Back to Journals » OncoTargets and Therapy » Volume 14
The Association Between Radioiodine Refractory in Papillary Thyroid Carcinoma, Sodium/Iodide Symporter Expression, and BRAFV600E Mutation
Authors Anekpuritanang T , Uataya M, Claimon A, Laokulrath N, Pongsapich W , Pithuksurachai P
Received 3 March 2021
Accepted for publication 5 June 2021
Published 29 June 2021 Volume 2021:14 Pages 3959—3969
DOI https://doi.org/10.2147/OTT.S308910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Arseniy Yuzhalin
Tauangtham Anekpuritanang,1 Maythad Uataya,2 Apichaya Claimon,3 Natthawadee Laokulrath,1 Warut Pongsapich,2 Paveena Pithuksurachai2
1Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, 10700, Thailand; 2Department of Otorhinolaryngology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, 10700, Thailand; 3Department of Radiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, 10700, Thailand
Correspondence: Paveena Pithuksurachai
Department of Otorhinolaryngology, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Wanglang Road, Bangkok Noi, Bangkok, 10700, Thailand
Tel +66 2 419 8045
Fax +66 2 419 8044
Email [email protected]
Objective: To study the association between radioiodine refractory papillary thyroid carcinoma, sodium/iodide symporter (NIS) expression, and the BRAFV600E mutation.
Methods: A study was conducted on 30 radioiodine refractory papillary thyroid carcinoma patients and 30 radioiodine-avid papillary thyroid carcinoma patients. The expressions of sodium/iodide symporter and BRAFV600E mutated protein were determined by immunohistochemistry using formalin-fixed, paraffin-embedded tissue.
Results: The mutated BRAFV600E protein was identified in 26 radioiodine refractory papillary thyroid carcinoma subjects (86.7%) and 22 radioiodine-avid papillary thyroid carcinoma subjects (73.3%), with no significant difference between the 2 groups (P = 0.3). Sodium/iodide symporter expression was detected in 4 of 30 cases (13.3%) from the radioiodine-avid papillary thyroid carcinoma group but was negative for all radioiodine refractory cases. There was no association between sodium/iodide symporter expression and radioiodine refractory papillary thyroid carcinoma (P = 0.11). Cases with positive NIS expression were likely negative for BRAFV600E mutation (3/4; P = 0.02).
Conclusion: Papillary thyroid carcinomas with BRAFV600E mutation were more likely to be negative for NIS expression. BRAFV600E mutation and NIS expressions cannot be used to predict radioiodine sensitivity.
Keywords: BRAF mutation, immunohistochemistry, papillary thyroid carcinoma, radioiodine therapy, sodium/iodide symporter
Introduction
In the past 35 years, the incidence of thyroid cancer in Thailand has risen 2.4-fold.1 The National Cancer Institute of Thailand reported more than 2800 new cases in 2018.2 Papillary thyroid carcinoma (PTC) accounts for 75% of all thyroid cancers, with surgical intervention and postoperative radioiodine ablation therapy (RAI) as the standard treatment. Patients with excellent responses to RAI have good prognoses and a low recurrence rate. In contrast, those resistant to RAI have a higher risk of local recurrences and metastases during the follow-up period, resulting in a higher mortality rate. Thus, additional treatments—such as re-operation, external beam radiation, chemotherapy, or targeted therapy—are usually employed.3–5 The term “radioiodine refractory papillary thyroid carcinoma” (RRPTC)6 can be applied to RAI-resistant patients, who account for 5–15% of PTC cases.7
Mutations in the BRAF proto-oncogene have been widely linked to malignant transformations of the thyroid gland.8 The most common mutation is the substitution of valine (V) by glutamic acid (E) at residue 600 of the BRAF protein. This results in BRAFV600E oncoprotein, which possesses elevated serine/threonine protein kinase activities and constitutively activates the mitogen-activated protein kinase signaling pathway in human cancer.9,10 Several reports have also shown an association of the BRAFV600E mutation with the aggressive clinicopathological characteristics of PTC, including lymph node metastasis, extra-thyroidal invasion, loss of radioiodine avidity, and disease recurrence.11
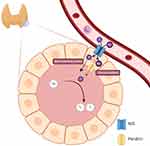
