Back to Journals » International Journal of General Medicine » Volume 17
The Association Between Epidermal Growth Factor rs3756261 A/G Gene Polymorphism and the Risk of Ankylosing Spondylitis in a Chinese Han Population
Received 26 November 2023
Accepted for publication 14 March 2024
Published 27 March 2024 Volume 2024:17 Pages 1213—1220
DOI https://doi.org/10.2147/IJGM.S448976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Hui Zhang, Wei Jiang
Department of Orthopedics, The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, Changzhou, People’s Republic of China
Correspondence: Wei Jiang, Email [email protected]
Background: Epidermal growth factor (EGF) is a potent pro-angiogenic molecule promoting the angiogenic phenotype of ankylosing spondylitis (AS). Studies demonstrated that EGF rs3756261 polymorphism was associated with the risk of inflammatory diseases, but not including AS.
Methods: To investigate the association between EGF rs3756261 polymorphism and the risk of AS, we genotyped the EGF rs3756261 polymorphism in 208 patients with AS and 412 controls in a Chinese Han population using a custom-by-design 48-Plex SNP scanTM Kit. The serum EGF levels were measured using an enzyme-linked immunosorbent assay in 208 AS patients and 412 controls.
Results: Our data indicated that EGF rs3756261 polymorphism was associated with an increased risk of AS in the Chinese Han population. Stratified analyses indicated that the EGF rs3756261 polymorphism elevated the risk of AS among the males, smokers, drinkers and those aged < 30 years. In addition, the EGF rs3756261 polymorphism was related to increased CRP and HLA-B27 levels in AS patients. Next, we found that the average serum levels of EGF were significantly higher in AS patients compared with controls. Meanwhile, EGF serum levels were significantly higher in AG genotype carriers when compared with AA genotype carriers in AS patients.
Conclusion: In conclusion, this study indicated that EGF rs3756261 polymorphism was associated with the risk of AS and EGF serum levels in a Chinese Han population.
Keywords: EGF, rs3756261 polymorphism, serum levels, ankylosing spondylitis, case–control study
Introduction
Ankylosing spondylitis (AS) is an inflammatory disease characterized by the clinical features of back pain and stiffness. It is caused by chronic inflammatory disorders, manifested as pathological bone formation, joint and stiffness of spine, spine fibrosis, leading to spine deformity, even disability.1–3 The morbidity of AS is high in young adults aged 18–22 years, especially the men.4 However, the pathogenesis of AS is not yet clear. Genetic factors play major roles in AS pathogenesis.5–8 Among all relevant genes, HLA-B27 is the most important genetic factor.9 Other susceptibility genes (such as endoplasmic reticulum aminopeptidase-1 and mevalonate kinase) have also been reported.10,11
Epidermal growth factor (EGF) is a powerful mitogen that activates DNA synthesis and cellular differentiation via mitogen-activated protein kinases (MAPKs) and other pathways.12,13 EGF is involved in angiogenesis in epidermal tissue.14,15 Studies have shown that a rapid decrease in salivary EGF levels can cause progression of the intraoral manifestations of Sjögren’s syndrome.16,17 In rheumatoid arthritis (RA) patients, high EGF levels were found in the synovial fluid, suggesting the involvement of EGF in the pathogenesis of arthritic diseases.18,19 EGF and epidermal growth factor receptor (EGFR) can be downregulated by dioscin, which exerts anti-inflammatory activity by regulating the c-Jun N-terminal kinase (JNK) signaling pathway.20 In the existing literature, a variety of EGF polymorphisms have been reported to be associated with etiology of inflammatory diseases. For instance, Wang et al revealed that EGF rs11568835 G/A polymorphism was associated with susceptibility to RA.21 A study of EGF gene rs11568943 and rs2237051 polymorphisms found no evidence of a haplotype association with psoriatic arthritis (PsA).22
Although EGF plays an important role in the immune response, no studies have examined the influence of the EGF rs3756261 A/G polymorphism on the susceptibility to AS. Functional variations in EGF gene may contribute to the development of AS.23 Here, we designed this case–control study to assess the association of the EGF rs3756261 polymorphism and AS risk in a Chinese Han population.
Methods
Subjects
In this case–control study, 208 AS patients and 412 sex- and age-matched controls were consecutively recruited from the Changzhou Second Hospital-Affiliated Hospital of Nanjing Medical University (Changzhou, China), between September 2014 and January 2018. A diagnosis of AS was established by using the classification criteria reported by the American College of Rheumatology (Modified New York Criteria).24 The controls were individuals receiving health examinations without AS, matched AS for age and sex, and recruited from the same institutions during the same time period. None of them had immune illnesses, chronic systemic disease, infection, or other serious diseases.
