Back to Journals » Journal of Inflammation Research » Volume 15
The Application of Extracellular Vesicles Mediated miRNAs in Osteoarthritis: Current Knowledge and Perspective
Authors Shang X, Fang Y, Xin W , You H
Received 25 January 2022
Accepted for publication 8 April 2022
Published 21 April 2022 Volume 2022:15 Pages 2583—2599
DOI https://doi.org/10.2147/JIR.S359887
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Xiaobin Shang,1,* Yan Fang,2,* Wenqiang Xin,3 Hongbo You1
1Department of Orthopaedics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 2Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 3Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, 352000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongbo You, Email [email protected]
Abstract: Osteoarthritis (OA) is a whole joint disease characterized by synovitis, cartilage destruction, and subchondral bone sclerosis and cyst. Despite decades’ study, effective treatment is rare for this chronic disease. Extracellular vesicles (EVs), including exosomes, microvesicles, and apoptosis bodies, are nano-sized vesicles with a cargo containing biologically active agents, such as nucleic acids, lipids, and proteins. As a group of short non-coding RNAs, microRNAs (miRNAs) can be delivered by parental cells secreted EVs. Negatively regulate the target mRNAs at the posttranscriptional level and regulate gene expression in recipient cells without modifying gene sequence. Recently, most studies focused on the function of EVs mediated miRNAs in the pathophysiological process of OA. However, all kinds of EVs specific and OA specific factors might influence the administration of EVs-miRNAs, especially the precise quantitative management. As a result, the flourishing of current research about EVs in the laboratory might not promote the relevant clinical transformation in OA treatment. In this review, we reviewed the present application of EVs-miRNAs in the therapeutic of OA and further analyzed the potential factors that might influence its application. Further progress in the quantitative management of EVs-miRNAs would accelerate the clinical transformation of miRNAs enriched EVs in the OA field.
Keywords: osteoarthritis, extracellular vesicles, microRNAs, quantitative management, clinical transformation
Introduction
Osteoarthritis (OA) is a common chronic inflammatory disease associated with the pathophysiological change in the whole joint tissues.1,2 Although it is considered a senile disease previously, the incidence is increasing even among young people due to unhealthy lifestyles.3–5 The main structural changes in the development and progress of OA are synovitis, cartilage destruction, subchondral bone sclerosis, and cyst, all of which could cause clinical symptoms from swelling and pain to joint deformity and until loss function of the joint. However, the pathological changes in cell level are still unclear, which is the main reason for the rare effective treatment to stop or reverse the progress of OA.6 It is well known that the knee joint could synthesize and secrete extracellular matrix (ECM) mainly comprised of Collagen 2 and proteoglycan. However, the healthy knee cartilage can be affected by abnormal mechanical stress, inflammation, and metabolic disorders, further inducing abnormal expression of Collagen X, Matrix metalloproteinases (MMPs), and A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), accelerating cartilage destruction. As a result, the homeostasis of the knee joint can be reversed from anabolic towards catabolic, which cannot avoid accelerating the degradation of cartilage accompanying subchondral bone sclerosis, angiogenesis, and synovitis.7 The leading clinical therapeutic for early-stage OA is to relieve pain, while joint replacement seems to be the only effective treatment at the end stage.8 As a result, the number of total joint replacements has skyrocketed recently. However, this surgery also has relevant risks like postoperative anemia, infection, periprosthetic fractures, and revision surgery which is unavoided after a long period of wear and tear.9 Hence, it is urgent to explore the pathogenesis of this disease further and find the revolutionary treatment considering the severe economic burden caused by an aging population in the following decades.
MiRNAs are a group of short non-coding RNAs which could negatively regulate the target mRNAs at the posttranscriptional level and regulate gene expression in cells without modifying the gene sequence.10 Previous studies have demonstrated that miRNAs participate in the modulation of different diseases such as cancer, cardiovascular disease, neurological disease, and also osteoarthritis.11 Hundreds of miRNAs have shown altered expression in OA compared to healthy joint tissue, so it is promising to target specific miRNAs to interfere with the progress of OA.12,13 Considering that the common is a multi-tissue organ, the interaction through miRNAs among different tissues such as cartilage-bone, cartilage-synovium and synovium-bone can be a refreshing approach to modulate the joint homeostasis. The issue is that the nuclease can quickly degrade miRNAs alone during the transportation process among tissues.14 So, there should be some delivery systems that could protect miRNAs from degradation in theory.
To bridge this gap, cell-secreted Extracellular Vesicles (EVs) with a cargo containing DNA, mRNA, protein, lipid, and miRNAs have intrigued great scientific interest.15 As nano-size membrane vesicles, EVs can be delivered quickly among tissues, even cross the microcrack channels and vessel channels between bone and cartilage interface, facilitating bone-cartilage communication.16 More importantly, the lipid bilayer of EVs could protect the miRNAs from degradation during the transportation process.17 With the intercellular communication capability, EVs have been exploited to deliver exogenous therapeutic reagents for decades.18 Especially, numerous studies have researched the role of EVs-mediated miRNAs regulation in the progress of OA and demonstrated encouraging results.19–21 However, most studies neglected the details of EVs’ quantification in the published papers when utilizing EVs as a transport agent of miRNAs directly to evaluate relevant function in the treatment of OA. For example, the information about the ratio of EVs and treated cells in vitro and the amount of EVs injected into the joint per time in vivo were sometimes lacking or vaguely described, which is hard for other laboratories to repeat a similar experiment and make interlaboratory comparisons of experimental results unwarrantable Meanwhile, differentiated quantitative management of EVs-miRNAs among studies, which could affect the standardization of its application, also impede the clinical transformation of EVs.
This review will first introduce the common knowledge about EVs and miRNAs, the biogenesis and function of EVs and miRNAs; then, we will review the current application of EVs-miRNAs in OA treatment. We will also analyze the possible factors that affect EVs-miRNAs administration and discuss different perspectives that might influence its clinical transformation.
Biogenesis and Function of miRNAs
Epigenetics, including DNA methylation, histone modification, and noncoding RNA regulation, are associated with the pathogenesis of many different diseases, such as cancer, cardiopathy, and inflammatory arthritis, including OA.22 As one kind of epigenetic regulation, miRNAs are a group of short single-stranded RNAs with a length of around 22 nucleotides.10 In brief, miRNAs are firstly transcripted in the nucleus, then generated pri-miRNAs are processed into smaller pieces by the ribonuclease Drosha, resulting in the formation of pre-miRNAs. After that, pre-miRNAs are transferred from the nucleus to the cytoplasm with the help of Exportin-5. After cleavage by the endonuclease Dicer, double-stranded pre-miRNAs are further processed into single double-stranded miRNAs. Finally, mature miRNAs are involved in an RNA-induced silencing complex (RISC), and the form of the miRNA implements functions–RISC complex, which could regulate the expression of target mRNAs by mRNA degradation and/or translational repression dependent on the degree of sequential complementarity between miRNAs and targets mRNAs (Figure 1).
