Back to Journals » Journal of Inflammation Research » Volume 13
The Association Between Sortilin and Inflammation in Patients with Coronary Heart Disease
Authors Han W, Wei Z, Zhang H, Geng C, Dang R, Yang M, Zhang J, Wang C, Jiang P
Received 28 November 2019
Accepted for publication 21 January 2020
Published 10 February 2020 Volume 2020:13 Pages 71—79
DOI https://doi.org/10.2147/JIR.S240421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Wenxiu Han,1 Zhijie Wei,2 Hailiang Zhang,1 Chunmei Geng,1 Ruili Dang,1 Mengqi Yang,1 Jun Zhang,3 Changshui Wang,1 Pei Jiang1
1Department of Pharmacy, Jining First People’s Hospital, Jining Medical University, Jining 272011, People’s Republic of China; 2Department of Medical Administration, Jining First People’s Hospital, Jining Medical University, Jining 272011, People’s Republic of China; 3Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China
Correspondence: Pei Jiang; Ruili Dang
Jining First People’s Hospital, Jining Medical University, Jining, People’s Republic of China
Tel/Fax +86 537 2106208
Email [email protected]; [email protected]
Purpose: Inflammation is a key contributor to coronary heart disease (CHD). Sortilin is a sorting receptor and has been identified as a critical regulator of inflammatory response. Therefore, our study aimed to determine the link between circulating sortilin levels, proinflammatory cytokine levels, and the occurrence of CHD.
Patients and Methods: Our study included 227 CHD patients and 101 matched healthy individuals. Circulating serum levels of sortilin and proinflammatory cytokines, including IL-1β, IL-6 and TNF-α, were assessed by a double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA). Linear regression and correlation analyses were used to estimate the associations between sortilin and proinflammatory cytokines. Moreover, six single-nucleotide polymorphisms (SNPs) spanning the sortilin and SORL1 genes were genotyped.
Results: Elevated levels of sortilin (P=0.027) and proinflammatory cytokines IL-1β (P=0.013), IL-6 (P=0.000) and TNF-α (P=0.010) were observed in CHD patients compared to those in healthy controls. Furthermore, sortilin levels were significantly positively correlated with IL-1β (r=0.252, P=0.0001), IL-6 (r=0.250, P=0.0001) and TNF-α (r=0.180, P=0.0064) levels. Notably, sortilin polymorphisms were revealed to be associated with the occurrence of CHD and varying sortilin levels. Subjects with the rs599839 AA risk genotype for CHD had significantly higher sortilin levels than those with the GG and GA genotypes (P=0.000); the same tendency was also observed in the levels of the proinflammatory cytokines IL-1β (P=0.003) and TNF-α (P=0.000). Similarly, GG carriers of rs464218 with increased sortilin levels were found to be at increased risk for CHD (P=0.014). The levels of IL-1β (P=0.025) and IL-6 (P=0.015) were also increased in these patients.
Conclusion: Our findings reveal that high sortilin levels may interact with inflammatory response to contribute to the occurrence of CHD. Considering that our clinical evidence suggests for the first time that sortilin involves in inflammatory response in CHD, the mechanistic role of sortilin in the progression of CHD deserves detailed investigation.
Keywords: sortilin, inflammation, SORL1, polymorphism, coronary heart disease
Introduction
Coronary heart disease (CHD) is the most common form of disease affecting the heart and is considered to be a major public health burden throughout the world. Atherosclerosis, as the pathological basis of CHD, has become a particular focus of attention worldwide. Atherosclerosis has long been considered a degenerative disease caused by the continuous accumulation of cholesterol in the arterial intima.1 However, more recent data have redefined atherosclerosis as a complex disorder of chronic inflammation.2,3 Accumulating evidence has been published supporting the role of inflammation in the initiation and progression of atherosclerosis. Luc et al reported that active inflammatory processes may trigger plaque rupture and enhance the risk of a clinically significant atherothrombotic event, according to histopathological and immunochemical observations.4 Furthermore, data are emerging regarding the role of inflammation in typical dyslipidemia associated with elevated very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL) and triglycerides as well as reduced high-density lipoprotein (HDL) levels.5 Importantly, proinflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α are secreted in all phases of atherosclerotic lesion progression,6,7 suggesting their potential role in the occurrence of CHD.