Each patient was interviewed by trained personnel using a pre-tested questionnaire to obtain information of demographic data and related risk factors for AS. The study was approved by the Ethics Committee of Changzhou Second Affiliated Hospital of Nanjing Medical University (Changzhou, China). All patients provided written informed consent to be included in the study. This study was complied with the Declaration of Helsinki.
Blood Sampling and Genotyping
Blood samples were collected using vacutainers and transferred to test tubes containing ethylenediamine tetra-acetic acid (EDTA). All blood samples were stored in a low-temperature refrigerator at −80°C. Genomic DNA was isolated from whole blood using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). SNP was genotyped using a custom-by-design 48-Plex SNP scanTM Kit (Genesky Biotechnologies Inc., Shanghai, China), which was based on double ligation and multiplex fluorescent polymerase chain reaction (PCR). We tested the 208 AS patients’ and 412 healthy controls’ EGF levels in blood serum using an enzyme-linked immunosorbent assay kit (Boster, Wuhan, China). The levels of EGF were calculated by referring to a standard curve, according to the manufacturer’s instructions. Genotyping for EGF rs3756261 polymorphism was performed using the following primers: GCAGATGCTATGGCTGATGA (forward) and GAAGTGTGATCTGCCCACCT (reverse) (Genesky Biotechnologies Inc., Shanghai, China). About 10% of selected samples were re-genotyped to verify the genotyping accuracy. The concordance of genotypes in the repeated samples was 100%.
Statistical Analyses
Differences in demographics, variables, and genotypes of the EGF rs3756261 A/G polymorphism were evaluated using a chi-squared test. The association between EGF rs3756261 polymorphism and risk of AS was estimated by calculating odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression analyses. The Hardy-Weinberg equilibrium (HWE) was tested by a goodness-of-fit chi-squared test to compare the observed genotype frequencies to the expected frequencies among controls. We analyzed the allele and genotype distributions of AS patients and controls. Stratified analyses according to drinking, smoking, sex, and age were conducted. The association of genotypes of EGF gene polymorphism with EGF serum levels was evaluated using the Student’s t-test. A cross-over analysis was used to assess the effects of the interaction between environmental factors (smoking or drinking) and genetic factors on the AS risk. All statistical analyses were done with SPSS 22.0 software (SPSS Inc., Chicago, USA) and GraphPad Prism (version 8.0).
Results
Characteristics of the Study Population
The demographic and clinical characteristics of all subjects are summarized in Table 1. Subjects were adequately matched for age and sex (P = 0.157 and 0.406, respectively). The genotype distributions of EGF rs3756261 A/G polymorphism in all subjects are illustrated in Table 2. The observed genotype frequencies for this polymorphism in controls were in HWE (P = 0.419).
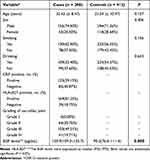 |
Table 1 Demographic Factors in Ankylosing Spondylitis and Control |
 |
Table 2 Logistic Regression Analysis of Associations Between EGF rs3756261 A/G Polymorphisms and Risk of Ankylosing Spondylitis |
Association Between EGF rs3756261 A/G Polymorphism and the Risk of AS
In this case–control study, data indicated that AG or AG+GG genotype was associated with an increased risk of AS (AG vs AA: OR, 1.64; 95% CI, 1.14–2.35; P = 0.007; AG+GG vs AA: OR, 1.67; 95% CI, 1.18–2.36; P = 0.004). In addition, G allele increased the risk of AS (G vs A: OR, 1.53; 95% CI, 1.14–2.06; P = 0.004). However, GG genotype was not related with the risk of AS (GG vs AA: OR, 1.98; 95% CI, 0.80–4.91; P = 0.132; GG vs AA+AG: OR, 1.68; 95% CI, 0.68–4.12; P = 0.253). Stratified analyses of sex, age, drinking, and smoking indicated that the EGF rs3756261 A/G polymorphism increased the risk of AS among the males, smokers, drinkers and those aged <30 years (Table 3).
 |
Table 3 Stratified Analyses Between EGF rs3756261 A/G and the Risk of Ankylosing Spondylitis |
Cross-Over Analysis
Due to the findings of stratified analyses, we performed the cross-over analysis to evaluate the potential interaction between exposure factors (smoking or drinking) and genetic factors (Table 4). Drinkers carrying the AG genotype were more easily to suffer from AS compared with non-drinkers carrying the AA genotype (OR, 1.71, 95% CI, 1.04–2.81; P = 0.035). This indicated that AG genotype could strongly interact with drinking. The similar phenomenon was also shown in smokers carrying the AG genotype (AG vs AA: OR, 2.08; 95% CI, 1.26–3.44; P = 0.004). These data indicated that there was a strong interaction between the AG genotype of rs3756261 and exposure factors (smoking or drinking).