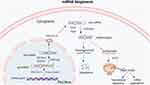 |
Figure 1 Schematic diagram of miRNAs’ biogenesis and its function in post-transcription. |
Although miRNAs have been reported to be involved in the pathophysiological process of OA via modulation of various biological processes, like proliferation, differentiation, migration, apoptosis, and autophagy, their comprehensive function of diverse miRNAs in OA is still elusive.23 For example, every miRNA is predicted to have hundreds of possible mRNA targets, and individual mRNA can be regulated by diverse miRNAs,24 leading to a complicated array of biological modulation on downstream signaling pathways. As a result, further research on miRNAs regulation is necessary to clarify its underlying mechanism in OA. Furthermore, EVs have been considered delivery vehicles that could protect RNA molecules from degradation during the transportation process within the extracellular environment.25
Biogenesis and Function of EVs
Since the nanosize membrane-bound vesicles were secreted from reticulocytes in 1983,26 these vesicles are released from most cells. They exist in various body fluids such as blood, urine, and saliva.26 Although most studies named the vesicles based on their size, biogenesis, and release pathways, the vesicles extracted so far are a mixture of subtypes due to the limitation of isolation methods.26,27 So far, these vesicles are recommended to be classified into exosomes, microvesicles, and apoptosis bodies, mainly according to the size of vesicles (Figure 2). However, all the methods such as ultracentrifugation, precipitation, size-based technique, and immunoaffinity purification cannot 100% distinguish subtypes of vesicles.28 Besides, recent studies have shown that the protein composition may be much more important than size in determining each subtype of vesicles.29 To address the confusion of naming, the International Society for Extracellular Vesicles (ISEV) suggested: “extracellular vesicles” (EVs) as the generic term of cell-secreted particles which are encapsulated by a lipid bilayer membrane and cannot replicate.30
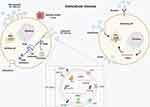 |
Figure 2 Schematic diagram of EVs’ biogenesis and its role in intercellular communication. |
In brief, the biogenesis of exosomes started from endocytosis followed by early endosomes transition to late endosomes, also called multivesicular bodies (MVBs), during which large intraluminal membrane vesicles (ILVs) are generated by internal budding of the endosome membrane. Finally, exosomes are released from the internal ILVs after the MVBs fuse with the plasma membrane and are further uptaken by receiving cells. Although endosomal sorting complexes required for transport (ESCRT) are thought to be involved in the assembly process of endosomes, the exosomal formation can be performed without the ESCRT complex under certain conditions.26 As a result, how the cargo of exosomes, like miRNAs, is integrated into exosomes during the biogenesis process. In addition, several factors, including miRNA motifs and miRNA-associated proteins like Argonaute 2, Alix, and MEX3C, might affect the miRNA sorting process.31 Furthermore, although the secreted exosomes can be uptaken by the target cells, exosomal secretory and uptake mechanisms are still under research. Unlike exosomes, microvesicles are generated by the outward budding and fission of the plasma membrane, then released into the extracellular space. Apoptotic vesicles released from apoptotic cells also called oncosomes in cancer cells, have a diameter ranging from 1000nm to 5000nm, sometimes even larger to 10 μm.32,33
Recent studies found EVs might play vital roles in the modulation of synovial inflammation and immune responses and promote tissues’ regeneration and repair, including cartilage and subchondral bone.34,35 Considering the function of EVs is mainly fulfilled by its cargo, and miRNAs could also modulate chondrogenesis and cartilage repair, it would be of great significance to clarify the regulation of EVs transferred miRNAs in the treatment of OA. In the following sections, details about the application of EVs-miRNAs in OA will be discussed.
The Application of EVs-miRNAs in OA Treatment
As aforementioned, numerous miRNAs have been reported to be involved in the pathophysiological process of OA by regulating relevant target mRNAs. For example, a study in 2019 identified 142 miRNAs and 2387 mRNAs differentially expressed between lesioned and preserved OA articular cartilage from 130 samples by RNA sequencing.36 Intriguingly, some miRNAs have been secreted and exist in synovial fluid, which could further affect cartilage homeostasis and synovial inflammation, even the subchondral bone remodeling considering the existence of microchannels across the bone-cartilage interface.37,38 However, it is well known that miRNAs are easily degraded by nuclease in the extracellular environment, so EVs may provide the protective film to potentiate the transportation of miRNAs intercellularly.
As a cell-free therapeutic method, many studies have reported the protective or destructive role of EVs by affecting cartilage repair,39 synovialis,40 and subchondral bone remodeling41 in the progress of OA and raised considerable interests in the scientific research field. Although most cells could secret EVs, most studies preferred to utilize different mesenchymal stem cells (MSCs) as the source of EVs due to the particular function such as differentiation, regeneration, and anti-inflammation of MSCs in the treatment of OA.34 Therefore, EVs from diverse MSCs may demonstrate a similar protective role in OA treatment due to inherited features from parental cells.21,35 Meanwhile, these vesicles have good stability and can also be used as a vehicle to deliver bioactive factors, during which miRNAs have shown exciting therapeutic effects.42 More importantly, previous studies reported that MSC-secreted EVs could stimulate tissue regeneration via their cargoes containing miRNA.43,44 Of the various kinds of MSCs, bone marrow mesenchymal stem cells (BMSCs) can be the most common cells in previous studies, due to the bone targeting ability45 and mature chondrogenic differentiation techniques.46 For example, hypoxic pretreatment of BMSCs secreted small extracellular vesicles could stimulate chondrocytes’ proliferation and migration and inhibit apoptosis via the miR-216a-5p/JAK2/STAT3 signaling pathway, which could lead to the cartilage repair in OA.47 Except for BMSCs, adipose tissue would be another source of MSCs (ADMSCs) due to the abundant and accessible source compared to the other MSCs,48 and EVs-miRNAs derived from ADMSCs have demonstrated effective anti-inflammatory and protective function on inflamed chondrocytes.49 Besides, EVs from induced pluripotent cell lines induced mesenchymal stem cells (iMSCs),50 umbilical cord mesenchymal stem cells (UCMSCs),51 embryonic mesenchymal stem cells (EMSCs),52 amniotic membrane-derived mesenchymal stromal cells (AMSCs),53 synovium mesenchymal stem cells (SMSCs),54 infrapatellar fat pad mesenchymal stem cells (IMSCs)55 and tendon stem cells (TSCs)56 all showed similar therapeutic functions in OA by transferring their cargoes containing miRNAs57 (Table 1).
 |
Table 1 Therapeutic Application of MSCs-Derived EVs-miRNAs in OA Treatment |
Compared to MSCs, the EVs from non-MSCs such as chondrocytes, synovial fibroblasts, osteoclasts, and osteoblasts are less studied. However, previous studies have demonstrated that their EVs could mediate the transportation of active biomolecules, including miRNAs, to affect adjacent tissues.70 More importantly, these non-MSCs are seeded in the periarticular tissue and could participate in the pathophysiological process of OA directly (Table 2).