Sortilin and sortilin-related receptor 1 (SORL1, also known as SORLA) are members of the Vps10p domain receptor family that were discovered in the 1990s.8,9 These two proteins have been extensively studied due to their functions as regulators of intracellular trafficking through their Vps10p domain. Due to playing an essential role in cell signaling by acting as sorting regulators or receptors/coreceptors, both sortilin and SORL1 are involved in many associated cellular disorders.10,11 Recently, a few studies in sortilin knockout mice have reported that sortilin is involved in the regulation of cytokine secretion during immune responses through the control of IL-6 and Interferon-γ (IFN-γ) exocytosis.12 In addition to binding to IFN-γ and IL-6, sortilin has also been demonstrated to bind to other cytokines, such as Interferon-α (IFN-α), interleukin-17A, interleukin-10, and interleukin-12, in immune cells.13 Interestingly, evidence in HEK293 cells has shown that the endocytic receptor SORL1 may impact cellular uptake as well as IL-6 signaling.14 Conversely, knockdown of SORL1 reduces extracellular levels of the proinflammatory cytokine IL-6 in astrocyte cultures.15 Therefore, it is feasible to speculate that the serum sortilin level may interact with inflammatory response and be related to CHD susceptibility. Additionally, clinical studies identified sortilin as a risk factor for cardiovascular disease,16 and SORL1 is also considered to contribute to the development of atherogenesis.17 However, the mechanism is not entirely clear.
Although some basic research into the relationship among sortilin, inflammation and CHD has been undertaken, direct clinical evidence of sortilin level as a marker for CHD risk, as well as of the role of inflammation as a potential confounder of such an association, is lacking. To provide clinical evidence for our hypothesis, we determined the circulating serum levels of sortilin and proinflammatory cytokines, including IL-1β, IL-6 and TNF-α, in 227 CHD patients and 101 healthy controls. Furthermore, we genotyped the sortilin and SORL1 SNPs in these patients to further verify our findings.
Materials and Methods
Participants
To investigate the involvement of sortilin in CHD patients, a total of 227 CHD patients and 101 matched healthy individuals were enrolled from the outpatient clinic of Jining First People’s Hospital in Shandong Province. This study was conducted in accordance with the Declaration of Helsinki and approved by the medical ethics committee of the Jining First People’s Hospital. The written informed consent was obtained from each participant prior to enrolment.
CHD was defined as significant coronary artery stenosis greater than 50% for at least one of the three major coronary arteries or major branches. The angiographic analyses were conducted and confirmed by at least two experienced cardiologists, who were blinded to the study’s objective and design. Participant enrolment was denied according to the presence of severe autoimmune disease, acute or chronic viral hepatitis, valvular heart disease, liver or renal failure, neoplastic disease, thyroid or adrenal gland disease and acute infectious disease. The drugs mainly used in CHD patients are as follows: 87% with lipid-lowering drug, 29% with angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker, 23% with diuretic and 31% with calcium channel blocker, respectively. Additionally, the 101 matched healthy controls without any medications during recent 3 months were verified as non-CHD adults after being assessed through a series of examinations, such as medical history, clinical physical examination, radiographic chest examination, and electrocardiogram.
