Back to Journals » Journal of Inflammation Research » Volume 17
The Apelin/APJ System: A Potential Therapeutic Target for Sepsis
Authors Song Q, Wang X, Cao Z, Xin C, Zhang J, Li S
Received 20 August 2023
Accepted for publication 1 January 2024
Published 17 January 2024 Volume 2024:17 Pages 313—330
DOI https://doi.org/10.2147/JIR.S436169
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Qing Song, Xi Wang,* Zhenhuan Cao,* Chun Xin,* Jingyuan Zhang,* Suwei Li
Intensive Care Unit, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, 116000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Suwei Li, Tel +86 180 9887 5867, Email [email protected]
Abstract: Apelin is the native ligand for the G protein-coupled receptor APJ. Numerous studies have demonstrated that the Apelin/APJ system has positive inotropic, anti-inflammatory, and anti-apoptotic effects and regulates fluid homeostasis. The Apelin/APJ system has been demonstrated to play a protective role in sepsis and may serve as a promising therapeutic target for the treatment of sepsis. Better understanding of the mechanisms of the effects of the Apelin/APJ system will aid in the development of novel drugs for the treatment of sepsis. In this review, we provide a brief overview of the physiological role of the Apelin/APJ system and its role in sepsis.
Keywords: apelin, sepsis, organ dysfunction, hemodynamics, fluid homeostasis
Introduction
Sepsis is a condition in which the body’s response to infection becomes uncontrolled. Sepsis can lead to life-threatening organ dysfunction and is one of the main causes of death for patients in the intensive care unit.1,2 Fluid resuscitation and vasopressors are the cornerstones for sepsis treatment. The major causes for circulatory failure and failed interventions for septic shock are microvascular leakage, irresponsiveness of arteries to vasopressors and myocardial injury. Despite the implementation of organ support measures like fluid resuscitation, vasoactive drugs, inotropic agents, mechanical ventilation and hemodialysis, the overall prognosis of sepsis has not shown improvement.3 An effective strategy for the prevention and treatment of sepsis is lacking.
 |
Figure 3 Changes of Apelin in serum of patients with sepsis. (A) Changes in serum Apelin in patients with septic myocardial injury. ©2022. Spandidos Publications. Reprinted from Luo Q, Liu G, Chen G, et al. Apelin protects against sepsis‑induced cardiomyopathy by inhibiting the TLR4 and NLRP3 signaling pathways. IntJ Mol Med. 2018;42(2):1161–1167.43 (B) Changes in serum Apelin in septic ARDS patients and the relationship between Apelin levels and prognosis. Copyright ©2022. Dove Medical Press. Reprinted from Yuan Y, Wang W, Zhang Y, et al. Apelin-13 attenuates lipopolysaccharide-induced inflammatory responses and acute lung injury by regulating PFKFB3-driven glycolysis induced by NOX4-dependent ROS. J Inflamm Res. 2022;15:2121–2139.44 (C) Changes in Apelin and ELA in the serum of septic patients, whose stability is reduced in the septic environment. Reprinted from Coquerel D, Lamoureux J, Chagnon F, et al. Apelin-13 in septic shock: effective in supporting hemodynamics in sheep but compromised by enzymatic breakdown in patients. Sci Rep. 2021;11(1):22770.46 *P<0.05, **P<0.01, ****P<0.0001. |
 |
Figure 4 The protective effect and mechanism of apelin on cardiac function. (A) Apelin increases MAP, CO, cardiac contractility, and improves hemodynamics in sheep with septic shock. Reprinted from Coquerel D, Lamoureux J, Chagnon F, et al. Apelin-13 in septic shock: effective in supporting hemodynamics in sheep but compromised by enzymatic breakdown in patients. Sci Rep. 2021;11(1):22770.46 (B) Apelin attenuates myocardial hydrolysis and neutralizes apoptosis in septic rats. ©2022. Spandidos Publications. Reprinted from Luo Q, Liu G, Chen G, et al. Apelin protects against sepsis‑induced cardiomyopathy by inhibiting the TLR4 and NLRP3 signaling pathways. IntJ Mol Med. 2018;42(2):1161–1167.43 (C) Apelin improves pyroptosis-related protein expression. (D) Potential mechanism of apelin protecting myocardial injury in sepsis.*P<0.05, ***P<0.001 compared with control; ###P<0.001 compared with CLP group (Upward black/red arrows indicate enhancement, downward black arrows indicate decrease, the horizontal red line indicates inhibition). |
 |
Figure 5 Apelin protects against septic lung injury. (A) Apelin reduces lung inflammation and edema, lung sections from various groups were examined at both 200x and 400x original magnifications, revealing intramural neutrophils within the alveolar walls, indicated by arrows in H&E-stained images. (B) Apelin reduces the expression of inflammatory factors in the lungs. (C) Apelin reduces the expression of NOX4 and alleviates oxidative stress, thereby reducing the activation of fructose-2,6-bisphosphate kinase 3. Copyright ©2022. Dove Medical Press. The above all figures are reprinted from Yuan Y, Wang W, Zhang Y, et al. Apelin-13 attenuates lipopolysaccharide-induced inflammatory responses and acute lung injury by regulating PFKFB3-driven glycolysis induced by NOX4-dependent ROS. J Inflamm Res. 2022;15:2121–2139.44 (D) Apelin protects the lungs by reducing inflammation, oxidative stress, and fibrosis. *P<0.05 compared with control; #P<0.05 compared with LPS group. (Upward red arrows indicate increase, downward black/red arrows indicate decrease, the horizontal red line indicates inhibition). |