The sodium/iodide symporter (NIS), a transmembrane glycoprotein normally located at the basolateral membrane of thyroid follicular cells, plays an important role in the iodine regulation of those cells (Figure 1). In thyroid cancer treated with RAI, NIS mediates the radioiodide-131 uptake by thyroid cancer cells.12 Abnormal localization of NIS can decrease the uptake, thus reducing the effect of RAI and causing disease recurrence.13,14 BRAF mutations have been reported to cause NIS repression,14 and they have been postulated as a mechanism of RAI resistance. In spite of many reports on mutated BRAFV600E in RRPTC patients, the effects of this mutation on disease outcomes are inconsistent.15,16 Moreover, there has been no Southeast Asian study on the associations between RRPTC, NIS expression, and the BRAFV600E mutation, which may be useful for the planning of postoperative treatments and assessing the likelihood of satisfactory outcomes. This study therefore aimed to determine the associations between RRPTC, NIS expression, and the BRAFV600E mutation.
Materials and Methods
Patients
A retrospective case-control study was conducted at the Department of Otorhinolaryngology and the Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. The sample size calculation was based on the prevalence of BRAF mutations in radioiodine refractory patients reported by Yang et al. Patients diagnosed with PTC who underwent a total thyroidectomy and RAI treatment were recruited and evenly divided between an RRPTC group and a control group. The diagnoses of the RRPTC group were based on the 2015 American Thyroid Association Guidelines for RRPTC. The cases in that group met at least one of these criteria: 1) malignant or metastatic disease without radioiodine uptake at the initial treatment; 2) tumor tissue with no ability to concentrate radioiodine after previous evidence of radioiodine uptake; 3) in cases with multiple metastasis, radioiodine uptake in some lesions but not in others; and 4) progress of metastatic disease, despite a significant concentration of radioiodine. As to the control group, it consisted of radioiodine avid papillary thyroid carcinoma (RAPTC) patients who had exhibited an excellent response to treatment. They met all of the following criteria: 1) no clinical evidence of a tumor; 2) no evidence of a tumor by RAI imaging and/or neck ultrasound; and 3) a low serum thyroglobulin (Tg) level in the absence of interference by antibodies.6 Then excluded were patients with diseases related to the BRAFV600E mutation, such as cardiofaciocutaneous syndrome, non-Hodgkin’s lymphoma, colorectal cancer, malignant melanoma, non-small cell lung cancer, lung adenocarcinoma, and brain tumor (glioblastoma, pilocytic astrocytoma, pleomorphic xanthoastrocytoma, and ganglioglioma).8,10,17–21 Likewise, patients were excluded if they had diseases related to an abnormality of the NIS protein (such as Hashimoto’s thyroiditis, congenital hypothyroidism, and Graves’ disease).22,23 Eventually, 60 patients were enrolled, with 30 patients each in the RRPTC and RAPTC (control) groups.
The patient characteristics and surgical information were retrieved from electronic medical records. The details related to age, sex, clinical stagings, gross extra-thyroidal extensions, and recurrent laryngeal nerve involvements. In addition, pathological data were retrieved from the hospital’s pathology archive. They comprised information on diagnoses; tumor sizes; and structural invasions such as angiolymphatic invasions, perineural invasions, and microscopic capsular invasions. Also recorded was the American Thyroid Association 2015 risk stratification (defined as low, intermediate, and high risk).
All of the hematoxylin and eosin slides from the thyroid samples were reviewed by 2 pathologists (TA and NL). The histological variant of each tumor was determined according to the WHO 2017 diagnostic criteria.24 One representative section from each case, containing both tumor and non-tumor thyroid tissue (for internal control), was selected for immunohistochemistry. A total of 60 specimens (30 each from the RRPTC and RAPTC groups) were sent for both NIS and BRAFV600E staining. The collection process is illustrated in Figure 2.