Blood Chemistry Analysis
Peripheral venous blood samples were collected after overnight fasting. Serum samples were isolated and stored at −80 °C until analysis. Serum levels of very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL) and triglycerides as well as high-density lipoprotein (HDL) were measured by commercially available kits supplied by ERBA Mannheim (Germany) and an ERBA autoanalyzer. Serum levels of sortilin, IL-1β, IL-6 and TNF-α were measured with a commercially available ELISA kit (Sigma-Aldrich, USA, for sortilin; R&D Systems, USA, for IL-1β, IL-6 and TNF-α) according to the manufacturer’s protocol. Briefly, a 96-well plate precoated with an anti-human monoclonal antibody was provided by the manufacturer. The diluted standards, control specimens and unknown samples were pipetted into the appropriate wells. Subsequently, a second antibody labeled with horseradish peroxidase (HRP) was added to form an antibody-antigen-HRP labeled antibody complex. After incubation and washing steps to remove the unbound substances, a substrate solution was added and reacted with the complex above to produce measurable signals. After stopping the reaction with the stop reagent, the optical density values were measured at 450 nm using a microplate reader (BioRad). All of the serum samples were routinely analyzed in duplicate, and the results were averaged.
Genetic Studies
A TIANamp Blood DNA kit (TIANGEN, China) was used to extract the genomic DNA from peripheral blood, and a NanoDrop-1000 (NanoDrop, USA) was used to detect the concentration and purity of sample DNA to ensure that the samples could be used for subsequent experiments. Six SNPs were selected in our study and primers for SNP typing were designed by the software Assay Design 3.1 (Table 1). After polymerase chain reaction amplification, products were treated with shrimp alkaline phosphatase to dephosphorylate any unincorporated dNTPs. Then, an extension reaction was performed using iPLEX extension reagents (Agena Bioscience, USA) according to the manufacturer’s protocol. After resin purification, the PCR products were spotted onto SpectroCHIPs using a Nanodispenser RS1000. The detection of primer extension products was performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, and the software Spectro-TYPER was used to automatically import and analyze the genotyping data with genotypes called based on the calculated mass of the extension products. Additionally, to validate the MassARRAY results, no less than 10% of DNA samples were randomly selected and genotyped again.
 |
Table 1 Primers of Target Gene Used in the PCR |
Statistical Analysis
The Hardy-Weinberg Equilibrium test was performed for every SNP in controls. Continuous variables were described as the mean ± standard deviation, and the differences were compared by Student’s t-test for normally distributed or nonparametric variables Mann–Whitney U-tests for skewed variables. Categorical variables were compared by the χ2 test. Correlations between sortilin and serum IL-1β, IL-6, and TNF-α were analyzed by Spearman correlation analysis. Significance was defined as a P value <0.05 in all analyses. All analyses were conducted using the statistical software package SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Basic Characteristics of the Subjects
Our study included 227 patients with CHD and 101 healthy control individuals. The demographic and clinical characteristics and blood lipids levels of the study participants are presented in Table 2. As shown in Table 2, there were no statistically significant differences in age, gender, BMI, smoking and drinking status between CHD patients and healthy control individuals (P>0.05).
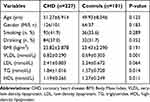 |
Table 2 Demographic and Clinical Characteristics of the Study Participants |
Correlation Between the Levels of Sortilin and the Three Proinflammatory Cytokines IL-1β, IL-6, TNF-α
Compared to healthy controls, CHD patients showed a significant increase in serum sortilin level (387.72 ± 151.0 ng/L vs 348.51 ± 139.9 ng/L, P = 0.027), as highlighted in Figure 1. Similarly, increased serum levels of IL-1β, IL-6 and TNF-α were also observed in CHD patients (IL-1β, 31.70 ± 12.789 ng/L vs 28.03 ± 11.185 ng/L, P=0.013; IL-6, 17.85 ± 10.705 ng/L vs 13.45 ± 9.605 ng/L, P = 0.000; TNF-α, 311.01 ± 167.5 ng/L vs 263.29 ± 119.6 ng/L, P = 0.010). Moreover, the serum sortilin level was significantly correlated with the IL-1β (r = 0.252, P = 0.0001), IL-6 (r = 0.250, P = 0.0001), and TNF-α (r = 0.180, P = 0.0064) levels (Figure 2).
 |
Figure 2 The correlations between serum levels of sortilin and IL-1β (A), IL-6 (B), and TNF-α (C) in CHD patients. **P < 0.01, ***P < 0.001. |
Hardy-Weinberg Equilibrium Test
The genotype distribution of all SNPs in the control groups conformed to the Hardy-Weinberg equilibrium according to the χ2 test (rs646776: χ2 = 0.130, P = 0.718; rs599839: χ2 = 3.050, P = 0.081; rs464218: χ2 = 0.669, P = 0.413; rs2070045: χ2 = 0.532, P = 0.466; rs2282649: χ2 = 2.096, P = 0.148; and rs1010159: χ2 = 1.726, P = 0.189).
