Back to Journals » International Journal of General Medicine » Volume 16
The Association Between Absolute Lymphocyte Count and Long-Term Mortality in Critically Ill Medical Patients: Propensity Score-Based Analyses
Authors Hsiao YC, Shen PY, Wong LT, Chan MC, Chao WC
Received 20 June 2023
Accepted for publication 17 August 2023
Published 22 August 2023 Volume 2023:16 Pages 3665—3675
DOI https://doi.org/10.2147/IJGM.S424724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Yi-Chun Hsiao,1,* Pei-Yi Shen,1,* Li-Ting Wong,2 Ming-Cheng Chan,3,4 Wen-Cheng Chao3– 6
1Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 2Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan; 3Department of Critical Care Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 4Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan; 5Big Data Center, Chung Hsing University, Taichung, Taiwan; 6Department of Automatic Control Engineering, Feng Chia University, Taichung, Taiwan
*These authors contributed equally to this work
Correspondence: Wen-Cheng Chao, Department of Critical Care Medicine, Taichung Veterans General Hospital, No, 1650, Section 4, Taiwan Boulevard, Xitun District, Taichung City, 40705, Taiwan, Email [email protected]
Objective: Absolute lymphocyte count (ALC) has been implicated with short-term outcomes in a number of diseases, and we aimed to investigate the association between week-one ALC and long-term mortality in patients who were admitted to the medical intensive care units (ICUs).
Methods: We enrolled patients who were admitted to the medical ICUs at the Taichung Veterans General Hospital, a referral centre located in central Taiwan, between 2015 and 2020 to conduct this retrospective cohort study. The outcome of interest was long-term all-cause mortality, and hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to determine the association. Furthermore, we employed propensity score-matching (PSM) and weighting techniques, consisting of inverse probability of treatment weighting (IPTW) and covariate balancing propensity score (CBPS), to confirm the association between ALC and mortality.
Results: A total of 5722 critically ill patients were enrolled, and the one-year mortality was 44.8%. The non-survivor group had a lower ALC (1549, 1027– 2388 vs 1948, 1373– 2743 counts/μL, p< 0.01) compared with those in the survivor group. Cox regression showed that low ALC was independently associated with mortality (adjHR 1.091, 95% CI 1.050– 1.134). Propensity score-based analyses demonstrated the robust association, with adjHRs in the original, PSM, IPTW, and CBPS populations of 1.327 (95% CI 1.224– 1.438), 1.301 (95% CI 1.188– 1.424), 1.292 (95% CI 1.186– 1.407), and 1.297 (95% CI 1.191– 1.412), respectively. Sensitivity analyses further showed that the association between low ALC and mortality existed in a dose-response manner.
Conclusion: We found that low ALC was associated with long-term mortality in critically ill patients; further studies are warranted to validate and translate these findings into clinical utility.
Keywords: absolute lymphocyte counts, critical illness, mortality, long-term outcome, propensity score
Background
Advances in critical care over the past two decades have led to improved survival and a steady increase in the number of patients discharged from the intensive care unit (ICU); therefore, studies exploring long-term outcomes are currently a research priority in critical care.1,2 Accumulating studies have found that critical illness may lead to prolonged physical, metabolic, and immunological impairment, a condition known collectively as post-intensive care syndrome (PICS).3,4 Hence, the identification of early predictors for long-term outcomes is an essential issue in critical care.
Absolute lymphocyte count (ALC) is a frequently assessed laboratory measurement varied with age and sex.5,6 Accumulating evidence have shown that low ALC was associated with poor outcomes, mainly short-term mortality, in a wide range of diseases, including sepsis, pneumonia, malignancy, heart failure, and coronavirus disease (COVID) infection.7–11 Recent studies have found that reduced ALC may correlate with impaired adaptive immunity, particularly T-cell immunity, and impaired adaptive immunity may further lead to an increased risk of infection after acute illness and poor long-term outcomes.12–14 Our recently published study also found that impaired T-cell immunity on day-8 correlated with high hospital mortality in critically ill elderly patients with sepsis.15 However, the clinical evidence on the association between early ALC and long-term mortality in critically ill patients remains unclear. In the present study, we linked the critical care database at Taichung Veterans General Hospital (TCVGH) with Taiwan’s nationwide death-registry file and used a propensity score-based approach to investigate the association between week-one ALC and long-term all-cause mortality in patients who were admitted to the medical ICUs.
