statin use with disability-free survival and cardiovascular disease among healthy older adults. J Am Coll Cardiol. 2020;76(1):17–27. doi:10.1016/j.jacc.2020.05.016
37. Zhou Z, Ryan J, Ernst ME, et al. Effect of statin therapy on cognitive decline and incident dementia in older adults. J Am Coll Cardiol. 2021;77(25):3145–3156. doi:10.1016/j.jacc.2021.04.075
38. Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol. 2018;14(1):57–70. doi:10.1038/nrneph.2017.155
39. Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88(88):3–11. doi:10.1016/j.phrs.2014.03.002
40. Kong Y, Feng W, Zhao X, et al. Statins ameliorate cholesterol-induced inflammation and improve AQP2 expression by inhibiting NLRP3 activation in the kidney. Theranostics. 2020;10(23):10415–10433. doi:10.7150/thno.49603
41. Agarwal R. Effects of statins on renal function. Mayo Clin Proc. 2007;82(11):1381–1390. doi:10.4065/82.11.1381
42. Tonelli M, Moyé L, Sacks FM, Cole T, Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14(6):1605–1613. doi:10.1097/01.ASN.0000068461.45784.2F
43. Elisaf M, Mikhailidis DP. Statins and renal function. Angiology. 2002;53(5):493–502. doi:10.1177/000331970205300501
44. Tsiara S, Elisaf M, Mikhailidis DP. Early vascular benefits of statin therapy. Curr Med Res Opin. 2003;19(6):540–556. doi:10.1185/030079903125002225
45. Deedwania PC, Stone PH, Fayyad RS, Laskey RE, Wilson DJ. Improvement in renal function and reduction in serum uric acid with intensive statin therapy in older patients: a post hoc analysis of the SAGE trial. Drugs Aging. 2015;32(12):1055–1065. doi:10.1007/s40266-015-0328-z
46. Park F. Activators of G protein signaling in the kidney. J Pharmacol Exp Ther. 2015;353(2):235–245. doi:10.1124/jpet.115.222695
47. Tonelli M. Do statins protect the kidney by reducing proteinuria? Ann Intern Med. 2006;145(2):147–149. doi:10.7326/0003-4819-145-2-200607180-00015
48. Bedi O, Dhawan V, Sharma PL, Kumar P. Pleiotropic effects of statins: new therapeutic targets in drug design. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(7):695–712. doi:10.1007/s00210-016-1252-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