Sortilin and SORL1 Polymorphisms
The genotypic frequencies of sortilin (rs646776, rs599839, and rs464218) and SORL1 (rs2070045, rs2282649, and rs1010159) polymorphisms are summarized in Table 3. There were significant differences in the frequency of the sortilin polymorphisms (for rs599839, χ2 = 34.261, P = 0.000; for rs464218, χ2 = 8.008, P = 0.018) between CHD patients and healthy controls, while no significant difference in sortilin polymorphism (rs646776) was observed (P > 0.05). For rs599839, when subdividing these samples into GG and GA + AA groups, an obvious difference between cases and controls was found (χ2 = 7.453, P = 0.006, OR = 8.378, 95% CI = 1.709–41.074). Similarly, for rs464218, a significant difference in the genotype frequency was also observed between CHD patients and healthy controls; the G allele (GG + GA) occurred significantly more frequently among the CHD patients than the controls (χ2 = 5.345, P = 0.021, OR = 0.573, 95% CI = 0.357–0.921). However, no significant differences in SORL1 polymorphisms were found between CHD patients and healthy controls (P > 0.05).
 |
Table 3 Genotype Distributions of Sortilin and SORL1 Gene Polymorphisms in CHD and Control Group |
Association of Sortilin Polymorphisms with Serum Levels of Sortilin, IL-1β, IL-6 and TNF-α
As shown in Figure 3, for rs599839, GG and GA carriers were combined because of the small number of GG carriers. The sortilin serum levels in the carriers of the risk genotype for CHD, AA, were significantly higher than those of the GG and GA carriers (391.25 ± 128.249 ng/L vs 314.70 ± 64.652 ng/L, P=0.000, Figure 3A), and the same trend was also observed for IL-1β (25.62 ± 7.311 ng/L vs 32.19 ± 13.020 ng/L, P = 0.003, Figure 3B) and TNF-α levels (233.80 ± 55.940 ng/L vs 317.60 ± 172.039 ng/L, P = 0.000, Figure 3D). Similarly, a significant difference was observed in the serum sortilin level among three different rs464218 genotypes (P = 0.039), and the sortilin level in CHD patients with the risk genotype, GG, was significantly higher than that in patients with the AA genotype (433.02 ± 118.100 ng/L vs 366.31 ± 116.403 ng/L, P = 0.014, Figure 3E); the same trend was evident in the IL-1β (38.80 ± 18.223 ng/L vs 28.58 ± 8.802 ng/L, P = 0.025, Figure 3F) and IL-6 levels (26.16 ± 17.343 ng/L vs 15.77 ± 7.149 ng/L, P = 0.015, Figure 3G).
Discussion
Sortilin, a 95-kDa protein expressed in a wide range of tissues including the brain, spinal cord, heart, and skeletal muscle, participates in intracellular protein sorting between the trans-Golgi network and endosomes.8 This sorting receptor is expressed at the cell surface and acts as an endocytotic receptor for various extracellular ligands in lipid metabolism18,19 and the neuronal system,20,21 where it facilitates the recycling or lysosomal degradation of internalized ligands. Preclinical in vivo evidence suggests that sortilin plays a significant role in the pathogenesis of vascular and metabolic disorders, including atherosclerosis, through contributions to arterial wall inflammation10,12 and calcification22 and dysregulation of lipoprotein metabolism.23,24 We found that patients with CHD presented a significantly higher sortilin level than healthy controls. Consistent with our results, recent clinical findings demonstrated increased sortilin levels in CHD patients with comorbid diabetes mellitus,25 suggesting its potential role as a biomarker for CHD. Although preclinical and clinical studies have demonstrated the vital role of sortilin in cardiovascular disease, the mechanism by which sortilin mediates CHD is unclear.