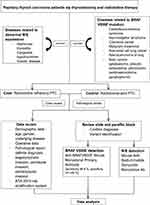 |
Figure 2 Methodology flow diagram. |
The study protocol was approved by the Siriraj Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (272/2560[EC4]). This research was conducted in accordance with the ethical principles of the Declaration of Helsinki. All personally identifiable information was removed and confidentially recorded as anonymous data. Since the level of research did not exceed the minimum risk to subjects, the requirement to obtain written informed consent was waived.
NIS Staining Protocol
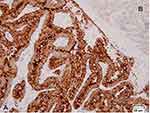
The NIS status was determined by immunohistochemistry using a mouse anti-sodium/iodide symporter monoclonal antibody (Clone FP5A, catalog number a.a.625–643; Chemical Express, USA). Four-µm thick, formalin-fixed, paraffin-embedded, tissue sections were deparaffinized in xylene and rehydrated in a graded series of ethanol. The slides were incubated in 3% H2O2 to deactivate the endogenous peroxidase. The antigen retrieval was done using a heat retrieval reagent at pH 6.0 for 32 minutes. The primary antibody was incubated at 37º C for 1 hour before being diluted (1:400) in a diluent buffer (Ab 1 µL + diluent 399 µL). The thyroid tissue from a patient with Graves’ disease was used as the positive control for the assay (Figure 3A). The normal thyroid tissue included in each slide served as an internal control (Figure 3B). The entire tumor area was reviewed and searched for tumor cells with a positive membranous staining pattern, which is associated with the iodide-accumulating ability of follicular cells (Figure 4A and B). The presence of only a cytoplasmic staining pattern was considered as negative for analysis (Figure 4C–E). Positive specimens were further semi-quantified by counting the number of positive cells per 10,000 tumor cells. This was achieved by first determining the number of tumor cells per high power field (400x; 0.01-mm field diameter) in each case. Then, the number of fields needed for 10,000 tumor cells was calculated on a case-by-case basis. All data were recorded for further analysis.
BRAFV600E Staining Protocol
Four-µm formalin-fixed paraffin-embedded sections were dried at 75 °C for 4 minutes and stained with anti-BRAFV600E mouse monoclonal primary antibody VE1 (catalog number 790–4855), using the protocol recommended by the vendor (Ventana Medical Systems, USA). Antibody incubation was followed by standard signal amplification, comprising a Ventana amplifier kit and ultraWash, and then counterstained with 1 drop of hematoxylin for 12 minutes and 1 drop of bluing reagent for 4 minutes. For the chromogenic detection, an ultraView Universal DAB detection kit (Ventana Medical Systems) was used. The slides were subsequently removed from the immunostainer before being washed in water with a drop of dishwashing detergent and mounted with a cover glass. A tissue sample from known BRAFV600E positive colorectal adenocarcinoma was used as a positive control, while the normal thyroid tissue was used as a negative control.
BRAFV600E Mutation Detection
An immunoreaction was scored as positive when unambiguous cytoplasmic staining for VE1 was observed in most of the tumor cells. Negative results included faint staining, isolated nuclear staining, weak staining of single interspersed cells, and non-specific monocyte/macrophage staining (Figure 5). This assay (sensitivity 98.8%; specificity 97–100%) was used to determine the BRAFV600E mutation status.25
Statistical Analysis
The statistical analyses were performed with IBM SPSS Statistics for Windows (version 22.0; IBM Corp., Armonk, NY, USA). Demographic data and continuous variables were presented by descriptive statistics (means and percentages). Categorical variables (including the associations between RRPTC, NIS expression, and the BRAFV600E mutation) were compared using chi-squared or Fisher’s exact tests, as appropriate. T-tests were used to evaluate the differences in the continuous variables of the 2 groups. A P value of < 0.05 was set as the cutoff point for rejecting null hypothesis.
