Back to Journals » International Journal of General Medicine » Volume 16
The Association Between Red Cell Distribution Width (RDW) and All-Cause Mortality in Elderly Patients with Hip Fractures: A Retrospective Cohort Study
Authors Wang NJ, Zhang YM, Zhang BF
Received 13 April 2023
Accepted for publication 8 August 2023
Published 17 August 2023 Volume 2023:16 Pages 3555—3566
DOI https://doi.org/10.2147/IJGM.S417079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Neng-Jun Wang,* Yu-Min Zhang,* Bin-Fei Zhang
Department of Joint Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin-Fei Zhang, Email [email protected]
Background: Red cell distribution width (RDW) may be related to the prognosis of hip fractures. The purpose of this study was to evaluate the association between (RDW) and all-cause mortality in elderly hip fractures.
Materials and Methods: Elderly patients aged ≥ 65 years who had a hip fracture were screened between January 1, 2015, and September 30, 2019. The age, gender of patients and other demographics, as well as history of allergy, injury mechanism, underlying illnesses at the time of admission, fracture classification, time from admission to operation, RDW, operation time, blood loss, infusion, transfusion, treatment strategy, and length in hospital stay and follow-up and other clinical characteristics were collected. Linear and nonlinear multivariate Cox regression models were used to identify the association between RDW and mortality in these patients. Analyses were performed using EmpowerStats and the R software.
Results: A total of 2587 patients were included in this retrospective cohort study. The mean follow-up period was 38.92 months. A total of 873 (33.75%) patients died due to all-cause mortality. The RDW was linearly associated with mortality in elderly patients with hip fractures. Linear multivariate Cox regression models showed that RDW was associated with mortality (hazard ratio [HR]=1.03, 95% confidence interval [CI]:1.02– 1.05, P < 0.0001) after adjusting for confounding factors. The mortality risk increased by 3% when RDW increased by 1 fL.
Conclusion: RDW is associated with mortality in elderly patients with hip fractures, and RDW could be considered a predictor of mortality risk.
Registration: ChiCTR2200057323.
Keywords: all-cause mortality, red cell distribution width, hip fracture
Introduction
Hip fracture is a traumatic event that occurs frequently in the elderly and is associated with substantial mortality, morbidity, and economic costs.1 The number of hip fractures has increased rapidly with aging of the global population.2 Research has projected that the number of hip fractures occurring worldwide each year will rise to 6.26 million by the year 2050.3 At the same time, with the increasing number of elderly people, a concomitant increase in avoidable deaths, disability, and medical costs due to hip fractures will occur.4 In the foreseeable future, hip fractures will pose a greater burden on health services.5,6 Therefore, researching hip fractures, particularly in the elderly, is important.
Patients with hip fractures suffer different adverse consequences even after treatment, such as postoperative death in the hospital, incomplete recovery of pre-fracture function, or transition from independent living to long-term care among survivors.7 Several factors influence the prognosis of hip fractures. Studies indicate that age and sex,8 surgery-related factors (eg, surgery type and time to surgery),9,10 comorbidities, and postoperative complications11 are associated with functional outcomes after hip fracture. Regarding time to surgery, timely operation within 48 hours of admission appears to be best practice.12 An early systemic review revealed that postoperative 30-day and 1-year mortality increased due to delayed surgery of over 48 hours.13 Meanwhile, different postoperative management measures may also have different effects on the prognosis of patients with hip fracture. Studies have shown that the patients with hip fracture involved in hospital healthcare would result in better quality of care and improved outcomes.14,15 Asplin et al pointed out that continued rehabilitation after discharge is necessary for the recovery of patients with hip fracture.16 However, understanding the extent of prognostic factors is still limited.17,18
Red cell distribution width (RDW) is a simple blood test parameter that reflects the degree of heterogeneity of erythrocyte volume in peripheral blood and is usually used in the diagnosis and differentiation of several types of anemias.19,20 In previous studies, higher levels of RDW have been considered an adverse prognostic factor in cardiovascular diseases, inflammation, and different types of cancers.21–24 In recent years, the relationship between RDW and clinical fractures or other traumas has been reported. For example, the risks of all-clinical fractures also increase with higher RDW values.25 Studies have suggested that RDW is independently associated with an increased risk of mortality following hip fractures. Yin et al found that hip fracture patients who experience a greater fluctuation in RDW during the hospital course are at a higher risk of 2-year all-cause mortality.26 Garbharran et al found that hip fracture patients with larger RDWs also had higher mortality rates, suggesting an association between larger RDWs and both short- (4 months) and long-term (1 year) mortality in patients with hip fracture.27 Therefore, we speculated that RDW may be related to the prognosis of hip fractures.