 |
Table 2 Therapeutic Application of non-MSCs-Derived EVs-miRNAs in OA Treatment |
As the only cells in cartilage, chondrocytes play a vital role in the homeostasis of joints and the progress of OA. It is well known that adjacent cells secreted EVs could affect the metabolism of chondrocytes and vice versa.70 For example, chondrocytes secreted EVs-miR‐95‐3p,77 and EVs-miR-848576 could promote chondrogenic differentiation of BMSCs by targeting HDAC2/8 and regulating Wnt/β-catenin pathways, respectively. Meanwhile, osteoarthritic chondrocytes secreted EVs aggravated OA’s synovitis and cartilage erosion via miR-449a-5p/ATG4B-mediated autophagy inhibition.40 In our study, we found that chondrocytes secreted EVs-miR-221 could significantly inhibit the bone formation capability of osteoblasts, which could partly demonstrate mechanotransduction between cartilage and subchondral bone via EVs-miR-221, considering the mechanosensitive character of miR-221.71,78,79
Pain and swelling caused by synovitis are the main symptoms in OA patients due to inflammatory response.80 Therefore, macrophages and fibroblast-like synoviocytes (FLSs) in synovial tissue may play a vital role in the pathologic process of OA. In 2021, Peng et al reported that M1-polarized macrophages, which are thought as proinflammatory cells, could promote inflammation in chondrocytes via EVs- miR-1246 mediated activation of the Wnt/β-catenin pathway.72 As the constituents of the intimal lining layer of the synovial membrane, FLSs secreted EVs could inhibit inflammation and apoptosis in chondrocytes and thus prevent cartilage degeneration via the overexpression of miR-126-3p. However, the exact mechanism is still unclear.73 More importantly, the EVs secreted by chondrocytes and synoviocytes can also exist in the synovial fluid. The miRNA expression stored in EVs isolated from synovial fluid of OA patients can also be a biomarker to observe the progress of OA.38
Subchondral bone has been admitted to playing a crucial role in the initiation and progress of OA.41 As the essential components of bone, both osteoblasts and osteoclasts have been reported to affect chondrocytes via EVs-miRNAs. For example, Wu et al reported that osteoblasts from OA sclerotic subchondral bone secreted EVs could promote cartilage degeneration by miR-210-5p.74 Similarly, osteoclast secreted exosomal let-7a-5p could alleviate the TGF-β-induced inhibition of chondrocyte hypertrophy by targeting Smad2.75 More importantly, microchannels such as microcracks, fissures, and vessels across the bone-cartilage interface potentiate the crosstalk between these two-part via EVs.16,81,82 Therefore, an extensive study on the microenvironment of subchondral bone would facilitate us to understand the pathophysiology of OA better.
The above-mentioned MSCs and non-MSCs secreted EVs potentiate miRNAs-mediated epigenetic regulation in OA. Significantly, these studies have demonstrated the vital role of EVs-mediated miRNAs in cartilage destruction and regeneration,20 synovial inflammation,38 bone remodeling,83 and the crosstalk among the above biological processes.37,40,75 Therefore, this may provide an important target to observe the pathological process of OA and even a crucial breakthrough to the treatment of OA.
EVs Specific Factors Affecting Its Application in OA
Although many preclinical studies reported promising effects of EVs-miRNAs in OA’s therapeutic and even reverse OA’s progress in animal experiments, reliable therapeutic effects in clinical trials are rare.21,57 As a result, the clinical transformation of EVs in the OA field is nearly no possibility. Up to date, only two trials (NCT04223622/ NCT05060107) about the application of EVs in OA are available according to the data retrieved from https://clinicaltrials.gov/. One of the most important reasons for this awkward situation is the lax management of EVs in previous studies, especially quantitative management.84 For example, some studies prefer to adopt the particle numbers as the unit to administrate the EVs dose during the experiment. In contrast, other studies would like to use protein levels to decide the amount of EVs. Therefore, it is necessary to clarify the behind factors that might interfere with its application, such as cell sources of EVs, extraction methods, and the complex biogenesis and secretion, delivery, and uptaken of EVs, all of which would compromise precise quantitative management of EVs-miRNAs and slowed clinical transformation of its therapeutic application in OA (Figure 3).
 |
Figure 3 Schematic diagram of potential factors that affect the management of EVs-miRNAs, which might further affect its clinical application in human OA. |
Cell Source
Although almost all the cells could secrete EVs, previous studies focused on MSCs derived from various tissues such as bone marrow, adipose tissue, umbilical cord, and embryonic tissue.19 However, although these MSCs secreted EVs shared similar therapeutic mechanisms by promoting proliferation, regeneration, and anti-inflammation, they demonstrate tissue specificity to treat some diseases, including OA. For example, EVs derived from BMSCs have been chiefly studied to treat disorders related to bone, such as cartilage defects or OA.85 And the potential explanation can be that the bone marrow source potentiates bone targeting ability.45 Similarly, EVs from UMSCs can be more inclined to treat diseases in gynecological and infant. However, the reason remains unclear57 and EVs from EMSCs might be more potent in promoting proliferation and differentiation than adult MSCs.86 Additionally, Ragni et al reported that UMSCs produce a higher rate of EVs/cells than BMSCs, which may be meaningful for future MSC type selection to induce the highest production rate to lower the clinical cost in clinical trials.87 However, the different yields of EVs among various cell sources may complicate the quantitative administration of EVs in practical application. As a result, it is unreasonable to compare EVs’ functions among different MSCs and make systematic comparisons of EVs’ application in OA treatment impossible. In addition, the contents of miRNAs between MSC-EVs and their parental MSCs are also different. For example, miRNA-Sequence indicated that several miRNAs from MSC-EVs were significantly different from that of MSCs.88 Therefore, the study-to-study comparability EVs-miRNAs in OA treatment can only be reliable among the same cell source.
Extraction Method
Up to date, many methods have been adopted to isolate and purify EVs from body fluid and conditional medium, including ultracentrifugation (UC), density gradient centrifugation (DGC), ultrafilter (UF), size exclusion chromatography (SEC), polymer-based (PEG) precipitation, commercial ExoQuick-TC reagent kit (EQ) and a combination of the above methods.89 Despite different nomenclature, the principle of these methods is based on the characters of EVs, such as size, density, surface proteins, or the combination of these characters. Each method could successfully isolate EVs with a similar particle size range detected by Nanoparticle Tracking Analysis (NTA), positive protein markers by the Western blot analysis, and typical morphology by Transmission Electron Microscope (TEM) visualization. However, the yield, lipoproteins and protein contaminants, extraction duration, and procedure cost are diverse among these methods.90 Besides, some immunoaffinity-based methods like immunoaffinity capture91 and microfluidics92 could effectively extract EVs with specific surface protein markers and avoid protein contaminants. Still, this kind of method might lose a lot of antibody-negative EVs and cannot reflect all EVs, no less the high cost and unique device required. According to a survey of all studies to isolate EVs from conditioned cell culture media in 2015, UC was adopted in 85% of these cases, while UF, PEG precipitation, and SEC were 18%, 14%, and 15% respectively.93 Further study in 2019 concluded that UC and density gradient centrifugation were still the first choices to isolate EVs.94 Nevertheless, the optimal protocol for the isolation of EVs is still lacking so far, and the combination of several methods is recommended to make up for the shortcomings of a single approach and make a balance between specificity and recovery.30 As a result, when involved in the quantitative management of EVs in studies, the isolation method must be considered because variable isolation methods would aggravate the uncertainties and inconsistencies of EVs studies across laboratories.