Results
The demographic data and characteristics of the 60 patients are detailed in Table 1. There was no statistically significant difference in the prevalences of the BRAFV600E mutation in the RRPTC and RAPTC groups (26/30 [86.7%] vs 22/30 [73.3%], respectively; P = 0.3; Table 2).
 |
Table 1 Demographic Data and Patient Characteristics |
 |
Table 2 BRAFV600E Mutation, NIS Status, and Radioiodine Refractory Status |
Four of 30 cases in the RAPTC group had a detectable NIS expression, defined as the presence of staining at the basolateral membrane of individual tumor cells, with or without cytoplasmic staining (Figure 3A and B). In contrast, all cases in the RRPTC group showed no detectable NIS expression. Nevertheless, statistical analysis could not reject the null hypothesis (P = 0.11). The results of the NIS expressions and BRAFV600E mutations of the RRPTC and RAPTC groups are summarized in Table 2.
A comparison of the BRAFV600E mutation status and NIS expression revealed that tumors with negative BRAFV600E were more likely to retain NIS expression (25% vs 2.1%; P = 0.02; Table 3). There was only 1 case with BRAFV600E that still retained NIS expression.
 |
Table 3 BRAFV600E Mutation and NIS Expression/Localization |
Discussion
In this study, the overall prevalence of BRAFV600E mutation was 80%, with a higher frequency in the RRPTC group than the RAPTC group. Nevertheless, the difference was not significant (86% vs 73%; P = 0.3).
The reported prevalences of BRAFV600E in PTC have varied from 31% to 87%,7,26 with some differences between Western countries (30–50%) and Asian countries (40–80%).27,28 The diversity in the frequency of the BRAF mutations may be associated with differences in the level of iodine intake.29,30 In Thailand, Khemka and coauthors reported a 56% prevalence of the BRAF mutation in PTC cases,[Unpublished dissertation] which was similar to the 54% reported by Pongsapich and colleagues.31 However, the true frequency of this mutation in Thai PTC remains unknown due to lack of large scale molecular studies. The sample size in our study was far too small to draw a conclusion on the prevalence of the disease.
Many studies have investigated the associations between the BRAFV600E mutation and the aggressive clinicopathological characteristics of PTC, such as extra-thyroidal extension, advanced pathological staging, lymph node metastasis, recurrence, and tumor persistence with loss of radioiodine avidity.8 Liu and associates conducted a meta-analysis of 63 studies that had examined the associations between the BRAFV600E mutation, prognostic factors, and poor outcomes in PTC. Their work showed that the BRAF mutation increased the risk of developing advanced disease and lymph node metastasis by about 1.5-fold, and the risk of an extra-thyroidal extension by about 2-fold. The recurrence rate was also doubled in patients with a BRAF mutation. Additionally, the overall survival of patients with the mutation was about a fifth of that for patients with the wild-type BRAF.11 Nonetheless, the correlation between the BRAFV600E mutation and the radioiodine-sensitivity status was still inconclusive. In another work, Yang et al reported an association between the BRAFV600E mutation and radioiodine refractory in metastatic PTC.32 Furthermore, Barollo and colleagues established that the BRAFV600E mutation was related to the iodine uptake status of recurrent PTC.33 In a study on the clinical outcomes of radioiodine therapy in low- and intermediate-risk PTC patients with the BRAFV600E mutation, Li and associates found no statistical difference between the BRAF-mutated group and the wild-type group.15 Additionally, some studies have reported no association between the BRAFV600E mutation and radioiodine sensitivity in non-distant metastasis PTC groups.34–37
Despite there being no correlation between the BRAFV600E mutation and the radioiodine refractory status of PTC in our study, there were significant differences in some clinical parameters. They were age, extra-thyroidal extension, recurrent laryngeal nerve involvement, perineural invasion, microscopic thyroidal capsular invasion, tumor size, and disease stage. These factors may predict the radioiodine refractory status, which is consistent with the work of Li et al.39 It is likely that the radioiodine refractoriness scores (for extra-thyroidal extension, pN staging, lymph node metastasis ≥ 4 nodes, lymph node metastasis, smoking and tumor type) could be highly and positively correlated with the prevalence of radioiodine refractory status.38 From our study, an age over 55 years, a tumor size exceeding 4 cm, and perineural invasion increased the chances of developing RRPTC by about 3- to 5-fold. In addition, patients with an advanced stage of PTC had an 8-fold greater chance of developing RRPTC. In comparison, patients diagnosed with PTC with metastasis, a high American-Thyroid-Association risk of recurrence and persistence disease, and pathological reports of recurrent laryngeal nerve involvement or microscopic capsular invasion, had a 13- to 18-fold increased chance of developing RRPTC. Likewise, patients having PTC with a gross extra-thyroidal extension had a 21-fold higher chance of developing RRPTC.