However, the relationship between RDW and prognosis of patients with hip fractures remains unclear. Therefore, this study assessed the influence of RDW on patient mortality over a long-term follow-up period. We hypothesized that there would be an association between RDW and mortality. In this retrospective cohort study, we aimed to identify the role of RDW in hip fractures.
Materials and Methods
Study Design
In our study, we recruited elderly patients who had a hip fracture between January 1, 2015, and September 30, 2019, at the largest trauma center in Northwest China.
This retrospective study was approved by the Ethics Committee of Xi’an Honghui Hospital (No. 202201009). All procedures involving human participants were performed in accordance with the 1964 Declaration of Helsinki and its amendments. The informed consent was obtained from all subjects.
Participants
Demographic and clinical data of the patients were obtained from their original medical records. The inclusion criteria were as follows: 1) age ≥ 65 years; 2) radiographic or computed tomography diagnosis of a femoral neck, intertrochanteric, or subtrochanteric fracture; 3) patients who were receiving surgical or conservative treatment in a hospital; 4) availability of clinical data in the hospital; and 5) patients able to be contacted by telephone. The exclusion criteria were as follows: 1) patients who could not be contacted; 2) patients who with anemia or cancer.
Hospital Treatment
The patients were examined using blood tests and ultrasonography to prepare for surgery to determine patient’s cardiopulmonary function and whether there was thrombosis in the lower limbs. Intertrochanteric fractures are often managed with closed/open reduction and internal fixation (ORIF) of the proximal femoral nail anti-rotation. Femoral neck fractures are often treated with hemiarthroplasty (HA) or total hip arthroplasty (THA) depending on the patient’s age. Prophylaxis for deep vein thrombosis through drug treatment and functional exercise was initiated on admission. Upon discharge, the patients were asked to return monthly to assess fracture union or function.
Follow-Up
After discharge, the patients’ family members were contacted by telephone from January to March 2022 to collect data on survival, survival time, and activities of daily living. This follow-up was conducted by two medical professionals with two weeks of training and one year of experience. Patients who could not be contacted initially were referred two other times. When the family members of the patients did not respond, we stopped and recorded the patients as lost to follow-up.
Endpoint Events
The endpoint event in this study was all-cause mortality after treatment. We defined all-cause mortality as death reported by patients’ family members.
Variables
The variables in our study were as follows: age, gender, occupation, history of allergy, injury mechanism, fracture classification; presence of hypertension, diabetes, coronary heart disease (CHD), arrhythmia, hemorrhagic stroke, ischemic stroke, cancer, multiple injuries, dementia, chronic obstructive pulmonary disease (COPD), hepatitis, and gastritis; age-adjusted Charlson comorbidity index (aCCI), time from injury to admission, time from admission to operation, RDW, operation time, blood loss, infusion, transfusion, treatment strategy, and length in hospital stay and follow-up. The dependent variable was all-cause mortality, while the independent variable was the RDW. The other variables were potentially confounding factors.
Statistics Analysis
Continuous variables are reported as the means ± standard deviations (Gaussian distribution) or medians (range, skewed distribution). Categorical variables are presented as numbers with proportions. A Chi-squared test (categorical variables), one-way analysis of variance (ANOVA [normal distribution]), or Kruskal–Wallis H-test (skewed distribution) was used to detect differences between different RDWs (according to anemia criteria). Univariate and multivariate Cox proportional hazards regression models (three models) were used to test the association between RDW and mortality. Model 1 was not adjusted for covariates. Model 2 was minimally adjusted for sociodemographic variables. Model 3 was fully adjusted for all covariates. To test the robustness of our results, we performed sensitivity analysis. We converted the RDW into a categorical variable according to the anemia criteria and calculated the P-value for trends to verify the results of RDW as a continuous variable, and we examined the possibility of nonlinearity. Because Cox proportional hazards regression model-based methods are often suspected to be unable to deal with nonlinear models, the nonlinearity between RDW and mortality was addressed using a Cox proportional hazards regression model with cubic spline functions and smooth curve fitting (the penalized spline method). If nonlinearity was detected, we first calculated the inflection point using a recursive algorithm and then constructed a two-piecewise Cox proportional hazards regression model on both sides of the inflection point.