Methods
Ethical Approval
The Institutional Review Board at the TCVGH approved this study (SE20249B-2) with a waiver of informed consent due to all of the data used were de-identified data. The study complies with the Declaration of Helsinki.
Patient Population and Definition of the Main Exposure
This study utilized a retrospective cohort design and included consecutively admitted critically ill patients at TCVGH, a 1624-bed referral centre in central Taiwan, during the period from 2015 to 2020.
The inclusion criteria were consecutive patients who were admitted to ICUs at TCVGH between 2015 and 2020. Given that we focused on the long-term outcome of patients who were admitted to medical ICUs in this study, the exclusion criteria were compromised of 1) patients who were admitted to surgical ICUs, 2) patients who were admitted to cardiac ICU, and 3) patients whose ICU admission was less than 24 hours. The index ICU admission for patients with multiple admissions was defined as their first admission. We used the average level of ALC in patients with more than two sets of ALC within the first week.
Primary Outcome
The outcome of interest was the time to all-cause mortality that was retrieved from the death registration profile of Taiwan’s National Health Insurance Database (NHID), with the censored date was defined as the date of death in the NHID or March 31, 2021. In brief, Taiwan has mandated a compulsory National Health Insurance (NHI) program since 1995, which encompasses the vast majority of the population; therefore, the date of death data utilized in this study should be accurate.16
Covariates
The critical care database at TCVGH was utilized to obtain essential clinical data, including demographic details, laboratory results, comorbidities evaluated using the modified Charlson Comorbidity Index (CCI),17 Acute Physiology and Chronic Health Evaluation (APACHE) II score, presence of septic shock that was defined by diagnosis of sepsis, serum lactate level greater than 18.0 mg/dL as well as usage of vasopressors, receiving mechanical ventilation for more than 3 days, underwent renal replacement therapy, receiving blood transfusion during ICU admission, and cumulative fluid balance status during day 1–3, which have been identified as mortality-relevant factors in our previous investigation and other studies.18,19 We also included the main organ dysfunction/failure as the covariate.
Statistical Analyses
We used the Kolmogorov–Smirnov test to examine the distribution and used the Mann–Whitney U-test and Fisher’s exact test, given that the distribution of few variables was skewed. Continuous data were represented as median (interquartile range, IQR), while categorical data were presented as percentages. Variables were included in the multivariable model if the associated univariable p value was < 0.20 and the variance inflation factor was < 10.20 We used a Cox proportional hazards model to determine the Hazard ratios (HRs) for all-cause mortality and 95% confidence intervals (CIs) after adjusting for potential confounders such as age, sex, comorbidities, and other factors. Statistical analyses were performed using R (version 3.6.0), and the significance level was set at 0.05.
Subgroup Analysis
To measure the significance of the modification effect of covariates, we employed the Wald test to examine whether the association between week-one ALC and long-term mortality might differ across the covariates in this study.
Propensity Score-Based Analyses
In this study, we used propensity score-matching (PSM) along with two other propensity score weighting methods, namely the inverse probability of treatment weight (IPTW) and covariate balancing propensity score (CBPS), to confirm the association between ALC and long-term all-cause mortality among the enrolled patients.21–23 We employed the optimal nearest neighbour matching algorithm, with a calliper distance of 0.15 in PSM, given that calliper distance <0.20 was considered acceptable in PSM studies with independent covariates, continuous covariates, and the binary outcome.24 However, PSM may exclude some patients from the analysis due to a lack of matched control subjects, and the exclusion of patients may lead to concerns regarding the representativeness of the study population.21,25 To address this limitation, we also used propensity score weighting methods, including IPTW and CBPS, which aim to include the entire population and adjust for potential confounders.22,23,26
Sensitivity Analyses
The sensitivity analysis was conducted to test the robustness of the association between a low ALC and mortality using distinct cut-off values to define the low ALC in both original and propensity score-matched populations. Given that we focused on the long-term outcome, we further attempted to verify the finding by excluding patients whose mortality was within 14 days after the ICU admission.