The location of the NIS protein expression is important in the process of iodide uptake. An overexpression of the protein at the basolateral membrane in thyroid cancer cells relative to the surrounding normal tissues indicates the avidity of the radioactive iodine treatment. However, in PTC with a low radioiodine uptake, the NIS protein is mainly localized with a subcellular distribution. Thus, it has been suggested that a low radioiodine uptake may not be due to a low NIS expression, but rather to impaired targeting of the plasma membrane or impaired intracellular retention of NIS.39–43 Therefore, our study defined NIS staining as positive when it was detected only at the basolateral membrane. All of our PTC specimens yielded a low NIS expression relative to normal thyroid tissue. Only 4 cases showed focal areas with a positive basolateral membrane staining of NIS, and all were in the RAPTC group (4/30). Our study failed to show statistical significance between NIS positivity and radioiodine-sensitivity status (P = 0.11), largely due to the global down-regulation of NIS in almost all of the PTC cases.44 It was inconclusive whether the abnormal location or the absence of the NIS expression impacted on the radioiodine refractory status. Previous studies of NIS expression in thyroid carcinoma utilized many kinds of immunohistochemistry antibodies. Different methods showed a wide spectrum of NIS detection rates, ranging between 12% and 100%. However, when detection was focused on a targeted area, as in the basolateral membrane, the NIS detection rates (0.8–58.8%) were lower than those for the overall areas.13,45 The same anti-NIS antibody (Clone FP5A) used in the current investigation was employed by Morari and associates. Their NIS detection rate was 12.2%, which is comparable with the 6.7% detection rate found by our work.13 Other studies used a TSA signal amplification method and added alternative anti-NIS to enhance the sensitivity; however, the detection rate was not significantly increased. This variation in detection rates might be related to tumor patterns, collection methods, ethnicity, and a limited capacity of the anti-NIS antibody. Recently, qPCR for SLC5A5 gene has been introduced for analysis to overcome the limitations and to provide additional information on NIS expressions. The results showed that SLC5A5 was strongly suppressed in comparison with normal thyroid tissue.46 However, each institution should strike a balance between factors such as the specimen-preservation technique, cost effectiveness, and generalized applicability, and select the optimum methods.
Furthermore, there have been studies on factors that may predict radioiodine-sensitivity status. Mian and colleagues found that RRPTC was related not only to the NIS transcription factor, but also to the Tg transcription, thyroid peroxidase transcription, and pendrin transcription factors. All of those factors were significantly decreased in the RRPTC group.47 Xing explained that NIS, the thyroid stimulating hormone receptor, thyroid peroxidase, Tg, transforming growth factor beta, and histone deacetylase affected the iodine uptake of thyroid cancer cells.48 Hongwei and coauthors indicated that MED16 reduction in PTC contributes to tumor progression and RAI resistance via activation of the transforming growth factor beta pathway.49
In the comparison of the BRAFV600E mutation status with NIS expression, tumors without the mutation were more likely to retain NIS expression (P = 0.02). The result suggests that the BRAF mutation may alter the NIS protein localization and expression in PTC; this corresponds with the findings of other studies.16,26 However, RRPTC was not significantly associated with the BRAFV600E mutation and NIS expression. There may be other factors or pathways that are associated with iodine uptake in thyroid cancer.