Inflammation is an immune response to harmful stimuli. Inflammation mechanisms are controlled by proinflammatory cytokines including IL-1β, IL-6 and TNF-α, inducing complex intracellular pathway signaling. Accumulating data are emerging regarding the role of IL-1β and TNF-α in atherosclerosis due to the recruitment of inflammatory cells to the site of injury or the promotion of adverse vascular smooth muscle cell remodeling.26,27 Indeed, increased circulating levels of the inflammatory cytokines TNF-α and IL-6 are observed in CHD patients.28,29 In our study, an elevated sortilin level was found in CHD patients. Considering that sortilin has been demonstrated to bind inflammatory cytokines, such as IFN-γ, IL-6, IFN-α, and IL-10 in immune cells, and is involved in the stimulation of IL-1β, C-C motif chemokine ligand 2, and TNF gene expression in murine microglia,30 it is reasonable to hypothesize that the sortilin level may interact with inflammatory response and be associated with the susceptibility to CHD. To test our hypothesis, serum levels of sortilin and three proinflammatory cytokines were determined by ELISA. Significant increases in serum sortilin, IL-1β, IL-6 and TNF-α levels were observed in CHD patients. To further estimate the associations between sortilin and proinflammatory cytokines, linear regression and correlation analyses were used. The analysis showed that the sortilin level was strongly positively correlated with the levels of three proinflammatory cytokines, IL-1β, IL-6 and TNF-α, supporting our hypothesis on the role of sortilin-mediated inflammation in the development of CHD. In support of our findings, recent studies on a C57BL/6 mouse model of atherosclerosis demonstrated that loss of sortilin impairs the secretion of IL-6 and IFN-γ cytokines in macrophages and Th1 cells,12 reducing the inflammatory component of vascular lesions and ultimately resulting in decreased atherosclerosis. Our study provides the first clinical evidence of the involvement of sortilin in inflammatory response during the pathogenesis of CHD.
To further validate the key role of sortilin in CHD, six sortilin and SORL1 SNPs were selected and genotyped using the Agena Biosciences MassArray platform. We discovered an association between the sortilin polymorphisms rs599839 and rs464218 and susceptibility to CHD. However, significant differences in SORL1 gene polymorphisms were not found between CHD patients and healthy controls. Notably, the genetic polymorphism analysis confirmed that the incidence of CHD was related to serum sortilin levels. Specifically, for rs599839, the serum sortilin level in the carriers of the CHD risk genotype, AA, was significantly higher than that in the GG and GA carriers. Likewise, for rs464218, individuals with the GG genotype with increased sortilin levels were found to be at increased risk of CHD. Interestingly, for rs599839, we discovered the same tendency that the CHD patients with the AA genotype had higher IL-1β and TNF-α levels than patients with the GG and GA genotype; for rs464218, increased IL-1β and IL-6 levels were also observed in patients with the risk genotype GG. No significant differences were observed in IL-6 (for rs599839) and TNF-α (for rs464218) levels between the two groups, respectively. These negative results can be explained by regional racial bias, a relatively small sample size. Additionally, the serum levels of inflammatory cytokines may be affected by the drugs. In summary, sortilin polymorphisms are associated with increased serum sortilin and proinflammatory cytokines levels and an increased risk of CHD, suggesting that the SNP effects are mediated by increased sortilin levels and subsequently elevated proinflammatory cytokine levels. It is noteworthy that genome-wide association studies have demonstrated an association between sortilin and CHD,31,32 but this association is due to its regulation of hepatic lipoprotein metabolism.23,33 Furthermore, other studies also demonstrated that the genetic variant rs599839 is prominently associated with CHD risk and that the genetic variants of chromosome 1p13 increase the risk of CHD via increased total cholesterol and LDL-cholesterol levels and decreased HDL-cholesterol levels.34 Overall, our findings emphasize that the key role of sortilin in CHD may be affected by intricate mechanisms, including inflammation and lipid metabolism.