miRNA Loading Approach
Both indirect and direct engineered methods are available to produce miRNAs-loaded EVs. In brief, an indirect way would engineer the parental cells by physical, chemical, or genetic manipulation, which could further secret miRNA-rich EVs.95 For example, oxygen condition is thought to be vital in the regulation of MSCs by affecting proliferation, differentiation, and self-renewal.96 Hypoxic pretreatment could induce BMSCs to secret EVs, which further stimulated the proliferation, migration, and inhibited apoptosis of chondrocytes via miR-216a-5p mediated modulation of JAK2/STAT3 signaling pathway.47 Kartogenin (KGN) was reported to promote the chondrogenic differentiation of MSCs in vitro.97 A subsequent study demonstrated that KGN pretreated human UCMSCs could induce chondrogenic differentiation of MSCs and promote cartilage repair in an animal model by sEV-miR-381-3p mediated inhibition of TAOK1.51 As for genetic manipulation, mimic54,60 or plasmid transfection,59 lentivirus transduction,47,65 and transgene98 have been used often in previous studies. As an artificial synthesized double-stranded miRNA molecule to mimic the function of endogenous miRNA duplexes, mimic transfection can be the most common method to increase miRNAs’ expression in parental cells with apparent advantages like a simple operation, high efficiency, and Cost-Effective.99 Therefore, miRNAs loaded EVs by mimic transfection appeared in many studies, also in the OA field. For example, miR-129-5p was overexpressed in human synovial mesenchymal stem cells shuttled by mimic transfection, and secreted exosomes were shown to relieve IL-1β induced inflammatory response and apoptosis of chondrocytes via targeting high mobility group protein −1 (HMGB1).60 Similarly, other miRNAs like miR-31,61 miR-136-5p,61 miRNA-100-5P,55,66 miR-320c,67 miR-140-5p,69 miR-126-3p,73 miR-210-5p,74 miR-8485,76 miR‐95‐3p77 have also been loaded in cells to release EVs-miRNAs by mimic transfection. However, mimic transfection cannot genuinely simulate endogenous miRNAs due to the mysterious chemical modifications by the manufacturers.100 More importantly, these exogenous miRNA mimics could lead to supraphysiological miRNA accumulation in parental cells, which can induce non–specific gene expression. As a result, parental cells modified by the transfection/transduction/transgene methods may also express EVs that are different by their miR content and the hundred of miR targets. Furthermore, the exact mechanism of how the loaded miRNAs by mimic transfection are assembled into EVs is still unclear, and this reminds us to be much more careful with this method. Intriguingly, the low concentration accumulation of miRNA by mimic transfection was not sufficient to inhibit the expression of the target gene.99 In contrast, the same amount of miRNA overexpression induced by plasmid transfection, lentivirus transduction, or transgene could effectively suppress target gene expression.101–103 Therefore, subtype analysis of different EVs can be crucial to managing the dose in EVs-miRNAs mediated OA treatment.
Compared to indirectly engineered methods, the direct engineered methods load miRNAs after the production of EVs. Briefly, the direct loading of miRNAs to EVs can be classified as physical methods including electroporation, sonication, incubation, freeze-thawing, and chemical methods containing saponin permeabilization and CaCl2- heat shock.95 However, direct methods are not perfect despite cheap and straightforward and are with the low efficient loading of miRNAs into EVs. More importantly, these chemical and physical shocks could change the natural properties of EVs, which might affect the transfer and uptake of EVs by recipient cells, and further the activity and function of the miRNAs inside. As far as we know, direct methods to load miRNAs in EVs have not been reported in the OA field.
Quantitative Method
According to the recommendation of the Minimal information for studies of extracellular vesicles 2018 (MISEV2018),30 either characteristic of the isolated EVs or the source material can be used to normalize the vesicles number when studying EVs functions. The characteristics of EVs consist of particle number, the total amount of proteins, nucleic acids, or lipids, and content or activity of specific EV-associated molecules in the EVs. In contrast, source characteristics are the amount of matrix where the EVs were extracted, for example, the volume of biofluid or cell culture medium, and the number of parental cells. According to the collected studies, particle counting by NTA and the total amount of proteins tested by the bicinchoninic acid (BCA) Protein Assay Kit are the most commonly used. In contrast, some studies did not describe the information about the normalization strategy. For example, Wang et al isolated EVs from the conditioned medium of chondrogenic progenitor cells (CPCs) by ultracentrifugation and measured the size distribution and the particle concentration of EVs by NTA with a Nanosight NS300 instrument. Then chondrocytes were treated with EVs resuspended in PBS (108 particles/mL) in vitro, and the surgical-induced OA model was injected intra-articularly with 8 μL EVs in vivo.104 In another study, Jin et al determined the protein concentration in EVs by BCA kit. After that, synovial fibroblast cells were treated with 2 µg EVs in the 6-well plates, and 250 ng EVs were injected into the knee joint of the surgical-induced OA model.65 More importantly, some studies are missing details about the ratio of EVs and treated cells and the duration of intervention in vitro experiments. As for the in vivo animal experiment, the different time and frequency of intervention in various OA models with varying animal species further complicate the function analysis of EVs. Although the ratio of particle to protein was recommended to be a means to compare sample purity and thus guide the quantitative management of EVs, one issue needs to be considered.105 Namely, the NTA technique cannot distinguish vesicles from non-vesicular particles, and the BCA method cannot measure the degree of protein contamination. To accurately regulate EVs-miRNAs, the quantity of EVs and the quantity of functional miRNA to target mRNA in recipient cells need to be precisely calculated in the particular tissue, considering the final applicable action comes from miRNA mediated target inhibition. Nevertheless, the optimal normalization strategy to quantify EVs-miRNAs is still lacking, making it difficult to compare two independent studies.106 Therefore, much more precise quantification methods of EVs-miRNA are required. In contrast, details about EVs-miRNAs preparation and isolation, quantification, and application should be provided in practice to potentiate the comparison of experimental results among studies comprehensively and objectively.
OA Specific Factor
The various routes of EVs administration are associated with the distribution and clearance rate of EVs in vivo, thus affecting the therapeutic effect of EVs in practical applications.89 Unlike other diseases, the only standard administration route of EVs is through intra-articular injection due to the external anatomical site of the knee joint in OA. Nevertheless, the natural exosomes with the limitation of poor targeting is a significant issue in OA treatment with EVs, especially how to improve the penetration ratio of EVs across the extracellular matrix and then uptaken by targeted cells accurately and efficiently. To this end, the chemical of transgenic surface modification of EVs has been thought as a possible way to achieve the precise target and accelerate the clinical transformation of EVs, despite the drawbacks such as potential change of surface molecules caused by chemical linking of targeting peptides, inefficient modification and cytotoxicity by transfection.89 For example, Xu et al demonstrated that KGN delivered by MSC-binding peptide E7 (E7) modified exosomes significantly increased the uptaken ratio by SMSCs and induced a higher degree of cartilage differentiation than KGN provided by exosomes without E7.107 However, the question remains, if and how valid are these surface modification methods for miRNA-loaded EVs in the therapeutic applications require further research.108 Besides, some drawbacks such as contamination and variable efficiency of modification with chemical processes and safety concerns with transgenic alteration would be the current issues before clinical applications.109 Therefore, engineered modified EVs can be promising in EVs delivery and targeting application, while blind application of surface modification should be avoided due to inadequate knowledge.
In addition, in vivo experiment with various animal OA models is a crucial tool to study the pathogenesis of disease and the therapeutic effect of and relevant treatment.110 Therefore, different animal models induced by surgical and chemical approaches might affect the therapeutic effect of EVs-miRNAs due to the different pathogenesis among other models. So far, surgical induced OA models by anterior cruciate ligament transection (ACLT),50 destabilized medial meniscus (DMM)47 and grooved cartilage defect,51 and chemical-induced OA models with sodium iodoacetate,111 sodium monoiodoacetate (MIA),112 and collagenase113 have been adopted in OA studies. As a heterogeneous disease, no single animal model could represent all aspects of the pathological progress of OA.114 These surgical models which could mimic posttraumatic OA are most commonly utilized in studies. However, the pathological progress of these models is much more rapid than that in humans, and this might suppress their response to some interventions.115 Similarly, their validity of chemical-induced OA models for OA has also been questioned mainly due to the widespread cell death and rapid joint destruction secondary to chemical injection like MIA, which might not happen in human OA.116 Furthermore, animal specials also matter when testing and comparing the EVs-miRNAs therapeutics among studies because of the considerable difference among animals on the anatomy, histology, and physiology.117,118 As a result, some drugs that are effective in small animal experiments may not be the same efficacy as humans.119
Therefore, the interpretation of the animal experimental result and relevant extrapolation to the human condition must be made with caution. Meanwhile, sufficient details about the EVs administration in the animal experiment are necessary to judge the appropriateness and biological relevance of the concluded result, which could also potentiate the independent replication of similar investigations by others. In addition, different animal OA models would be recommended to test the therapeutic effect of EVs to avoid the resulting bias caused by the different models. For example, Woo et al used the MIA rat and DMM mouse models to demonstrate the therapeutic function of hASC-EVs, which might provide more substantial evidence than a single OA model.120
Discussion and Future Perspective
EVs have been intensely studied in different diseases, including OA, as a cell-free delivery vehicle, during the past decades.15 Meanwhile, miRNAs transferred by EVs have also demonstrated a vital role in regulating the disease progress by inhibiting the expression of relevant targets, thus affecting different biological processes such as proliferation, migration, differentiation, inflammation, autophagy, and apoptosis. However, current research remains in the laboratory stage and cannot be effectively translated into clinical trials.84 One of the most important reasons is the chaos of EVs administration in the studies, especially the quantitative management. Although the MISEV201429 and MISEV201830 have provided strict guidelines about the isolation, identification, and application of EVs, the experimental details about the EVs are sometimes not displayed in literature as shown in Tables 1 and 2.