 |
Figure 6 Apelin reduces septic liver damage. (A) Apelin reduces the levels of AST and IL-6 in the serum of mice with septic shock. (B) Apelin improves hepatic edema and macrophage infiltration in septic shock mice, reducing the expression of inflammatory factors.(C) Apelin alleviates hepatocyte apoptosis in septic shock mice and LPS-induced apoptosis in Huh-7 cells; TUNEL staining is shown in green, and DAPI staining is shown in blue. The above all figures are reprinted from Zhou H, Yang R, Wang W, et al. Fc-apelin fusion protein attenuates lipopolysaccharide-induced liver injury in mice. Sci Rep. 2018;8(1):11428.106 (D) Mechanism diagram illustrating the protective effects of Apelin on septic liver injury. *P<0.05, **P<0.01, ***P<0.001, n.s, no significant difference (Upward red arrows indicate increase, downward black arrows indicate decrease, the horizontal red line indicates inhibition). |
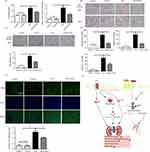 |
Figure 7 The role of ELA in alleviating AKI and regulating body fluid homeostasis. (A) ELA increases creatinine clearance and rescues kidney function in mice with septic shock. (B–D) ELA reduced the expression of renal inflammatory factors and the infiltration of macrophages, and reduced renal edema (Red arrows indicate vacuolation and yellow arrows indicate nuclear pyknosis in the LPS group). (E) ELA alleviates tubular cell apoptosis in septic shock mice; green represents TUNEL staining, and blue represents DAPI staining. The above all figures are reprinted from Xu F, Zhou H, Wu M, et al. Fc-Elabela fusion protein attenuates lipopolysaccharide-induced kidney injury in mice. Biosci Rep. 2020;40(9).114 (F) The protective mechanism of ELA on the kidneys and the regulating mechanism of body fluids.*P<0.05, ***P<0.001, n.s, no significant difference (Upward black/red arrows indicate increase, downward black arrows indicate decrease, The horizontal red line and the vertically downward red line indicate inhibition). |
APJ is a G protein-coupled receptor (GPCR) that is structurally similar to the type 1 receptor of angiotensin II.4 The first identified endogenous ligand for APJ is Apelin, which was extracted from bovine stomach.5 In 2013, the second ligand, Elabela (ELA), was discovered.6 In rodents and human, the Apelin/APJ system is widely expressed in many organs and tissues, including the heart, kidney, brain, lung, stomach, blood vessels, spinal cord, endothelium, and adipose tissue. Due to level of Apelin in plasma being lower than that in tissues,7,8 and APJ and Apelin expressed in similar locations, Apelin may not be a major circulating hormone, and its secretion mechanism is probable paracrine or autocrine. The main subtypes of Apelin are Apelin-36, Apelin-17, and Apelin-13.9 Apelin-13 has the highest biological activity among all subtypes with a variety of effects and is the dominant type in the human circulatory system.10 The major subtypes of ELA are ELA-32, ELA-21, and ELA-11.11 ELA plays an important role in heart development. In zebrafish lacking ELA, embryos died early because of weakness or absence of heart, and addition of ELA reversed these abnormalities.6 Mice lacking ELA exhibited abnormal heart development and embryonic death.12 ELA is present in human plasma, but the expression of ELA in human tissues is not fully recognized.13 The Apelin /APJ system is involved in regulating physiological processes such as myocardial contractility and vascular tone, body fluid homeostasis, renal function, the inflammatory response and energy metabolism.
The extensive physiological effects of the Apelin/APJ system are closely related to many diseases such as cancer, heart failure, hypertension, atherosclerosis, diabetes, and neurological diseases. Numerous trials have demonstrated the beneficial impact of the Apelin/APJ system on sepsis, especially in improving hemodynamic disturbances and regulating fluid balance, indicating the Apelin/APJ system may represent a novel therapeutic target for sepsis. Research on the beneficial role of the Apelin/APJ system in various disorders has led to the development of various analogs of Apelin and ELA. Here we review the recent literature on the roles and mechanisms of the Apelin/APJ system in relation to its protective effects against sepsis. We also discuss the agonists and antagonists of APJ and their potential in sepsis treatment.
Physiological Roles of the Apelin/APJ System in the Cardiovascular System and the Kidney
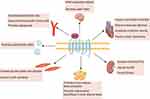
The Apelin/APJ system is involved in many physiological processes (Figure 1). In this review, we focus our discussion on its functions in cardiovascular homeostasis and the kidney. These effects, including regulatory effects on vascular tone, myocardial contractility, anticoagulation and humoral balance, may represent potential therapeutic value for sepsis.