Results
Baseline Characteristics of the Included Critically Ill Patients
Figure 1 shows the enrollment of subjects in this study (Figure 1). A total of 5722 patients were included for analyses, and the one-year mortality was 44.8% (2568/5722). The non-survivor group was older (68, 57–80 vs 71, 60–82 years, p<0.01), more likely to be male (66.6% vs 60.9%, p<0.01), and had a lower body mass index (BMI) (22.7, 20.1–25.9 vs 23.6, 20.8–26.7, p<0.01), and higher Charlson Comorbidity Index (CCI) compared with those in the survivor group (Table 1). The critical illness-relevant variables, including APACHE II score, presence of septic shock, use of mechanical ventilation for more than three days, positive cumulative fluid balance during day 1–3, leukocytosis, anaemia, and thrombocytopenia, were more severe in the non-survivor group than in the survivor group. Notably, we identified a lower absolute lymphocyte count (1549, 1027–2388 vs 1948, 1373–2743 counts/μL, p<0.01) in the non-survivor group compared with that in the survivor group.
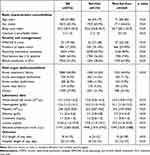 |
Table 1 Characteristics of Critically Ill Patients Categorized by One-Year Mortality |
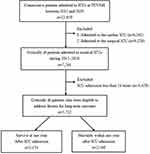 |
Figure 1 Flowchart of subject enrollment. Abbreviations: ICUs, intensive care units; TCVGH, Taichung Veterans General Hospital. |
Mortality Association of ALC and the Subgroup Analysis for Modification Effect
We used Cox regression to investigate the independent risk factors for long-term mortality among the 5722 critically ill patients and found that a lower ALC was independently associated with increased long-term mortality (adjHR 1.091, 95% CI 1.050–1.134) after adjustment for covariates (Table 2). We further examined the modification effects of covariates and found that the strength of association between ALC and long-term mortality appeared to be stronger among patients with younger age, fewer comorbidities, and lower severities of critical illness (Table 3). In detail, the strength of association between low ALC and long-term mortality were slightly stronger among critically ill patient with lower APACHE II, without mechanical ventilation, negative cumulative fluid balance during day 1–3, high level of haemoglobin and low level of serum creatinine, compared with the corresponding values in the comparison groups.
 |
Table 2 Cox Proportional Hazard Regression Analysis for Long-Term Mortality Among the 5722 Critically Ill Patients |
 |
Table 3 Stratified Analyses of Modification Effect on the Association Between the Absolute Lymphocyte Count Cut by 1500 Counts/μL and Risk of Mortality |
Propensity Score-Matching and Weighting Analyses
We further used propensity score-matching and weighting methods to address the association between the low week-one ALC (<1500 counts/μL) and long-term mortality (Figure 2). A total of 3916 patients were eligible for the PSM analysis (Table 4), and the Supplemental Table 1, Supplemental Table 2 and Supplemental Figure 1 illustrate the high quality of matching demonstrated by the measurement of the standardized mean difference (SMD) and significance of variables between the two groups in the four distinct cohorts. Table 4 summarises the consistent strength of the association between low ALC and risk for long-term mortality in the four populations. The adjusted HRs in the original PSM, IPTW and CBPS populations were 1.327 (95% CI 1.224–1.438), 1.301 (95% CI 1.188–1.424), 1.292 (95% CI 1.186–1.407), and 1.297 (95% CI 1.191–1.412), respectively.
 |
Table 4 Cox Proportional Hazard Regressions for Estimation of the Association Between the Level of Week-One Absolute Lymphocyte Count and Long-Term All-Cause Mortality in Distinct Patient Populations |
 |
Figure 2 Flowchart of propensity score matching. Abbreviations: ICUs, intensive care units; TCVGH, Taichung Veterans General Hospital. |
Sensitivity Analyses Using Distinct Cut-Off Values for Low ALC
We conducted sensitivity analyses using distinct cut-off values to define the low ALC in both original and propensity score-matched populations (Figure 3). We found that the association between low ALC and increased mortality existed in a dose-response manner in both the original and propensity score-matched populations.
 |
Figure 3 The strength of association between long-term mortality and week-one absolute lymphocyte count with distinct cut-off values. (A) Original population. (B) Propensity-score matched population. |
Sensitivity Analyses by Excluding Patients Whose Mortality Was Within 14 Days
We have excluded patients whose ICU admission was less than 24 hours. To further verify the lasting effect of ALC on mortality, we further excluded patients who died within 1–3, 1–5, 1–7, 1–10 and 1–14 days after ICU admission and found the robust association between ALC and mortality after excluding patient died within two weeks (Table 5).