How to isolate the purified EVs is still a vexing problem. Unfortunately, there is no optimal isolation method available up to date, while a combination of several ways is often recommended.121 When EVs are used to transfer miRNAs, the loading methods can affect the final function of EVs-miRNAs due to the limited knowledge of the biogenesis of EVs and the assembly of miRNAs in EVs. Meanwhile, the surface modification of EVs to promote the delivery of EVs is still under research and should be used with caution due to the potential damage to its surface. As for the quantitative method of EVs, the particle concentration and protein concentration are still the most common methods to manage EVs’ dose in the experiment. The ratio of particle number and protein concentration is recommended to estimate the purity of EVs. However, whether the ratio remains static during disease development would also affect the dosage calculation of EVs.
No matter which quantitative method is adopted, to avoid a highly supraphysiologic dose, the ratio of how many EVs per recipient cell was used and the amount of EVs produced per cell need to be calculated and quantify how many folds it is.122 It would be difficult to attribute the observed effects to EVs if they appeared to be highly supraphysiologic. The standard mimic transfection method would increase the miRNA for hundreds or thousands of times higher expression in parental cells, so the transfection may also stimulate cells to secret EVs with a heterogeneous population that is different not only by their miRNA content but also by the hundreds of miR targets that will be modified by the parental cell. As a result, the miRNA of interest inside EVs does not strictly mean that the miRNA mediates the effect. The convincing demonstration would be to remove the miRNA from the miRNA-modified EVs after its production and see the effect’s abrogation. However, most studies preferred to utilize the miRNA inhibitor to suppress the activity and function of miRNAs after the output of EVs-miRNAs,55,68 not before. In contrast, others used EVs inhibitors like GW4869, a neutral sphingomyelinase-targeting inhibitor to inhibit ESCRT-mediated exosome biogenesis.17,123
Another issue would be how to obtain a large yield of EVs for therapeutic purposes due to the limited number of MSCs or somatic cells in human bodies. One potential method can be the immortalized cell line CEVEC’s amniocytic production line cells, which have demonstrated a promising source for functional, miRNA-loaded EVs production.124 In addition, 3D cell niche engineering would be another method to produce large-scale EVs with enriched content for therapeutic purposes by stimulating the body’s microenvironment under accurate control of physical, chemical, and biological conditions.125 Meanwhile, optimized PEG prepetition protocol followed by ultracentrifugation has also been demonstrated to enrich functional active EVs from many conditioned medium.126 However, EV purity is always a problem for the PEG protocol alone.
In addition to the above factors, preparing conditioned medium or various body fluids before EVs isolation and storing EVs after the isolation are still challenges to preserve EVs-miRNAs’ structural stability and biological activities. Furthermore, how miRNAs are assembled in the endogenous biogenesis of EVs, how many EVs-miRNAs can be uptaken by the recipient cells, and how effective the digested miRNAs regulate downstream targets would finally affect the precise management of EVs-miRNAs. Last but not least, calculating the physiological dose of EVs-miRNAs and clarifying the pharmacodynamics and biological distribution of the injected EVs in the animal experiment might be another breakthrough in future research.
Precise quantitative management of EVs-miRNAs can accelerate its therapeutic application but also be helpful to develop its diagnostic function as a biomarker during disease progression. Due to the protective environment provided by EVs, many studies have highlighted the diagnostic advantages of EVs-miRNAs compared to the free-circulating miRNAs.127,128 Furthermore, the expression level of EVs-miRNAs can be easily tested by collecting intra-articular synovial fluid and intuitively reflecting the joint condition due to its physical association with periarticular tissues like synovial membrane and articular cartilage. For example, Kolhe et al found altered profiles of exosomal miRNAs in synovial fluid of OA patients and demonstrated this alteration could be gender specific.129
Conclusion
EVs-mediated miRNAs regulation remains an area of great interest in treating different diseases, including OA, and has demonstrated positive preclinical results. Future studies need to pay more attention to the quantitative management of EVs-miRNAs. In this case, progress in the quantitative direction of EVs-miRNAs would accelerate the clinical transformation of miRNAs enriched EVs in the diagnosis and therapeutic of OA. Moreover, other molecules like chemical drugs,58,62,107,130 growth factors,113,131 and lncRNAs132,133 can also be delivered by EVs and demonstrated to be effective in OA treatment. In addition, further research on the quantitative management of EVs-miRNAs and a sufficient understanding of EVs biology will enhance its diagnostic and therapeutic application in the OA field. This review summarized and analyzed the application of EVs-miRNA secreted by MSCs and non-MSCs in OA treatment. Still, some limitations on our understanding of their physiological roles in humans remain. For example, although there are initial indications that EV-mediated pathways operate in vivo, the actual nature of the EVs involved in these effects still needs to be clarified. Meanwhile, the chaos of quantitative management of EVs-miRNAs and animal models, among other research, has seriously affected the clinical application of EVs. More importantly, despite decades’ research, our understanding of the exact mechanism of EVs’ biogenesis and secretion, transportation, and uptake is still in its infancy. As a result, further study on EVs mediated miRNA regulation in the intercellular interaction is required.
Funding
There is no funding to report.
Disclosure
No potential conflicts of interest for this work were reported by the authors.
References
1. van den Bosch MHJ. osteoarthritis year in review 2020: biology. Osteoarthritis Cartilage. 2021;29(2):143–150. doi:10.1016/j.joca.2020.10.006
2. Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384:51–59. doi:10.1056/NEJMcp1903768
3. Kulkarni K, Karssiens T, Kumar V, Pandit H. Obesity and osteoarthritis. Maturitas. 2016;89:22–28. doi:10.1016/j.maturitas.2016.04.006
4. Fazio G, Vernuccio D, Di Gesaro G, et al. Obesity: a new pathology to pay attention to in young people. Curr Pharm Des. 2010;16(4):463–467. doi:10.2174/138161210790232167
5. Kushner RF, Choi SW. Prevalence of unhealthy lifestyle patterns among overweight and obese adults. Obes Silver Spring Md. 2010;18(6):1160–1167. doi:10.1038/oby.2009.376
6. Hunter DJ, Bierma Zeinstra S. Osteoarthritis. Lancet Lond Engl. 2019;393:1745–1759. doi:10.1016/S0140-6736(19)30417-9
7. Shang X, Böker KO, Taheri S, Hawellek T, Lehmann W, Schilling AF. The interaction between MicroRNAs and the Wnt/β-Catenin signaling pathway in osteoarthritis. Int J Mol Sci. 2021;22(18):1–16. doi:10.3390/ijms22189887
8. Arden NK, Perry TA, Bannuru RR, et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol. 2021;17:59–66. doi:10.1038/s41584-020-00523-9
9. Gandhi R, Perruccio AV, Mahomed NN. Surgical management of hip osteoarthritis. CMAJ Can Med Assoc J. 2014;186:347–355. doi:10.1503/cmaj.121584
10. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207. doi:10.1016/j.jaci.2017.08.034
11. Li Y, Kowdley KV. MicroRNAs in common human diseases. Genomics Proteomics Bioinformatics. 2012;10(5):246–253. doi:10.1016/j.gpb.2012.07.005
12. Swingler TE, Niu L, Smith P, et al. The function of MicroRNAs in cartilage and osteoarthritis. Clin Exp Rheumatol. 2019;37(Suppl 120):40–47.