All analyses were performed using statistical software packages R (http://www.R-project.org, R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions Inc., Boston, MA, USA). Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Statistical significance was set at P < 0.05 (two-sided).
Results
Patient Characteristics
From the initial 2887 participants who had hip fractures between January 2015 and September 2019, 2587 met the study criteria and were enrolled in our study. The mean follow-up period was 38.92 months. A total of 873 (33.75%) patients died due to all-cause mortality. Among them, the number of male deaths was 335 (38.37%), and the number of female deaths was 538 (61.63%). RDWs were divided into five groups. Table 1 lists the demographic and clinical characteristics of all 2587 patients, which includes comorbidities, factors associated with injuries, and treatment.
 |
Table 1 The Demographic and Clinical Characteristics |
Univariate Analysis of the Association Between Variables and Mortality
We performed univariate analysis to identify potential confounding factors and the relationship between variables and mortality (Table 2). Significant variables in the univariate analysis (P < 0.01) were included in the multivariate Cox regression (age, gender, injury mechanism, fracture classification, hypertension, CHD, arrhythmia, ischemic stroke, cancer, dementia, COPD, hepatitis, aCCI, time to admission, time to operation, treatment strategy, operation time, infusion, and length in hospital).
 |
Table 2 Effects of Factors on Mortality Measured by Univariate Analysis |
Multivariate Analysis of RDW and Mortality
We used three models (Table 3) to assess the correlation between RDW and mortality. Linear regression was performed when the RDW was considered as a continuous variable. The fully adjusted model showed a 3% increase in mortality risk (HR=1.03, 95% CI: 1.02–1.05; P < 0.0001) when RDW increased by 1 fL after controlling for confounding factors. When the RDW was considered as a categorical variable, we found statistically significant differences in RDW among the three models (P < 0.0001). In addition, the P-value for trends also showed a linear correlation in the three models (P < 0.0001).
 |
Table 3 Univariate and Multivariate Results by Cox Regression |
However, we found that the change interval was slow in this subgroup (Table 3). This instability indicates the possibility of a nonlinear correlation.
Curve Fitting and Analysis of Threshold Effect
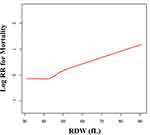
There may be a curved association between RDW and mortality after adjusting for confounding factors (Figure 1). We compared two fitting models to explore the curved associations (Table 4). Unfortunately, the curvilinear relationship between RDW and mortality could not be verified using the present data.
 |
Table 4 Nonlinearity of RDW versus Mortality |
Figure 2 presents the Kaplan–Meier survival curve.
 |
Figure 2 Kaplan-Meier survival. |
Discussion
Our retrospective cohort study reveals a significant linear relationship between RDW and all-cause mortality in elderly hip fracture patients. The results showed that the case fatality rate increased by 3% when the RDW increased by 1 fL (HR=1.03; 95% CI: 1.02–1.05; P < 0.0001), which highlighted the potential of RDW as a valuable clinical predictor for mid-term all-cause mortality in elderly patients with hip fractures. Therefore, the RDW after admission can be considered a clinical predictor of mid-term all-cause mortality in elderly patients with hip fractures.