 |
Table 5 Association Between Absolute Lymphocyte Count Lower Than 1500 per μL and Long-Term Mortality in Critically Ill Patients After Excluding Those Who Died Within Particular Durations |
Discussion
Long-term outcome in critical care is currently a growing area of research, and in this investigation we aimed to explore the association between week-one ALC and long-term mortality. We analyzed data from 5722 critically ill patients and found that a lower ALC was an independent risk factor for long-term mortality in critically ill patients. Furthermore, the strength of association between ALC and long-term mortality was stronger among patients with younger ages, fewer comorbidities, and lower severities of critical illness. The propensity score-matching and weighting analyses demonstrated a consistent association between ALC and morality. Our findings provided clinical evidence of the crucial role in the long-term outcome of ALC, which is a frequently measured but somehow ignored parameter in critical care.
Critically ill patients are characterized by a complex morbidity and disease course, and they remain vulnerable after discharge from the ICU, with a greater risk of developing complications, a phenomenon known as PICS.4 Advances in critical care management in the past two decades have resulted in an increasing number of patients who survive from critical illness; therefore, the long-term outcomes of critically ill patients are a vital area of research that deserves greater attention.1,27 Of particular importance is the identification of early determinants for long-term outcomes, which consists of not only risk stratification but also guidance for the development of the early therapeutic approach. For example, the long-term mortality impact of ALC may serve as an immunological indicator, and those with high risk might be benefited from early therapeutic approaches, such as vigilance and prevention of opportunistic infection.28
The low ALC may reflect an altered immunological and metabolic status in critical illness leading to a deleterious long-term outcome. In the present study, we focused on patients who were admitted to medical ICUs, with the majority of patients having sepsis, although the exclusion of patients who were admitted to cardiac and surgical ICUs may limit the generalizability of our findings. Similarly, Adrie et al investigated associations between short-term outcomes, including ICU-associated infection and 28-day mortality, and low ALC, classified by 500, 1000 and 1500 counts/μL, among 753 patients admitted to 4 French ICUs, with the majority (79%, 596/753) were Medical admission.7 Adrie et al found that no increase of ALC on day-3 after ICU admission was associated with ICU-associated infection (sub-distribution HR 1.37, 95% CI 1.12–1.67) and 28-day mortality (sub-distribution HR 1.67, 95% CI 1.37–2.03), and the present study with high number of enrolled patients and long follow-up period further identified the independent association between ALC and long-term outcome after adjustment of covariates in patients who were admitted to the medical ICUs.7
A number of studies, including studies on sepsis and COVID, have shown that elevated cytokine levels resulting from monocyte/macrophage may lead to increased apoptosis and depletion of lymphocytes.12,29,30 Cheng et al explored transcriptional and metabolic profiling among patients with sepsis and found that the shift from oxidative phosphorylation to aerobic glycolysis and impaired oxidative phosphorylation in leukocytes were implicated with post-septic immunoparalysis, which may lead to an increased susceptibility to secondary infections and a poor outcome.31 Edwards et al used lymphocyte respirometry to explore mitochondrial bioenergetic in 93 surgical patients and found a phenotype with hypometabolic lymphocytes sampled from lymphopenic patients.32 Our recently published study, using whole transcriptome to address immune-metabolism features on day-8 in immunocompromised patients with sepsis, also found an impaired T cell-associated pathway, as well as a dysregulated respiratory electron transport chain and cellular respiration pathway among immunocompromised patients with sepsis.33 Collectively, the aforementioned evidence shows that altered immunological and metabolic status may lead to impaired lymphocytic immunity in critical illness, and the findings in this study further provided clinical data supporting the role of low ALC.
Intriguingly, the analyses of the interaction effect found that the strength of the association between ALC and long-term mortality was higher in patients with younger age, fewer comorbidities, and lower severities including absence of septic shock, no need for mechanical ventilation, high level of haemoglobin, and low serum creatinine (Table 3). We postulated that critically ill patients with older age, CCI, and severities could have a tendency to have high short-term mortality; therefore, the long-term mortality impact of ALC might be mitigated by the mortality compete effect in patients with older age, CCI, and severities. Our previous study found that anaemia was associated with long-term mortality in critically ill patients, and more studies are needed to elucidate the biological mechanism underlying the interaction effect between low ALC and anaemia with respect to long-term mortality.34 We think the aforementioned analysis further validated the association between week-one ALC and long-term mortality in critically ill patients, particularly those without anaemia.