The present study had limitations. Firstly, the sample size was smaller than those used by some other studies. In addition, we collected specimens from only primary tumors. We assumed that the refractory status of the primary tumor or metastasis was a result of the severity and sensitivity to RAI of the primary tumor. This was consistent with the finding of a recent study by Gomes-Lima et al: that the BRAFV600E mutation was present in both primary and metastatic refractory thyroid tumors.50
Conclusions
Although RRPTC is not significantly associated with the BRAFV600E mutation and NIS expression when compared with RAPTC, a relationship between abnormal NIS distribution and the BRAFV600E mutation in PTC can be observed. Some characteristics and pathological features of tumor are correlated with RRPTC: extra-thyroidal extension, recurrent laryngeal nerve involvement, perineural invasion, micro-capsular invasion, tumor size, and disease stage. Hence, those factors may be helpful in predicting the radioiodine activity of PTC. BRAFV600E and NIS immunohistochemistry alone do not predict radioiodine sensitivity in PTC.
Acknowledgments
The authors gratefully acknowledge Dr. Chulaluck Komoltree, of the Division of Clinical Epidemiology, Department of Health Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University, for her assistance with the sample size calculation and statistical analyses. We also thank Miss Jeerapa Kerdnoppakhun of the Department of Otorhinolaryngology, Faculty of Medicine Siriraj Hospital, for her secretarial support.
Funding
This research was partly supported by the Faculty of Medicine Siriraj Hospital, Mahidol University (grant no. R016132006).
Disclosure
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
1. Sriplung H, Wiangnon S, Sontipong S, Sumitsawan Y, Martin N. Cancer incidence trends in Thailand, 1989–2000. Asian Pac J Cancer Prev. 2006;7(2):239–244.
2. Imsamran W, Adisai P, Pongsatorn S, et al. Cancer in Thailand 2013–2015. National Cancer Institute; 2018.
3. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381(9871):1058–1069. doi:10.1016/S0140-6736(13)60109-9
4. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623–632. doi:10.1056/NEJMoa1209288
5. Faugeras L, Pirson AS, Donckier J, et al. Refractory thyroid carcinoma: which systemic treatment to use? Ther Adv Med Oncol. 2018;10:1758834017752853. doi:10.1177/1758834017752853
6. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
7. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. doi:10.1677/erc.1.0978
8. Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45(4):346–356. doi:10.1097/PAT.0b013e328360b61d
9. Ritterhouse LL, Barletta JA. BRAF V600E mutation-specific antibody: a review. Semin Diagn Pathol. 2015;32(5):400–408. doi:10.1053/j.semdp.2015.02.010
10. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi:10.1038/nature00766
11. Liu C, Chen T, Liu Z. Associations between BRAF(V600E) and prognostic factors and poor outcomes in papillary thyroid carcinoma: a meta-analysis. World J Surg Oncol. 2016;14(1):241. doi:10.1186/s12957-016-0979-1
12. Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154(1):65–82. doi:10.1016/0304-4157(93)90017-I
13. Morari EC, Marcello MA, Guilhen AC, et al. Use of sodium iodide symporter expression in differentiated thyroid carcinomas. Clin Endocrinol (Oxf). 2011;75(2):247–254. doi:10.1111/j.1365-2265.2011.04032.x
14. Riesco-Eizaguirre G, Rodriguez I, De la Vieja A, et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. 2009;69(21):8317–8325. doi:10.1158/0008-5472.CAN-09-1248