Previous research has explored the associations between RDW and various conditions such as cancer,21,28 heart failure,29,30 and cardiovascular disease.31,32 Recently, some researchers have reported correlations between RDW and trauma, osteoporosis, fracture, and fracture complications. Sakai et al found that mortality in the elevated RDW group was statistically higher than that in the non-elevated RDW group among osteoporotic vertebral fracture patients who received conservative treatment, indicating a correlation between larger RDWs and poor outcomes.33 Li et al used the osteoporotic fracture index (malnutrition, poor physical performance, and fatigue) to delineate frailty and found that a larger RDW can be regarded as a frailty indicator in the elderly.34 In a past retrospective cohort study, Marom et al found the independent association between higher baseline RDW on admission and higher rates of all-cause mortality in the first 3, 6, and 12 months following proximal femoral fracture surgery.35 It is noteworthy that a relationship between RDW and hip fractures in the elderly has also been found. In a prospective cohort study of 1333 participants with a 2-year follow-up, Yin et al considered hip fracture patients with larger fluctuations in RDW between admission and discharge to be at a higher risk for all-cause mortality.26 In some follow-up studies with small sample sizes, an association between baseline RDW and short-term mortality in patients with hip fractures has been reported. For example, in a prospective cohort study with 4-month and 1-year follow-ups of 698 hip fracture patients, Garbharran et al reported that the group with larger admission RDWs also had a higher mortality rate.27 Additionally, Lv et al conducted the first study to explore the association between RDW and long-term all-cause mortality.36 They indicated that increased RDWs were significantly associated with an increased risk of all-cause mortality in a non-anemic hip fracture population. There have been some studies on the relationship between RDW and hip fractures, but studies on the relationship between RDW and all-cause mortality of hip fractures are still relatively scarce. More studies are needed to confirm the association between RDW and all-cause mortality after hip fractures with larger sample sizes. This study evaluated the association between RDW at admission and hip fracture prognoses in a large sample population and extended previous observations demonstrating the prognostic significance of increased RDWs in patients with hip fracture.
The underlying mechanism linking RDW with adverse outcomes after hip fractures remains unclear. However, researchers have found that larger RDWs can be regarded as a frailty indicator in the elderly.34 The presence of frailty predicts a substantially elevated risk of adverse outcomes, including fractures, cardiovascular events, and even mortality.37–39 Frailty has been regarded as an important factor affecting the prognosis of hip fractures. Low et al indicated that premorbid frailty is the strongest independent predictor of adverse outcomes, including poor functional independence measure efficiency and inability to recover pre-fracture mobility and return to community dwelling.40 Krishnan et al found that mortality and length of hospital stay were significantly higher in the high frailty group than in the low frailty group, indicating that frailty is associated with adverse outcomes after hip fracture.41 As a blood test parameter that reflects the degree of heterogeneity of erythrocyte volume in peripheral blood, RDW is not only used in the diagnosis and differentiation of several types of anemia, but is also associated with aggravated inflammation, greater disease burden, and higher oxidative stress.19,20,42 These factors may be potential mechanisms of frailty development.43–45 Considering these findings, large RDWs could be considered a predictor of poor prognosis after hip fracture, which was also confirmed in our study.
Although the linear relationship between RDW and the prognosis of hip fracture has been confirmed in this study, we also inferred the possibility of a curvilinear relationship by subgroup analysis and curve fitting; however, the inflection point on the curve was not found. At present, a linear relationship is more suitable for explaining the relationship between RDW and the prognosis of hip fractures.
To identify confounding factors in our study and draw more reliable conclusions, we first determined the factors influencing hip fracture prognosis and those influencing RDW. Previous studies showed that age,46 sex,47 frailty,40 fracture type,48 complication,49 CHD,50 cancer,51 dementia,52 time from injury to surgery,53 treatment strategy54 influence the prognosis of hip fractures. In addition, at the univariate analysis stage, we found additional factors that were statistically significant, such as ischemic stroke, operation time, and infusion volume. Meanwhile, considering the factors influencing RDW, COPD,55 cancer56 and other factors were incorporated into the model. Therefore, most confounding factors were controlled.
Our study has several limitations. First, loss to follow-up is unavoidable. Because our study had a retrospective design, there was some degree of loss to follow-up. In order to obtain as much information as possible on the prognosis of hip fracture patients, we called those who could not be contacted initially two more times by telephone. Second, our study was limited in its ability to infer causation; therefore, further studies on the causal relationship between RDW and all-cause mortality in elderly patients with hip fractures are required. Third, since the sample in the current study came from Western China, the generalizability of our present findings may be limited by ethnicity and region. Therefore, caution should be exercised when extrapolating these conclusions to other populations or countries.