Randomized Controlled Trials (RCTs) are considered the gold standard in clinical research but they are expensive, time-consuming, and may not be feasible for every research question. Real-world data, on the other hand, can provide insights into a wide range of patient populations and can reveal previously unknown associations in actual clinical practice.35,36 However, real-world data are subject to various biases and confounders that may affect their validity, and therefore, careful analysis is required to interpret findings. We used PSM, IPTW, and CBPS to reduce the potential confounding effects in this study using RWD. PSM is widely used to balance confounding factors, but may inevitably involve discarding a certain portion of the samples without appropriate matching, particularly with unbalanced samples. For example, the number of matched patients of using ALC 1500 and 1000 counts/μL to divide the ALC were 1958 and 926, respectively (Supplemental Tables 1 and 3). Therefore, the targeted population might be distinct from the originally claimed population, and the selected patient population could raise the concern of generalizability of the results.25 In contrast, PS weighting methods, including IPTW and CBPS, use the data from the original population. In brief, IPTW focuses on reweighting the data to create a pseudo-population with balance in covariate distributions, while CBPS adjusts for confounding by modelling the propensity score and using it as a covariate in a regression model.22,23 The number of subjects in the present study was high, and the standardized mean differences of variables between the two groups using ALC 1500 as a cut-off value were apparently low (Supplemental Figure 1). Therefore, we identified a highly consistent strength of association between ALC and mortality among the three propensity score-based analyses.
There were several limitations in this study. First, the study enrolled patients who were admitted to medical ICUs at the study hospital, and more studies are warranted for the generalizability of the findings to other settings and specific populations, such as patients with stroke/sepsis. Second, given this study was not a randomized study, the adjusted association observed cannot be taken to indicate causal effect. Thus, the findings should be taken as exploratory and hypothesis-generating. Third, the potential existence of unmeasured confounders, such as solid/haematological transplant recipients.
Conclusions
In conclusion, our study identified a lower week-one ALC as an independent risk factor for long-term mortality in patients who were admitted to the medical ICUs. These findings indicate that ALC may serve as a potential early predictor of long-term outcomes in critically ill patients. Further research is needed to explore the mechanisms underlying the association between ALC and long-term mortality and to determine whether interventions targeting ALC could improve outcomes in critically ill patients.
Funding
This study was supported by Taichung Veterans General Hospital (TCVGH-112G213, TCVGH-1124401C, TCVGH-1124401D, NSTC 112-2321-B-075A-001-1-1, and NSTC 112-2314-B-075A-001 -MY2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare no competing interests.
References
1. Prescott HC, Iwashyna TJ, Blackwood B., et al. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am J Respir Crit Care Med. 2019;200(8):972–981. doi:10.1164/rccm.201812-2383CP
2. Drake C, Wald HL, Eber LB, Trojanowski JI, Nearing KA, Boxer RS. Research priorities in post-acute and long-term care: results of a stakeholder needs assessment. J Am Med Dir Assoc. 2019;20(7):911–915. doi:10.1016/j.jamda.2019.02.018
3. Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5(2):90–92. doi:10.1515/jtim-2016-0016
4. Voiriot G, Oualha M, Pierre A, et al. Chronic critical illness and post-intensive care syndrome: from pathophysiology to clinical challenges. Ann Intensive Care. 2022;12(1):58. doi:10.1186/s13613-022-01038-0
5. Lin Y, Kim J, Metter EJ, et al. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun Ageing. 2016;13(1):24. doi:10.1186/s12979-016-0079-7
6. Perry WA, Chow JK, Snydman DR. Difference in absolute lymphocyte count among male and female heart transplant recipients. Clin Transplant. 2021;35(9):e14412. doi:10.1111/ctr.14412
7. Adrie C, Lugosi M, Sonneville R, et al. Persistent lymphopenia is a risk factor for ICU-acquired infections and for death in ICU patients with sustained hypotension at admission. Ann Intensive Care. 2017;7(1):30. doi:10.1186/s13613-017-0242-0