13. Asahara H. Current status and strategy of MicroRNA research for cartilage development and osteoarthritis pathogenesis. J Bone Metab. 2016;23(3):121–127. doi:10.11005/jbm.2016.23.3.121
14. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of MRNAs and MicroRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb1596
15. Wu P, Zhang B, Ocansey DKW, Xu W, Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. 2021;269:120467. doi:10.1016/j.biomaterials.2020.120467
16. Taheri S, Yoshida T, Böker KO, et al. Investigating the microchannel architectures inside the subchondral bone in relation to estimated hip reaction forces on the human femoral head. Calcif Tissue Int. 2021;1(5):510–524. doi:10.1007/s00223-021-00864-x
17. Kuang Y, Zheng X, Zhang L, et al. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of MiR-25. J Extracell Vesicles. 2020;10(1):1–20. doi:10.1002/jev2.12024
18. van Dommelen SM, Vader P, Lakhal S, et al. Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release Off J Control Release Soc. 2012;161(2):635–644. doi:10.1016/j.jconrel.2011.11.021
19. Zhang B, Tian X, Qu Z, Liu J, Yang L, Zhang W. Efficacy of extracellular vesicles from mesenchymal stem cells on osteoarthritis in animal models: a systematic review and meta-analysis. Nanomed. 2021;16:1297–1310. doi:10.2217/nnm-2021-0047
20. Song H, Zhao J, Cheng J, et al. Extracellular vesicles in chondrogenesis and cartilage regeneration. J Cell Mol Med. 2021;25(11):4883–4892. doi:10.1111/jcmm.16290
21. Mohd Noor NA, Abdullah Nurul A, Zain AM, Wan Nor MR, Aduni WK, Azlan M. Extracellular vesicles from mesenchymal stem cells as potential treatments for osteoarthritis. Cells. 2021;10(6):1–22. doi:10.3390/cells10061287
22. Hammaker D, Firestein GS. Epigenetics of inflammatory arthritis. Curr Opin Rheumatol. 2018;30(2):188–196. doi:10.1097/BOR.0000000000000471
23. Wu Y, Lu X, Shen B, Zeng Y. The therapeutic potential and role of MiRNA, LncRNA, and CircRNA in osteoarthritis. Curr Gene Ther. 2019;19(4):255–263. doi:10.2174/1566523219666190716092203
24. Chen L, Heikkinen L, Wang C, Yang Y, Sun H, Wong G. Trends in the development of MiRNA bioinformatics tools. Brief Bioinform. 2019;20(5):1836–1852. doi:10.1093/bib/bby054
25. Ng CY, Chai JY, Foo JB, et al. potential of exosomes as cell-free therapy in articular cartilage regeneration: a review. Int J Nanomed. 2021;16:6749–6781. doi:10.2147/IJN.S327059
26. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. doi:10.1146/annurev-cellbio-101512-122326
27. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
28. Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):E307. doi:10.3390/cells8040307
29. Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the international society for extracellular vesicles. J Extracell Vesicles. 2014;3(1):26913. doi:10.3402/jev.v3.26913
30. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
31. Qiu G, Zheng G, Ge M, et al. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of MicroRNAs. Stem Cell Res. Ther. 2018;9(1):320. doi:10.1186/s13287-018-1069-9
32. Théry C, Boussac M, Véron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309–7318. doi:10.4049/jimmunol.166.12.7309
33. Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi:10.1016/j.semcdb.2015.02.010
34. Zhao X, Zhao Y, Sun X, Xing Y, Wang X, Yang Q. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol. 2020;8:575057. doi:10.3389/fbioe.2020.575057
35. Tan SSH, Tjio CKE, Wong JRY, et al. Mesenchymal stem cell exosomes for cartilage regeneration: a systematic review of preclinical in vivo studies. Tissue Eng Part B Rev. 2021;27(1):1–13. doi:10.1089/ten.TEB.2019.0326
36. de Almeida RC, Ramos YF, Mahfouz A, et al. RNA sequencing data integration reveals an MiRNA interactome of osteoarthritis cartilage. Ann Rheum Dis. 2019;78(2):270–277. doi:10.1136/annrheumdis-2018-213882
37. Kato T, Miyaki S, Ishitobi H, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. doi:10.1186/ar4679
38. Gao K, Zhu W, Li H, et al. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod Rheumatol. 2020;30(4):758–764. doi:10.1080/14397595.2019.1651445
39. Taghiyar L, Jahangir S, Khozaei Ravari M, Shamekhi MA, Eslaminejad MB. Cartilage repair by mesenchymal stem cell-derived exosomes: preclinical and clinical trial update and perspectives. Adv Exp Med Biol. 2021;12:73–93. doi:10.1007/5584_2021_625
40. Ni Z, Kuang L, Chen H, et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10(7):522. doi:10.1038/s41419-019-1739-2
41. Hu W, Chen Y, Dou C, Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis. 2020;413:413–422. doi:10.1136/annrheumdis-2020-218089
42. Duan L, Xu X, Xu L, et al. Exosome-mediated drug delivery for cell-free therapy of osteoarthritis. Curr Med Chem. 2020. doi:10.2174/0929867327666201118161232
43. Goldie BJ, Dun MD, Lin M, et al. Activity-associated MiRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014;42(14):9195–9208. doi:10.1093/nar/gku594
44. Ferguson SW, Wang J, Lee CJ, et al. The MicroRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018;8(1):1419. doi:10.1038/s41598-018-19581-x
45. Borgovan T, Crawford L, Nwizu C, Quesenberry P. Stem cells and extracellular vesicles: biological regulators of physiology and disease. Am J Physiol Cell Physiol. 2019;317(2):C155–C166. doi:10.1152/ajpcell.00017.2019
46. Li X, Teng Y, Liu J, Lin H, Fan Y, Zhang X. Chondrogenic differentiation of BMSCs encapsulated in chondroinductive polysaccharide/collagen hybrid hydrogels. J Mater Chem B. 2017;5(26):5109–5119. doi:10.1039/c7tb01020f
47. Rong Y, Zhang J, Jiang D, et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering MiR-216a-5p. Acta Biomater. 2021;122:325–342. doi:10.1016/j.actbio.2020.12.034
48. Damia E, Chicharro D, Lopez S, et al. Adipose-derived mesenchymal stem cells: are they a good therapeutic strategy for osteoarthritis? Int J Mol Sci. 2018;19(7):E1926. doi:10.3390/ijms19071926
49. Ragni E, Colombini A, Viganò M, et al. Cartilage protective and immunomodulatory features of osteoarthritis synovial fluid-treated adipose-derived mesenchymal stem cells secreted factors and extracellular vesicles-embedded MiRNAs. Cells. 2021;10(5):1072. doi:10.3390/cells10051072
50. Yang Y, Zhu Z, Gao R, et al. Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic Acid/PEG hydrogel for osteoarthritis improvement. Acta Biomater. 2021;128:163–174. doi:10.1016/j.actbio.2021.04.003
51. Jing H, Zhang X, Luo K, et al. MiR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials. 2020;231:119682. doi:10.1016/j.biomaterials.2019.119682
52. Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi:10.1016/j.biomaterials.2017.11.028
53. Ragni E, Papait A, Perucca Orfei C, et al. Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-MiRNAs: anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl Med. 2021;10(7):1044–1062. doi:10.1002/sctm.20-0390