In conclusion, our study showed that RDW was associated with mortality in elderly patients with hip fracture, and RDW could be considered a predictor of mortality risk.
Abbreviations
RDW, Red Cell Distribution Width; HR, hazard ratio; CI, confidence interval; HA, hemiarthroplasty; THA, total hip arthroplasty; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; aCCI, Charlson comorbidity index; ORIF, open reduction internal fixation.
Data Sharing Statement
The data were provided by Xi’an Honghui Hospital. According to relevant regulations, the data cannot be shared, but could be requested from correspondence author.
Ethics Approval and Consent to Participate
The Ethics Committee of the Honghui Hospital, Xi’an Jiaotong University approved this study (No. 202201009).
Acknowledgments
This study is registered with the Chinese Clinical Trial Registry (ChiCTR) as number ChiCTR2200057323. The authors thank Editage Academic Services for the English language editing and review services.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Foundation of Xi’an Municipal Health Commission (No. 2021ms10) and the Key Research and Development Program of Shaanxi Province (Program No. 2021SF-250 and 2022SF-377).
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364–370. doi:10.1046/j.1532-5415.2003.51110.x
2. Hannan MT, Broe KE, Cupples LA, Dufour AB, Rockwell M, Kiel DP. Height loss predicts subsequent Hip fracture in men and women of the Framingham Study. J Bone Miner Res. 2012;27(1):146–152. doi:10.1002/jbmr.557
3. Cooper C, Campion G, Melton LJ. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2(6):285–289. doi:10.1007/BF01623184
4. Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res. 1990;252:163–166. doi:10.1097/00003086-199003000-00024
5. White SM, Griffiths R. Projected incidence of proximal femoral fracture in England: a report from the NHS Hip Fracture Anaesthesia Network (HIPFAN). Injury. 2011;42(11):1230–1233. doi:10.1016/j.injury.2010.11.010
6. Boufous S, Finch CF, Lord SR. Incidence of hip fracture in New South Wales: are our efforts having an effect? Med J Aust. 2004;180(12):623–626. doi:10.5694/j.1326-5377.2004.tb06124.x
7. Sheehan KJ, Williamson L, Alexander J, et al. Prognostic factors of functional outcome after hip fracture surgery: a systematic review. Age Ageing. 2018;47(5):661–670. doi:10.1093/ageing/afy057
8. Smith T, Pelpola K, Ball M, Ong A, Myint PK. Pre-operative indicators for mortality following hip fracture surgery: a systematic review and meta-analysis. Age Ageing. 2014;43(4):464–471. doi:10.1093/ageing/afu065
9. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332. doi:10.1136/bmj.c2332
10. Maggi S, Siviero P, Wetle T, Besdine RW, Saugo M, Crepaldi G. A multicenter survey on profile of care for hip fracture: predictors of mortality and disability. Osteoporos Int. 2010;21(2):223–231. doi:10.1007/s00198-009-0936-8
11. Roche JJ, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331(7529):1374. doi:10.1136/bmj.38643.663843.55
12. Seong YJ, Shin WC, Moon NH, Suh KT. Timing of hip-fracture surgery in elderly patients: literature review and recommendations. Hip Pelvis. 2020;32(1):11–16. doi:10.5371/hp.2020.32.1.11
13. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146–154. doi:10.1007/BF03016088
14. Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet. 2015;385(9978):1623–1633. doi:10.1016/S0140-6736(14)62409-0
15. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in Hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49–55. doi:10.1097/BOT.0b013e3182a5a045
16. Asplin G, Carlsson G, Zidén L, Kjellby-Wendt G. Early coordinated rehabilitation in acute phase after hip fracture - A model for increased patient participation. BMC Geriatr. 2017;17(1):240. doi:10.1186/s12877-017-0640-z