Conclusion
Our study demonstrates that CHD patients have higher sortilin and proinflammatory cytokine levels than healthy controls. Additionally, the sortilin level is positively related to proinflammatory cytokine levels, suggesting that sortilin may interact with inflammatory response to contribute to the occurrence of CHD. Moreover, individuals with a CHD risk allele presented increased sortilin levels, further validating the vital role of sortilin in CHD. Collectively, our study for the first time provided clinical evidence that sortilin involves in inflammatory response in CHD. CHD is a multifactorial disease, and the sortilin serum levels indeed do not rule out the effect of the drugs used in patients with non-first-episode CHD. Therefore, replication with large clinical samples, more mechanistic approach and more detail animal and cell experiments are needed to explore the mechanistic pathway between sortilin and inflammation in our study.
Ethics Approval and Informed Consent
Our study was approved by the medical ethics committee of the Jining First People’s Hospital, and the written informed consent was obtained from each participant prior to enrolment.
Consent for Publication
Not applicable.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank all participants for their contributions and all organizations providing technical and financial support for this study.
Author Contributions
Wenxiu Han and Zhijie Wei are co-first authors. PJ was responsible for the conception and design of the study. HLZ, CMG, CSW and MQY collected the samples and analyzed the basic clinical data; RLD, HLZ, CMG and MQY performed the genotyping; WXH and ZJW were involved the statistical analysis and drafting of the paper; RLD, JZ, ZJW and PJ interpreted the results and undertook critical revision of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Dai W, Li Y, Lv YN, et al. The roles of a novel anti-inflammatory factor, milk fat globule-epidermal growth factor 8, in patients with coronary atherosclerotic heart disease. Atherosclerosis. 2014;233(2):661–665. doi:10.1016/j.atherosclerosis.2014.01.013
2. Patel S, Celermajer DS, Bao S. Atherosclerosis-underlying inflammatory mechanisms and clinical implications. Int J Biochem Cell Biol. 2008;40(4):576–580. doi:10.1016/j.biocel.2007.11.017
3. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi:10.1161/CIRCULATIONAHA.105.537878
4. Luc G, Bard JM, Juhan-Vague I, et al. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23(7):1255–1261. doi:10.1161/01.ATV.0000079512.66448.1D
5. Escarcega RO, Garcia-Carrasco M, Fuentes-Alexandro S, et al. Insulin resistance, chronic inflammatory state and the link with systemic lupus erythematosus-related coronary disease. Autoimmun Rev. 2006;6(1):48–53. doi:10.1016/j.autrev.2006.07.001
6. Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35(27):1782–1791. doi:10.1093/eurheartj/ehu203
7. Schulte S, Sukhova GK, Libby P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol. 2008;172(6):1500–1508. doi:10.2353/ajpath.2008.070776
8. Petersen CM, Nielsen MS, Nykjaer A, et al. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272(6):3599–3605. doi:10.1074/jbc.272.6.3599
9. Jacobsen L, Madsen P, Moestrup SK, et al. Molecular characterization of a novel human hybrid-type receptor that binds the alpha2-macroglobulin receptor-associated protein. J Biol Chem. 1996;271(49):31379–31383. doi:10.1074/jbc.271.49.31379
10. Patel KM, Strong A, Tohyama J, et al. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116(5):789–796. doi:10.1161/CIRCRESAHA.116.305811
11. Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39(2):168–177. doi:10.1038/ng1943
12. Mortensen MB, Kjolby M, Gunnersen S, et al. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J Clin Invest. 2014;124(12):5317–5322. doi:10.1172/JCI76002