15. Li J, Yang T, Zhao T, Liang J, Lin YS. Clinical outcome of radioiodine therapy in low-intermediate risk papillary thyroid carcinoma with BRAF(V600E) mutation. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38(3):346–350. doi:10.3881/j.issn.1000-503X.2016.03.019
16. Dong H, Shen WZ, Yan YJ, Yi JL, Zhang L. Effects of BRAF(V600E) mutation on Na(+)/I(-) symporter expression in papillary thyroid carcinoma. J Huazhong Univ Sci Technol Med Sci. 2016;36(1):77–81. doi:10.1007/s11596-016-1545-3
17. Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer. 2006;5:2. doi:10.1186/1476-4598-5-2
18. Sanchez-Torres JM, Viteri S, Molina MA, Rosell R. BRAF mutant non-small cell lung cancer and treatment with BRAF inhibitors. Transl Lung Cancer Res. 2013;2(3):244–250. doi:10.3978/j.issn.2218-6751.2013.04.01
19. Rothschild SI. Targeted therapies in non-small cell lung cancer-beyond EGFR and ALK. Cancers (Basel). 2015;7(2):930–949. doi:10.3390/cancers7020816
20. Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95(24):1878–1890. doi:10.1093/jnci/djg123
21. Suzuki Y, Takahashi-Fujigasaki J, Akasaki Y, et al. BRAF V600E-mutated diffuse glioma in an adult patient: a case report and review. Brain Tumor Pathol. 2016;33(1):40–49. doi:10.1007/s10014-015-0234-4
22. Caillou B, Troalen F, Baudin E, et al. Na+/I- symporter distribution in human thyroid tissues: an immunohistochemical study. J Clin Endocrinol Metab. 1998;83(11):4102–4106. doi:10.1210/jcem.83.11.5262
23. Jhiang SM, Cho JY, Ryu KY, et al. An immunohistochemical study of Na+/I- symporter in human thyroid tissues and salivary gland tissues. Endocrinology. 1998;139(10):4416–4419. doi:10.1210/endo.139.10.6329
24. Katabi N, Lewis JS. Update from the 4th Edition of the World Health Organization classification of head and neck tumours: what Is New in the 2017 WHO Blue Book for tumors and tumor-like lesions of the neck and lymph nodes. Head Neck Pathol. 2017;11(1):48–54. doi:10.1007/s12105-017-0796-z
25. Sun J, Zhang J, Lu J, et al. Immunohistochemistry is highly sensitive and specific for detecting the BRAF V600E mutation in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2015;8(11):15072–15078.
26. Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas. Cancer. 2009;115(5):972–980. doi:10.1002/cncr.24118
27. Song YS, Lim JA, Park YJ. Mutation profile of well-differentiated thyroid cancer in Asians. Endocrinol Metab (Seoul). 2015;30(3):252–262. doi:10.3803/EnM.2015.30.3.252
28. Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine. 2012;91(5):274–286. doi:10.1097/MD.0b013e31826a9c71
29. Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94(5):1612–1617. doi:10.1210/jc.2008-2390
30. Kim HJ, Park HK, Byun DW, et al. Iodine intake as a risk factor for BRAF mutations in papillary thyroid cancer patients from an iodine-replete area. Eur J Nutr. 2018;57(2):809–815. doi:10.1007/s00394-016-1370-2
31. Pongsapich W, Chongkolwatana C, Poungvarin N, et al. BRAF mutation in cytologically indeterminate thyroid nodules: after reclassification of a variant thyroid carcinoma. Onco Targets Ther. 2019;12:1465–1473. doi:10.2147/OTT.S190001
32. Yang K, Wang H, Liang Z, Liang J, Li F, Lin Y. BRAFV600E mutation associated with non–radioiodine-avid status in distant metastatic papillary thyroid carcinoma. Clin Nucl Med. 2014;39(8):675–679. doi:10.1097/RLU.0000000000000498
33. Barollo S, Pennelli G, Vianello F, et al. BRAF in primary and recurrent papillary thyroid cancers: the relationship with (131)I and 2-[(18)F]fluoro-2-deoxy-D-glucose uptake ability. Eur J Endocrinol. 2010;163(4):659–663. doi:10.1530/EJE-10-0290