17. Young Y, Xiong K, Pruzek RM, Brant LJ. Examining heterogeneity of functional recovery among older adults with hip fractures. J Am Med Dir Assoc. 2010;11(2):132–139. doi:10.1016/j.jamda.2009.11.007
18. Penrod JD, Litke A, Hawkes WG, et al. Heterogeneity in hip fracture patients: age, functional status, and comorbidity. J Am Geriatr Soc. 2007;55(3):407–413. doi:10.1111/j.1532-5415.2007.01078.x
19. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. doi:10.3109/10408363.2014.992064
20. Pedrazzani C, Tripepi M, Turri G, et al. Prognostic value of red cell distribution width (RDW) in colorectal cancer. Results from a single-center cohort on 591 patients. Sci Rep. 2020;10(1):1072. doi:10.1038/s41598-020-57721-4
21. Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int. 2018;18:61. doi:10.1186/s12935-018-0558-3
22. Zhao T, Cui L, Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark. 2016;16(4):507–512. doi:10.3233/CBM-160591
23. Song B, Shi P, Xiao J, et al. Utility of red cell distribution width as a diagnostic and prognostic marker in non-small cell lung cancer. Sci Rep. 2020;10(1):15717. doi:10.1038/s41598-020-72585-4
24. Herraez I, Bento L, Del Campo R, et al. Prognostic role of the red blood cell distribution width (RDW) in Hodgkin Lymphoma. Cancers. 2020;12(11):3262. doi:10.3390/cancers12113262
25. Kim KM, Lui LY, Cauley JA, et al. Red cell distribution width is a risk factor for hip fracture in elderly men without anemia. J Bone Miner Res. 2020;35(5):869–874. doi:10.1002/jbmr.3963
26. Yin P, Lv H, Li Y, et al. Hip fracture patients who experience a greater fluctuation in RDW during hospital course are at heightened risk for all-cause mortality: a prospective study with 2-year follow-up. Osteoporos Int. 2018;29(7):1559–1567. doi:10.1007/s00198-018-4516-7
27. Garbharran U, Chinthapalli S, Hopper I, George M, Back DL, Dockery F. Red cell distribution width is an independent predictor of mortality in hip fracture. Age Ageing. 2012;42(2):258–261. doi:10.1093/ageing/afs176
28. Cheng J, Wang S, Jia J, Chen Q, Song Y, Li J. Association between pre-treatment and post-treatment 3-month red cell distribution width with three-year prognosis of prostate cancer. J Inflamm Res. 2021;14:6115–6127. doi:10.2147/JIR.S342272
29. Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659–666. doi:10.1016/j.ahj.2009.07.024
30. Lippi G, Turcato G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width in heart failure: a narrative review. World J Cardiol. 2018;10(2):6–14. doi:10.4330/wjc.v10.i2.6
31. Tajuddin SM, Nalls MA, Zonderman AB, Evans MK. Association of red cell distribution width with all-cause and cardiovascular-specific mortality in African American and white adults: a prospective cohort study. J Transl Med. 2017;15(1):208. doi:10.1186/s12967-017-1313-6
32. Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers. 2017;2017:7089493. doi:10.1155/2017/7089493
33. Sakai Y, Wakao N, Matsui H, Watanabe T, Iida H, Katsumi A. Elevated red blood cell distribution width is associated with poor outcome in osteoporotic vertebral fracture. J Bone Miner Metab. 2021;39(6):1048–1057. doi:10.1007/s00774-021-01242-1
34. Li CM, Chao CT, Chen SI, Han DS, Huang KC. Elevated red cell distribution width is independently associated with a higher frailty risk among 2932 community-dwelling older adults. Front Med. 2020;7:470. doi:10.3389/fmed.2020.00470
35. Marom O, Paz I, Topaz G, Ohana N, Yaacobi E. Red cell distribution width-A mortality predictor in older adults with proximal femoral fracture. Arch Gerontol Geriatr. 2022;100:104623. doi:10.1016/j.archger.2022.104623
36. Lv H, Zhang L, Long A, et al. Red cell distribution width as an independent predictor of long-term mortality in hip fracture patients: a prospective cohort study. J Bone Miner Res. 2016;31(1):223–233. doi:10.1002/jbmr.2597