13. Yabe-Wada T, Matsuba S, Takeda K, et al. TLR signals posttranscriptionally regulate the cytokine trafficking mediator sortilin. Sci Rep. 2016;6:26566. doi:10.1038/srep26566
14. Larsen JV, Petersen CM. SorLA in interleukin-6 signaling and turnover. Mol Cell Biol. 2017;37(11):e00641. doi:10.1128/MCB.00641-16
15. Sullivan SE, Liao M, Smith RV, et al. Candidate-based screening via gene modulation in human neurons and astrocytes implicates FERMT2 in Abeta and TAU proteostasis. Hum Mol Genet. 2019;28(5):718–735. doi:10.1093/hmg/ddy376
16. Goettsch C, Iwata H, Hutcheson JD, et al. Serum sortilin associates with aortic calcification and cardiovascular risk in men. Arterioscler Thromb Vasc Biol. 2017;37(5):1005–1011. doi:10.1161/ATVBAHA.116.308932
17. T Cuenco K, Lunetta KL, Baldwin CT, et al. Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch Neurol. 2008;65(12):1640–1648. doi:10.1001/archneur.65.12.1640
18. Nielsen MS, Jacobsen C, Olivecrona G, et al. Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem. 1999;274(13):8832–8836. doi:10.1074/jbc.274.13.8832
19. Nilsson SK, Christensen S, Raarup MK, et al. Endocytosis of apolipoprotein A-V by members of the low density lipoprotein receptor and the VPS10p domain receptor families. J Biol Chem. 2008;283(38):25920–25927. doi:10.1074/jbc.M802721200
20. Hu F, Padukkavidana T, Vaegter CB, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68(4):654–667. doi:10.1016/j.neuron.2010.09.034
21. Carlo AS, Gustafsen C, Mastrobuoni G, et al. The pro-neurotrophin receptor sortilin is a major neuronal apolipoprotein E receptor for catabolism of amyloid-beta peptide in the brain. J Neurosci. 2013;33(1):358–370. doi:10.1523/JNEUROSCI.2425-12.2013
22. Goettsch C, Hutcheson JD, Aikawa M, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016;126(4):1323–1336. doi:10.1172/JCI80851
23. Kjolby M, Andersen OM, Breiderhoff T, et al. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12(3):213–223. doi:10.1016/j.cmet.2010.08.006
24. Strong A, Ding Q, AC E, et al. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J Clin Invest. 2012;122(8):2807–2816. doi:10.1172/JCI63563
25. Oh TJ, Ahn CH, Kim BR, et al. Circulating sortilin level as a potential biomarker for coronary atherosclerosis and diabetes mellitus. Cardiovasc Diabetol. 2017;16(1):92. doi:10.1186/s12933-017-0568-9
26. Escarcega RO, Lipinski MJ, Garcia-Carrasco M, et al. Inflammation and atherosclerosis: cardiovascular evaluation in patients with autoimmune diseases. Autoimmun Rev. 2018;17(7):703–708. doi:10.1016/j.autrev.2018.01.021
27. Sarzi-Puttini P, Atzeni F, Shoenfeld Y, et al. TNF-alpha, rheumatoid arthritis, and heart failure: a rheumatological dilemma. Autoimmun Rev. 2005;4(3):153–161. doi:10.1016/j.autrev.2004.09.004
28. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145–156. doi:10.1161/CIRCRESAHA.115.306656
29. Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578–589. doi:10.1093/eurheartj/eht367
30. Patel AB, Tsilioni I, Leeman SE, et al. Neurotensin stimulates sortilin and mTOR in human microglia inhibitable by methoxyluteolin, a potential therapeutic target for autism. Proc Natl Acad Sci U S A. 2016;113(45):E7049- E7058. doi:10.1073/pnas.1604992113
31. Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357(5):443–453. doi:10.1056/NEJMoa072366
32. Schunkert H, Konig IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–338. doi:10.1038/ng.784
33. Musunuru K, Strong A, Frank-Kamenetsky M, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466(7307):714–719. doi:10.1038/nature09266
34. Kleber ME, Renner W, Grammer TB, et al. Association of the single nucleotide polymorphism rs599839 in the vicinity of the sortilin 1 gene with LDL and triglyceride metabolism, coronary heart disease and myocardial infarction. The Ludwigshafen risk and cardiovascular health study. Atherosclerosis. 2010;209(2):492–497. doi:10.1016/j.atherosclerosis.2009.09.068
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