34. Zhu G, Deng Y, Pan L, et al. Clinical significance of the BRAFV600E mutation in PTC and its effect on radioiodine therapy. Endocr Connect. 2019;8(6):754–763. doi:10.1530/EC-19-0045
35. Zoghlami A, Roussel F, Sabourin JC, et al. BRAF mutation in papillary thyroid carcinoma: predictive value for long-term prognosis and radioiodine sensitivity. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(1):7–13. doi:10.1016/j.anorl.2013.01.004
36. Shen G, Kou Y, Liu B, Huang R, Kuang A. BRAFV600E mutation does not significantly affect the efficacy of radioiodine therapy in patients with papillary thyroid carcinoma without known distant metastases. Clin Nucl Med. 2018;43(7):e215–e219. doi:10.1097/RLU.0000000000002142
37. Li J, Liang J, Zhao T, Lin Y. Noninferior response in BRAFV600E mutant nonmetastatic papillary thyroid carcinoma to radioiodine therapy. Eur J Nucl Med Mol Imaging. 2016;43(6):1034–1039. doi:10.1007/s00259-015-3305-1
38. Li G, Lei J, Song L, et al. Radioiodine refractoriness score: a multivariable prediction model for postoperative radioiodine-refractory differentiated thyroid carcinomas. Cancer Med. 2018;7(11):5448–5456. doi:10.1002/cam4.1794
39. Dohán O, Baloch Z, Bánrévi Z, Livolsi V, Carrasco N. Rapid communication: predominant intracellular overexpression of the Na(+)/I(-) symporter (NIS) in a large sampling of thyroid cancer cases. J Clin Endocrinol Metab. 2001;86(6):2697–2700. doi:10.1210/jcem.86.6.7746
40. Aashiq M, Silverman DA, Na’ara S, Takahashi H, Amit M. Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers (Basel). 2019;11(9):1382. doi:10.3390/cancers11091382
41. Paladino S, Melillo RM. Editorial: novel mechanism of radioactive iodine refractivity in thyroid cancer. J Natl Cancer Inst. 2017;109(12). doi:10.1093/jnci/djx106
42. Dohan O, Carrasco N. Advances in Na(+)/I(-) symporter (NIS) research in the thyroid and beyond. Mol Cell Endocrinol. 2003;213(1):59–70. doi:10.1016/j.mce.2003.10.059
43. De La Vieja A, Dohan O, Levy O, Carrasco N. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev. 2000;80(3):1083–1105. doi:10.1152/physrev.2000.80.3.1083
44. de Morais RM, Sobrinho AB, de Souza Silva CM, de Oliveira JR, da Silva ICR, de Toledo Nóbrega O. The role of the NIS (SLC5A5) gene in papillary thyroid cancer: a systematic review. Int J Endocrinol. 2018;2018:9128754. doi:10.1155/2018/9128754
45. Tavares C, Coelho MJ, Eloy C, et al. NIS expression in thyroid tumors, relation with prognosis clinicopathological and molecular features. Endocr Connect. 2018;7(1):78–90. doi:10.1530/EC-17-0302
46. Damanakis AI, Eckhardt S, Wunderlich A, et al. MicroRNAs let7 expression in thyroid cancer: correlation with their deputed targets HMGA2 and SLC5A5. J Cancer Res Clin Oncol. 2016;142(6):1213–1220. doi:10.1007/s00432-016-2138-z
47. Mian C, Barollo S, Pennelli G, et al. Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol (Oxf). 2008;68(1):108–116. doi:10.1111/j.1365-2265.2007.03008.x
48. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. doi:10.1038/nrc3431
49. Gao H, Bai P, Xiao L, et al. Mediator complex subunit 16 is down-regulated in papillary thyroid cancer, leading to increased transforming growth factor-β signaling and radioiodine resistance. J Biol Chem. 2020;295(31):10726–10740. doi:10.1074/jbc.RA119.012404
50. Gomes-Lima CJ, Shobab L, Wu D, et al. Do molecular profiles of primary versus metastatic radioiodine refractory differentiated thyroid cancer differ? Front Endocrinol (Lausanne). 2021;12:623182. doi:10.3389/fendo.2021.623182
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.