37. Bilotta C, Nicolini P, Casè A, Pina G, Rossi S, Vergani C. Frailty syndrome diagnosed according to the Study of Osteoporotic Fractures (SOF) criteria and adverse health outcomes among community-dwelling older outpatients in Italy. A one-year prospective cohort study. Arch Gerontol Geriatr. 2012;54(2):e23–8. doi:10.1016/j.archger.2011.06.037
38. Chao CT, Wang J, Chien KL. Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17(1):130. doi:10.1186/s12933-018-0772-2
39. Flaatten H, De Lange DW, Morandi A, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med. 2017;43(12):1820–1828. doi:10.1007/s00134-017-4940-8
40. Low S, Wee E, Dorevitch M. Impact of place of residence, frailty and other factors on rehabilitation outcomes post hip fracture. Age Ageing. 2021;50(2):423–430. doi:10.1093/ageing/afaa131
41. Krishnan M, Beck S, Havelock W, Eeles E, Hubbard RE, Johansen A. Predicting outcome after hip fracture: using a frailty index to integrate comprehensive geriatric assessment results. Age Ageing. 2014;43(1):122–126. doi:10.1093/ageing/aft084
42. Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol a Biol Sci Med Sci. 2010;65(3):258–265. doi:10.1093/gerona/glp163
43. Pérez-Ros P, Vila-Candel R, López-Hernández L, Martínez-Arnau FM. Nutritional status and risk factors for frailty in community-dwelling older people: a cross-sectional study. Nutrients. 2020;12(4):1041. doi:10.3390/nu12041041
44. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi:10.1016/j.arr.2016.08.006
45. Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi:10.2147/CIA.S158513
46. Roberts SE, Goldacre MJ. Time trends and demography of mortality after fractured neck of femur in an English population, 1968–98: database study. BMJ. 2003;327(7418):771–775. doi:10.1136/bmj.327.7418.771
47. Pereira SR, Puts MT, Portela MC, Sayeg MA. The impact of prefracture and hip fracture characteristics on mortality in older persons in Brazil. Clin Orthop Relat Res. 2010;468(7):1869–1883. doi:10.1007/s11999-009-1147-5
48. Vaseenon T, Luevitoonvechkij S, Wongtriratanachai P, Rojanasthien S. Long-term mortality after osteoporotic hip fracture in Chiang Mai, Thailand. J Clin Densitom. 2010;13(1):63–67. doi:10.1016/j.jocd.2009.10.003
49. Rostagno C, Buzzi R, Campanacci D, et al. In hospital and 3-month mortality and functional recovery rate in patients treated for hip fracture by a multidisciplinary team. PLoS One. 2016;11(7):e0158607. doi:10.1371/journal.pone.0158607
50. Frost SA, Nguyen ND, Black DA, Eisman JA, Nguyen TV. Risk factors for in-hospital post-hip fracture mortality. Bone. 2011;49(3):553–558. doi:10.1016/j.bone.2011.06.002
51. Kim SM, Moon YW, Lim SJ, et al. Prediction of survival, second fracture, and functional recovery following the first hip fracture surgery in elderly patients. Bone. 2012;50(6):1343–1350. doi:10.1016/j.bone.2012.02.633
52. Hou M, Zhang Y, Chen AC, et al. The effects of dementia on the prognosis and mortality of hip fracture surgery: a systematic review and meta-analysis. Aging Clin Exp Res. 2021;33(12):3161–3172. doi:10.1007/s40520-021-01864-5
53. Wang ZC, Chen X, Wu YX, et al. Effect of preoperative waiting time on prognosis of elderly patients with hip fracture. Zhongguo Gu Shang. 2022;35(4):361–366. doi:10.12200/j.issn.1003-0034.2022.04.012
54. Magnusson KA, Gunnarsson B, Sigurdsson GH, Mogensen B, Olafsson Y, Karason S. Treatment and outcome of patients with hip fracture. Laeknabladid. 2016;102(3):119–125. doi:10.17992/lbl.2016.03.69
55. Kalemci S, Akin F, Sarihan A, Sahin C, Zeybek A, Yilmaz N. The relationship between hematological parameters and the severity level of chronic obstructive lung disease. Pol Arch Intern Med. 2018;128(3):171–177. doi:10.20452/pamw.4198
56. Chen J, Liu Z, Gao G, et al. Efficacy of circulating microRNA-130b and blood routine parameters in the early diagnosis of gastric cancer. Oncol Lett. 2021;22(4):725. doi:10.3892/ol.2021.12986
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.