54. Wang K, Li F, Yuan Y, et al. Synovial mesenchymal stem cell-derived EV-Packaged MiR-31 downregulates histone demethylase KDM2A to prevent knee osteoarthritis. Mol Ther Nucleic Acids. 2020;22:1078–1091. doi:10.1016/j.omtn.2020.09.014
55. Wu J, Kuang L, Chen C, et al. MiR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of MTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi:10.1016/j.biomaterials.2019.03.022
56. Wang Y, He G, Guo Y, et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J Cell Mol Med. 2019;23:5475–5485. doi:10.1111/jcmm.14430
57. Cai J, Wu J, Wang J, et al. Extracellular vesicles derived from different sources of mesenchymal stem cells: therapeutic effects and translational potential. Cell Biosci. 2020;10(1):69. doi:10.1186/s13578-020-00427-x
58. Li S, Stöckl S, Lukas C, et al. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating MiR-126-3p. Stem Cell Res Ther. 2021;12(1):252. doi:10.1186/s13287-021-02317-6
59. Tao S-C, Huang J-Y, Gao Y, et al. Small extracellular vesicles in combination with sleep-related CircRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact Mater. 2021;6(12):4455–4469. doi:10.1016/j.bioactmat.2021.04.031
60. Qiu M, Liu D, Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. doi:10.1016/j.lfs.2020.118987
61. Chen X, Shi Y, Xue P, Ma X, Li J, Zhang J. Mesenchymal stem cell-derived exosomal MicroRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res Ther. 2020;22:256. doi:10.1186/s13075-020-02325-6
62. Qiu B, Xu X, Yi P, Hao Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the MiR-124/NF-KB and MiR-143/ROCK1/TLR9 signalling pathways. J Cell Mol Med. 2020;24(18):10855–10865. doi:10.1111/jcmm.15714
63. Ragni E, Perucca Orfei C, De Luca P, et al. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-MiRNAs: the example of joint disease. Stem Cell Res Ther. 2020;11(1):165. doi:10.1186/s13287-020-01677-9
64. Zhao C, Chen J-Y, Peng W-M, Yuan B, Bi Q, Xu Y-J. Exosomes from adipose derived stem cells promote chondrogenesis and suppress inflammation by upregulating MiR 145 and MiR 221. Mol Med Rep. 2020;21(4):1881–1889. doi:10.3892/mmr.2020.10982
65. Jin Z, Ren J, Qi S. Human bone mesenchymal stem cells-derived exosomes overexpressing MicroRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int Immunopharmacol. 2020;78:105946. doi:10.1016/j.intimp.2019.105946
66. Luo P, Jiang C, Ji P, Wang M, Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via MiR-100-5p/MTOR. Stem Cell Res Ther. 2019;10(1):216. doi:10.1186/s13287-019-1341-7
67. Sun H, Hu S, Zhang Z, Lun J, Liao W, Zhang Z. Expression of exosomal MicroRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem. 2019;120(1):171–181. doi:10.1002/jcb.27289
68. Wang R, Xu B, Xu H. TGF-Β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived MiR-135b. Cell Cycle Georget Tex. 2018;17. doi:10.1080/15384101.2018.1556063.
69. Tao S-C, Yuan T, Zhang Y-L, Yin W-J, Guo S-C, Zhang C-Q. Exosomes derived from MiR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi:10.7150/thno.17133
70. Wu X, Wang Y, Xiao Y, Crawford R, Mao X, Prasadam I. Extracellular vesicles: potential role in osteoarthritis regenerative medicine. J Orthop Transl. 2020;21:73–80. doi:10.1016/j.jot.2019.10.012
71. Shang X, Böker KO, Taheri S, Lehmann W, Schilling AF. Extracellular vesicles allow epigenetic mechanotransduction between chondrocytes and osteoblasts. Int J Mol Sci. 2021;22(24):13282. doi:10.3390/ijms222413282
72. Peng S, Yan Y, Li R, Dai H, Xu J. Extracellular vesicles from M1-polarized macrophages promote inflammation in the temporomandibular joint via MiR-1246 activation of the Wnt/β-Catenin pathway. Ann N Y Acad Sci. 2021;1503(1):48–59. doi:10.1111/nyas.14590
73. Zhou Y, Ming J, Li Y, et al. Exosomes derived from MiR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021;7(1):1–15. doi:10.1038/s41420-021-00418-y
74. Wu X, Crawford R, Xiao Y, Mao X, Prasadam I. Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells. 2021;10(2):251. doi:10.3390/cells10020251
75. Dai J, Dong R, Han X, et al. Osteoclast-derived exosomal Let-7a-5p targets smad2 to promote the hypertrophic differentiation of chondrocytes. Am J Physiol Cell Physiol. 2020;319(1):C21–C33. doi:10.1152/ajpcell.00039.2020
76. Li Z, Wang Y, Xiang S, et al. Chondrocytes-derived exosomal MiR-8485 regulated the Wnt/β-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem Biophys Res Commun. 2020;523:506–513. doi:10.1016/j.bbrc.2019.12.065
77. Mao G, Hu S, Zhang Z, et al. Exosomal MiR-95-5p Regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J Cell Mol Med. 2018;22(11):5354–5366. doi:10.1111/jcmm.13808
78. Hecht N, Johnstone B, Angele P, Walker T, Richter W. Mechanosensitive MiRs regulated by anabolic and catabolic loading of human cartilage. Osteoarthritis Cartilage. 2019;27(8):1208–1218. doi:10.1016/j.joca.2019.04.010
79. Stadnik PS, Gilbert SJ, Tarn J, et al. Regulation of MicroRNA-221, −222, −21 and −27 in articular cartilage subjected to abnormal compressive forces. J Physiol. 2021;599:143–155. doi:10.1113/JP279810
80. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18. doi:10.1186/s13075-017-1229-9
81. Yuan XL, Meng HY, Wang YC, et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthritis Cartilage. 2014;22:1077–1089. doi:10.1016/j.joca.2014.05.023
82. Pan J, Zhou X, Li W, Novotny JE, Doty SB, Wang L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res. 2009;27(10):1347–1352. doi:10.1002/jor.20883
83. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214. doi:10.1038/s41598-017-15376-8
84. Munir J, Yoon JK, Ryu S. Therapeutic MiRNA-enriched extracellular vesicles: current approaches and future prospects. Cells. 2020;9(10):2271. doi:10.3390/cells9102271
85. De Bari C, Roelofs AJ. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol. 2018;40:74–80. doi:10.1016/j.coph.2018.03.009
86. Hawkins KE, Corcelli M, Dowding K, et al. Embryonic stem cell-derived mesenchymal stem cells (MSCs) have a superior neuroprotective capacity over fetal MSCs in the hypoxic-ischemic mouse brain. Stem Cells Transl Med. 2018;7(5):439–449. doi:10.1002/sctm.17-0260
87. Ragni E, Banfi F, Barilani M, et al. Extracellular vesicle-shuttled MRNA in mesenchymal stem cell communication. STEM CELLS. 2017;35(4):1093–1105. doi:10.1002/stem.2557
88. Shao L, Zhang Y, Lan B, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Bio Med Res Int. 2017;2017:4150705. doi:10.1155/2017/4150705
89. Zhang Y, Bi J, Huang J, Tang Y, Du S, Exosome: LP. A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917–6934. doi:10.2147/IJN.S264498
90. Brennan K, Martin K, FitzGerald SP, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020;10:1039. doi:10.1038/s41598-020-57497-7
91. Koliha N, Wiencek Y, Heider U, et al. Bead-based platform highlights the diversity of extracellular vesicles. J Extracell Vesicles. 2016;5(1):29975. doi:10.3402/jev.v5.29975