 |
Table 4 Genetic (G) and Environmental (E) Factors 2*4 Fork Analysis |
Correlation Between the EGF rs3756261 A/G Polymorphism and Clinical Characteristics of AS
We evaluated the association between the EGF rs3756261 A/G polymorphism and clinical characteristics of patients with AS (Table 5). We found that the EGF rs3756261 A/G polymorphism was associated with increased CRP and HLA-B27 levels in AS patients. However, no significant relationship was found in the analyses of grading of sacroiliac joint.
 |
Table 5 The Associations Between EGF rs3756261 A/G Polymorphism and Clinical Characteristics of Ankylosing Spondylitis |
Association of EGF rs3756261 A/G Polymorphism with the Serum EGF Levels
The average serum levels of EGF were significantly higher in AS patients compared with controls (Table 1). We compared serum EGF levels on the basis of genotypes of EGF rs3756261 A/G polymorphism and demonstrated that, in AS patients, EGF serum levels were significantly higher in AG genotype carriers than AA genotype carriers (P < 0.05, Figure 1).
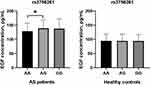 |
Figure 1 Serum EGF levels among AS patients and controls in each genotype of rs3756261 polymorphism (* indicated P < 0.05). |
Discussion
AS is a chronic inflammatory disorder characterized mainly by new bone formation that results in ankylosis of the sacroiliac and intervertebral joints. Angiogenesis plays an important role in the pathogenesis of chronic inflammatory disorders such as RA and PsA.22,25 Sacroiliitis is the primary clinical feature of AS patients. Fibrovascular synovial tissue infiltrated with inflammatory cells makes incursions into the joint, implying that new vessel formation plays a pivotal role in the pathogenesis of sacroiliitis.26 Many cases of sacroiliitis and peripheral arthritis in AS indicate that vascularity is increased in the synovial tissues.27,28 The inflammatory processes of the synovial membranes of peripheral arthritis in AS are similar to those of RA.27,29 Furthermore, Francois et al showed that changes in synovitis and subchondral bone marrow might result in joint destruction.30 The cytokines involved in angiogenesis included VEGF and EGF, which were also upregulated in AS, RA, and psoriasis.22 Together, these findings suggested that EGF may be associated with the pathogenesis of AS.
EGF gene is located on chromosome 4q25-27 and contains 24 exon and 23 introns. Rs3756261 A/G polymorphism is located on the promoter region of EGF gene. Butt et al investigated the relationship between EGF gene polymorphisms and PsA risk but observed no association of EGF gene loci with PsA susceptibility.22 Wang et al addressed the association between EGF rs11568835 G/A and rs3756261 A/G polymorphisms and RA risk, and found that EGF rs3756261 A/G polymorphism was not associated with susceptibility to RA.21 As these inflammatory diseases may share common susceptibility loci, investigating the association of EGF rs3756261 A/G polymorphism with AS risk is warranted. Thus, we performed this case–control study and found that the EGF rs3756261 A/G polymorphism was associated with an increased risk of AS in a Chinese Han population. This finding was not observed in the study by Wang et al, and we assumed that EGF rs3756261 A/G polymorphism may be a specific locus for some disorders such as AS. We found that individuals carrying AG genotype or G allele were more likely to develop AS. To our knowledge, this is the first study to reveal an association between the EGF rs3756261 A/G polymorphism and the risk of AS.
Next, we performed subgroup analyses of sex, age, drinking, and smoking, and found EGF rs3756261 A/G polymorphism increased the risk of AS among the males, smokers, drinkers, and subjects aged <30 years. It is likely that interactive contributions of genetic and environmental factors may contribute to the development of AS. To further evaluate the impact of the interactions between environmental and genetic factors on AS susceptibility, we performed cross-over analyses, and data suggested that drinkers or smokers carrying the AG genotype were more prone to from the occurrence of AS. It indicated that the interaction between drinking and smoking and AG genotype increased the risk of AS. A previous study by Ding et al also observed the interaction between FCRL4 gene and suspected environmental factors in patients with AS.31 Those studies provide evidence that AS is a chronic inflammatory disease, which is induced by genetic and environmental factors. In addition, we explored the association of EGF rs3756261 A/G polymorphism and clinical characteristics of AS, and found that this SNP was related to increased levels of CRP and HLA-B27 in AS patients.