8. Cilloniz C, Peroni HJ, Gabarrus A, et al. Lymphopenia is associated with poor outcomes of patients with community-acquired pneumonia and sepsis. Open Forum Infect Dis. 2021;8(6):ofab169. doi:10.1093/ofid/ofab169
9. Zhao J, Huang W, Wu Y, et al. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: a systematic review and meta-analysis. Cancer Cell Int. 2020;20(1):15. doi:10.1186/s12935-020-1094-5
10. Vaduganathan M, Ambrosy AP, Greene SJ, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. 2012;5(6):750–758. doi:10.1161/CIRCHEARTFAILURE.112.970525
11. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Journal of Intensive Care. 2020;8(1):36. doi:10.1186/s40560-020-00453-4
12. Finfer S, Venkatesh B, Hotchkiss RS, Sasson SC. Lymphopenia in sepsis-an acquired immunodeficiency? Immunol Cell Biol. 2022;101(6):535–544. doi:10.1111/imcb.12611
13. Cheng Z, Abrams ST, Toh J, et al. The critical roles and mechanisms of immune cell death in sepsis. Front Immunol. 2020;11:1918. doi:10.3389/fimmu.2020.01918
14. Monneret G, Venet F, Kullberg BJ, Netea MG. ICU-acquired immunosuppression and the risk for secondary fungal infections. Med Mycol. 2011;49(Suppl 1):S17–23. doi:10.3109/13693786.2010.509744
15. Chen IC, Chen HH, Jiang YH, Hsiao TH, Ko TM, Chao WC. Whole transcriptome analysis to explore the impaired immunological features in critically ill elderly patients with sepsis. J Transl Med. 2023;21(1):141. doi:10.1186/s12967-023-04002-z
16. Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175(9):1527–1529. doi:10.1001/jamainternmed.2015.3540
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
18. Messmer AS, Zingg C, Muller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients-A systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48(12):1862–1870. doi:10.1097/CCM.0000000000004617
19. Wang TJ, Pai KC, Huang CT, et al. A positive fluid balance in the first week was associated with increased long-term mortality in critically Ill patients: a retrospective cohort study. original research. Front Med. 2022;9:727103. doi:10.3389/fmed.2022.727103
20. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(1):17. doi:10.1186/1751-0473-3-17
21. Dehejia RH, W S. Propensity score-matching methods for nonexperimental causal studies. Review of Economics and Statistics. 2002;84(1):151–161. doi:10.1162/003465302317331982
22. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi:10.1002/sim.6607
23. Imai K, Ratkovic M. Covariate balancing propensity score. J R Stat Soc Series B Stat Methodol. 2014;76(1):243–263. doi:doi:10.1111/rssb.12027
24. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. doi:10.1002/pst.433
25. Okoli GN, Sanders RD, Myles P. Demystifying propensity scores. Br J Anaesth. 2014;112(1):13–15. doi:10.1093/bja/aet290
26. Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26(4):1654–1670. doi:10.1177/0962280215584401
27. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi:10.1097/CCM.0b013e318232da75
28. Hill AD, Fowler RA, Pinto R, Herridge MS, Cuthbertson BH, Scales DC. Long-term outcomes and healthcare utilization following critical illness--a population-based study. Crit Care. 2016;20(1):76. doi:10.1186/s13054-016-1248-y
29. Akbari H, Tabrizi R, Lankarani KB, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258:118167. doi:10.1016/j.lfs.2020.118167
30. Girardot T, Rimmelé T, Venet F, Monneret G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis. 2017;22(2):295–305. doi:10.1007/s10495-016-1325-3
31. Cheng SC, Scicluna BP, Arts RJ, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol. 2016;17(4):406–413. doi:10.1038/ni.3398
32. Edwards MR, Sultan P, Del Arroyo AG, et al. Metabolic dysfunction in lymphocytes promotes postoperative morbidity. Clin Sci. 2015;129(5):423–437. doi:10.1042/CS20150024
33. Cheng PL, Chen HH, Jiang YH, et al. Using RNA-seq to investigate immune-metabolism features in immunocompromised patients with sepsis. Front Med. 2021;8:747263. doi:10.3389/fmed.2021.747263
34. Lin IH, Liao PY, Wong LT, Chan MC, Wu CL, Chao WC. Anaemia in the first week may be associated with long-term mortality among critically ill patients: propensity score-based analyses. BMC Emerg Med. 2023;23(1):32. doi:10.1186/s12873-023-00806-w
35. Hernan MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi:10.1093/aje/kwv254
36. Hong JC. Strategies to turn real-world data into real-world knowledge. JAMA Netw Open. 2021;4(10):e2128045. doi:10.1001/jamanetworkopen.2021.28045
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