92. Chen C, Skog J, Hsu C-H, et al. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip. 2010;10(4):505–511. doi:10.1039/b916199f
93. Gardiner C, Di Vizio D, Sahoo S, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5(1):32945. doi:10.3402/jev.v5.32945
94. Royo F, Théry C, Falcón-Pérez JM, Nieuwland R, Witwer KW. Methods for separation and characterization of extracellular vesicles: results of a worldwide survey performed by the ISEV rigor and standardization subcommittee. Cells. 2020;9(9):E1955. doi:10.3390/cells9091955
95. Esmaeili A, Hosseini S, Baghaban Eslaminejad M. Engineered-extracellular vesicles as an optimistic tool for MicroRNA delivery for osteoarthritis treatment. Cell Mol Life Sci CMLS. 2021;78:79–91. doi:10.1007/s00018-020-03585-w
96. Hu X, Wu R, Shehadeh LA, et al. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genomics. 2014;15(1):303. doi:10.1186/1471-2164-15-303
97. Johnson K, Zhu S, Tremblay MS, et al. A stem cell-based approach to cartilage repair. Science. 2012;336:717–721. doi:10.1126/science.1215157
98. Szuplewski S, Kugler J-M, Lim SF, Verma P, Chen Y-W, Cohen SM. MicroRNA transgene overexpression complements deficiency-based modifier screens in drosophila. Genetics. 2012;190(2):617–626. doi:10.1534/genetics.111.136689
99. Jin HY, Gonzalez-Martin A, Miletic AV, et al. Transfection of MicroRNA mimics should be used with caution. Front Genet. 2015;6:340. doi:10.3389/fgene.2015.00340
100. Thomson DW, Bracken CP, Szubert JM, Goodall GJ. On measuring MiRNAs after transient transfection of mimics or antisense inhibitors. PLoS One. 2013;8:e55214. doi:10.1371/journal.pone.0055214
101. Xiao C, Srinivasan L, Calado DP, et al. Lymphoproliferative disease and autoimmunity in mice with increased MiR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi:10.1038/ni1575
102. Kang SG, Liu W-H, Lu P, et al. MicroRNAs of the MiR-17 92 family are critical regulators of T (FH) differentiation. Nat Immunol. 2013;14:849–857. doi:10.1038/ni.2648
103. Jin HY, Oda H, Lai M, et al. MicroRNA-17~92 plays a causative role in lymphomagenesis by coordinating multiple oncogenic pathways. EMBO J. 2013;32(17):2377–2391. doi:10.1038/emboj.2013.178
104. Wang R, Jiang W, Zhang L, et al. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res Ther. 2020;11(1):93. doi:10.1186/s13287-020-01594-x
105. Webber J, Clayton A. How Pure Are Your Vesicles? J Extracell Vesicles. 2013;2(1):19861. doi:10.3402/jev.v2i0.19861
106. Paolini L, Zendrini A, Radeghieri A. Biophysical properties of extracellular vesicles in diagnostics. Biomark Med. 2018;12(4):383–391. doi:10.2217/bmm-2017-0458
107. Xu X, Liang Y, Li X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. doi:10.1016/j.biomaterials.2020.120539
108. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi:10.7150/thno.21945
109. Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Mol Ther J Am Soc Gene Ther. 2013;21(1):185–191. doi:10.1038/mt.2012.180
110. Kim JE, Song D-H, Kim SH, Jung Y, Kim SJ. Development and characterization of various osteoarthritis models for tissue engineering. PLoS One. 2018;13(3):e0194288. doi:10.1371/journal.pone.0194288
111. He L, He T, Xing J, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11(1):276. doi:10.1186/s13287-020-01781-w
112. Zhang S, Teo KYW, Chuah SJ, et al. Temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi:10.1016/j.biomaterials.2019.02.006
113. Ruiz M, Toupet K, Maumus M, Rozier P, Jorgensen C, Noël D. TGFBI secreted by mesenchymal stromal cells ameliorates osteoarthritis and is detected in extracellular vesicles. Biomaterials. 2020;226:119544. doi:10.1016/j.biomaterials.2019.119544
114. McCoy AM. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol. 2015;52(5):803–818. doi:10.1177/0300985815588611
115. Teeple E, Jay GD, Elsaid KA, Fleming BC. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 2013;15(2):438–446. doi:10.1208/s12248-013-9454-x
116. Little CB, Zaki S. What constitutes an “animal model of osteoarthritis”–the need for consensus? Osteoarthritis Cartilage. 2012;20(4):261–267. doi:10.1016/j.joca.2012.01.017
117. Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19(4):493–499. doi:10.1016/j.knee.2011.07.005
118. Pedersen DR, Goetz JE, Kurriger GL, Martin JA. Comparative digital cartilage histology for human and common osteoarthritis models. Orthop Res Rev. 2013;2013(5):13–20. doi:10.2147/ORR.S38400
119. Pelletier J-P, Boileau C, Altman RD, Martel-Pelletier J. Experimental models of osteoarthritis: usefulness in the development of disease-modifying osteoarthritis drugs/agents. Therapy. 2010;7(6):621–634. doi:10.2217/thy.10.75
120. Woo CH, Kim HK, Jung GY, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9(1):1735249. doi:10.1080/20013078.2020.1735249
121. Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21(18):E6466. doi:10.3390/ijms21186466
122. Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the MicroRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111(41):14888–14893. doi:10.1073/pnas.1408301111
123. Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 2020;9(1):1703244. doi:10.1080/20013078.2019.1703244
124. Zeh N, Schneider H, Mathias S, et al. Human CAP cells represent a novel source for functional, MiRNA-loaded exosome production. PLoS One. 2019;14(8):e0221679. doi:10.1371/journal.pone.0221679
125. Cha JM, Shin EK, Sung JH, et al. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci Rep. 2018;8(1):1171. doi:10.1038/s41598-018-19211-6
126. Ludwig A-K, De Miroschedji K, Doeppner TR, et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J Extracell Vesicles. 2018;7(1):1528109. doi:10.1080/20013078.2018.1528109
127. Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of MiRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3(1):23743. doi:10.3402/jev.v3.23743
128. Maehara M, Toyoda E, Takahashi T, Watanabe M, Sato M. Potential of exosomes for diagnosis and treatment of joint disease: towards a point-of-care therapy for osteoarthritis of the knee. Int J Mol Sci. 2021;22(5):2666. doi:10.3390/ijms22052666
129. Kolhe R, Hunter M, Liu S, et al. Gender-specific differential expression of exosomal MiRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):2029. doi:10.1038/s41598-017-01905-y
130. Liu C, Li Y, Yang Z, Zhou Z, Lou Z, Zhang Q. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomed. 2020;15(3):273–288. doi:10.2217/nnm-2019-0208
131. Xu T, Xu M, Bai J, et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology. 2019;71(1):57–65. doi:10.1007/s10616-018-0264-y
132. Bai J, Zhang Y, Zheng X, et al. LncRNA MM2P-induced, exosome-mediated transfer of sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020;11(9):763. doi:10.1038/s41419-020-02945-5
133. Tan F, Wang D, Yuan Z. The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the MiR-106b-5p/TIMP2 axis. Inflammation. 2020;43(4):1498–1509. doi:10.1007/s10753-020-01227-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