Last, we measured the serum EGF levels among AS patients and controls, and found that AS patients showed higher EGF levels in comparison with healthy controls. However, Przepiera‑Będzak et al revealed that serum EGF levels were similar in AS and controls.23 Potential factors may account for inconsistent findings of these studies. First, ethnic group of these patients was different. Przepiera‑Będzak et al investigated this issue in a Polish population,23 while this study explored it in a Chinese Han population. Second, the sample sizes were significantly distinct. Limited sample sizes in the study by Przepiera‑Będzak et al may produce false-negative findings. In addition, we also found that EGF serum levels were significantly higher in AG genotype when compared with AA genotype carriers in AS patients, suggesting that EGF rs3756261 A/G polymorphism was related to the levels of EGF. Thus, we assumed that EGF rs3756261 A/G polymorphism may increase the risk of AS via affecting the EGF serum levels, which needs further studies to verify it.
Overall, the results obtained in our study might be helpful for early screening of individuals at high-risk of AS in the Chinese Han population. However, several limitations of the present study need to be addressed. First, this was a hospital-based case–control study; thus, selection bias was unavoidable. Second, the polymorphism investigated, based on functional consideration, may not offer a comprehensive view of the genetic variabilities of EGF gene. Third, environmental factors differed between Chinese and other populations; The EGF gene may be associated with different degrees of genetic risk in different ethnic groups with diverse environmental exposures. Fourth, the potential mechanisms of EGF rs3756261 A/G polymorphism affecting the incidence of AS should be investigated. Last, the most important factor was MRI bone marrow edema, which was not investigated in this study.
In conclusion, this study indicated that EGF rs3756261 A/G polymorphism was associated with an increased risk of AS and in a Chinese Han population. Drinking and smoking may be risk exposure factors to take a combined action with EGF rs3756261 A/G polymorphism in patients with AS. In addition, the EGF level of AS patients was found to be higher compared to the healthy controls.
Data Sharing Statement
The data of this research has been presented in the article.
Funding
This work was supported by grants from the Major Science and Technology Project of Changzhou Health Commission (ZD202013).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Bakland G, Gran JT, Becker-Merok A, NordvÅG BY, Nossent JC. Work disability in patients with ankylosing spondylitis in Norway. J Rheumatol. 2011;38(3):479–484. doi:10.3899/jrheum.100686
2. Smith JA. Update on ankylosing spondylitis: current concepts in pathogenesis. Curr Allergy Asthma Rep. 2014;15(1). doi:10.1007/s11882-014-0489-6
3. Mermerci Başkan B, Pekin Doğan Y, Sivas F, Bodur H, Özoran K. The relation between osteoporosis and Vitamin D levels and disease activity in ankylosing spondylitis. Rheumatol Int. 2009;30(3):375–381. doi:10.1007/s00296-009-0975-7
4. Ahsan T, Erum U, Jabeen R, Khowaja D. Ankylosing Spondylitis: a Rheumatology Clinic Experience. Pak J Med Sci. 1969;32(2). doi:10.12669/pjms.322.9366
5. Brown MA, Wordsworth BP. Genetics in ankylosing spondylitis - current state of the art and translation into clinical outcomes. Best Pract Res Clin Rheumatol. 2017;31(6):763–776. doi:10.1016/j.berh.2018.09.005
6. Mahmoudi M, Aslani S, Nicknam MH, Karami J, Jamshidi AR. New insights toward the pathogenesis of ankylosing spondylitis; genetic variations and epigenetic modifications. Mod Rheumatol. 2017;27(2):198–209. doi:10.1080/14397595.2016.1206174
7. Robinson PC, Brown MA. Genetics of ankylosing spondylitis. Mol Immunol. 2014;57(1):2–11. doi:10.1016/j.molimm.2013.06.013
8. Tam LS, Gu J, Yu D. Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol. 2010;6(7):399–405. doi:10.1038/nrrheum.2010.79
9. Chen B, Li J, He C, et al. Role of Hla-B27 in the pathogenesis of ankylosing spondylitis (review). Mol Med Rep. 2017;15(4):1943–1951. doi:10.3892/mmr.2017.6248
10. Babaie F, Ebrazeh M, Hemmatzadeh M, et al. Association analysis of Erap1 gene single nucleotide polymorphism in susceptibility to ankylosing spondylitis in Iranian population. Immunol Lett. 2018;201:52–58. doi:10.1016/j.imlet.2018.11.002
11. Yildiz F, Dinkci S, Erken E. Mevalonate kinase gene polymorphisms in ankylosing spondylitis patients: a cross-sectional study. Arch Rheumatol. 2023;38(2):238–248. doi:10.46497/ArchRheumatol.2023.9468
12. Yarden Y. The Egfr family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–8. doi:10.1016/s0959-8049(01)00230-1
13. Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17(3):309–317. doi:10.1210/me.2002-0368
14. Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11(4):689–708. doi:10.1677/erc.1.00600
15. Yarden Y, Sliwkowski MX. Untangling the Erbb signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. doi:10.1038/35052073
16. Azuma N, Katada Y, Kitano S, et al. Rapid decrease in salivary epidermal growth factor levels in patients with sjogren’s syndrome: a 3-year follow-up study. Mod Rheumatol. 2015;25(6):876–882. doi:10.3109/14397595.2015.1034941
17. Azuma N, Katada Y, Kitano S, et al. Correlation between Salivary Epidermal Growth Factor Levels And Refractory Intraoral Manifestations In Patients with Sjogren’s Syndrome. Mod Rheumatol. 2014;24(4):626–632. doi:10.3109/14397595.2013.850766
18. Nah SS, Won HJ, Ha E, et al. Epidermal growth factor increases prostaglandin E2 production Via ERK1/2 MAPK and NF-Kappab pathway in fibroblast like synoviocytes from patients with rheumatoid arthritis. Rheumatol Int. 2010;30(4):443–449. doi:10.1007/s00296-009-0976-6
19. Kusada J, Otsuka T, Matsui N, Hirano T, Asai K, Kato T. Immuno-reactive human epidermal growth factor (H-Egf) in rheumatoid synovial fluids. Nihon Seikeigeka Gakkai zasshi. 1993;67(9):859–865.
20. Petersson C, Hakansson A. A prospective study of infectious morbidity and antibiotic consumption among children in different forms of municipal day-care. Scand J Infect Dis. 1989;21(4):449–457. doi:10.3109/00365548909167451
21. Wang L, Bo L, Yan T, Zhang H, Zhou G, Liu R. Egf Rs11568835 G/a polymorphism is associated with increased risk of rheumatoid arthritis. Biomarkers. 2014;19(7):563–566. doi:10.3109/1354750x.2014.946450
22. Butt C, Lim S, Greenwood C, Rahman P. VEGF, FGF1, FGF2 and EGF gene polymorphisms and psoriatic arthritis. BMC Musculoskelet Disord. 2007;8(1):1. doi:10.1186/1471-2474-8-1
23. Przepiera-Bedzak H, Fischer K, Brzosko M. Serum VEGF, EGF, Basic FGF, and Acidic FGF Levels and their association with disease activity and extra‑articular symptoms in ankylosing spondylitis. Pol Arch Med Wewn. 2016;126(4):290–292. doi:10.20452/pamw.3341
24. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. a proposal for modification of the New York Criteria. Arthritis Rheum. 1984;27(4):361–368. doi:10.1002/art.1780270401
25. Koch AE. Review: angiogenesis: implications for Rheumatoid Arthritis. Arthritis Rheum. 1998;41(6):951–962. doi:10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D
26. Seo JS, Lee SS, Kim SI, et al. Influence of VEGF gene polymorphisms on the severity of ankylosing spondylitis. Rheumatology. 2005;44(10):1299–1302. doi:10.1093/rheumatology/kei013
27. Kidd BL, Moore K, Walters MT, Smith JL, Cawley MI. Immunohistological features of synovitis in ankylosing spondylitis: a comparison with rheumatoid arthritis. Ann Rheum Dis. 1989;48(2):92–98. doi:10.1136/ard.48.2.92
28. Revell PA, Mayston V. Histopathology of the synovial membrane of peripheral joints in ankylosing spondylitis. Ann Rheum Dis. 1982;41(6):579–586. doi:10.1136/ard.41.6.579
29. Ceponis A, Konttinen YT, Imai S, et al. Synovial lining, endothelial and inflammatory mononuclear cell proliferation in synovial membranes in psoriatic and reactive arthritis: a comparative quantitative morphometric study. Br J Rheumatol. 1998;37(2):170–178. doi:10.1093/rheumatology/37.2.170
30. Francois RJ, Gardner DL, Degrave EJ, Bywaters EG. Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis. Arthritis Rheum. 2000;43(9):2011–2024. doi:10.1002/1529-0131(200009)43:9<2011::AID-ANR12>3.0.CO;2-Y
31. Ding N, Hu Y, Zeng Z, et al. Case-only designs for exploring the interaction between Fcrl4 Gene and suspected environmental factors in patients with ankylosing spondylitis. Inflammation. 2014;38(2):632–636. doi:10.1007/s10753-014-9970-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