The regulation of vascular tone by the Apelin/APJ system is determined by effector cells. In endothelial cells, the Apelin/APJ system regulates vasodilation through the NO/L-arginine system, which is activated by NOS phosphorylation in a rapid, transient, and dose-dependent manner.14,15 In vascular smooth muscle cells (VSMCs), Apelin functions in vasoconstriction through the phosphorylation of myosin light chain (MLC), also in a dose-dependent manner.16 ELA also promotes vasodilation but via a different mechanism that does not involve NO and only partially involves endothelial cells.17,18
The Apelin/APJ system is essential for normal vascular development. Apelin knockout mouse embryos exhibited vascular stenosis and impaired retinal angiogenesis.19,20 Hypoxia is an inducer of Apelin expression, and the hypoxia-inducible factor 1α (HIF1α) transcription factor promotes Apelin gene transcription.21,22 Additionally, the hypoxia-induced proliferation of endothelial cells can be prevented by inhibition of the Apelin signaling pathway.21 These findings indicate that the Apelin/APJ system may be a regulator of both the normal developmental and pathophysiological processes of blood vessels.
Apelin can improve myocardial contractility.23 The Apelin peptide has been shown to promote inotropic effects in humans at subnanomolar concentrations. In clinical studies of healthy volunteers, an intracoronary bolus injection of Apelin-36 increased cardiac contractility and injection of Apelin-36 and [Pyr1]-Apelin-13 in the antecubital vein had the same effect.24–26 Exogenous infusion of Apelin restored myocardial contractility in Apelin knockout mice and increased myocardial shortening in healthy rats.23,27 These effects were observed in isolated perfused rat cardiac myocytes in a dose-dependent manner.23,28 [Pyr1]-Apelin-13 increased cardiac output without left ventricular hypertrophy, unlike other positive inotropes.29 Furthermore, the Apelin/APJ system exhibited an antagonistic effect on the renin-angiotensin system,30 and deficiency of the Apelin gene in mice exacerbated Ang II–induced cardiac dysfunction.31
The Apelin/APJ system is expressed in human platelets and exhibits anticoagulant effects in vitro.32,33 Animal experiments have demonstrated anti-thrombotic effects of Apelin in vivo. Apelin gene–deficient mice showed a shortened bleeding time, increased platelet aggregation, and rapid formation of small vein thrombosis. Apelin-13 infusion prolonged bleeding time in both Apelin gene–deficient mice and wild-type mice.33 Furthermore, Apelin inhibited thrombin and collagen–induced thrombocyte activation, but not ADP and TXA2–induced thrombocyte activation.33 The antithrombotic properties of Apelin have not yet been validated in humans.
In rats, Apelin induces relaxation of renal afferent and efferent arterioles pretreated with Ang II and reduces the intracellular Ca2+ level, which is dependent on the integrity of the arteriolar endothelial cells and NO.34 Furthermore, the vasodilator effect of Apelin increases renal medullary blood flow, which is beneficial for diuresis. In rodents, Apelin directly inhibits the insertion of aquaporin 2 into the apical plasma membrane of the collecting duct, thereby promoting water excretion.34,35 Animal studies have shown that the Apelin/APJ system can improve kidney disease by preventing renal fibrosis, ischemia-reperfusion injury, and renal vascular calcification.36–39
The Involvement of the Apelin/APJ System in Septic Conditions
Apelin is an inotropic agent with anti-inflammatory effects and calcium sensitization and antioxidant properties. Studies in preclinical models of sepsis have indicated excellent protective effects of the Apelin/APJ system against sepsis. Here, we outline the potential therapeutic values of Apelin in sepsis, focusing on its protective effects on cardiovascular homeostasis (Figure 2).
Role of Plasma Apelin in Sepsis Diagnosis and Prognostic Prediction
The common biomarkers of sepsis are C-reactive protein (CRP) and procalcitonin (PCT). Both are significantly elevated in sepsis.40 Various studies have demonstrated changes in Apelin in sepsis and septic shock, highlighting its potential diagnostic and prognostic role.
Several studies have used enzyme-linked immunosorbent assay to determine the serum level of Apelin in sepsis patients. Safaa et al41 studied plasma Apelin content in 80 neonates and found that the average serum value of Apelin in septic neonates (1214.7 ± 273.06 pg/mmol) was significantly higher than that in healthy neonates(116.27 ± 21.96 pg/mmol). Furthermore, neonatal survivors of early-onset sepsis had lower Apelin levels than non-survivors. Another study noted a substantial increase (eight-fold) in plasma Apelin levels among neonates with sepsis compared with healthy neonates.42 Luo et al43 measured Apelin levels in the serum of 73 adults, including 40 patients with septic myocardial injury and 33 healthy volunteers (Figure 3A). The serum Apelin levels in the septic group were significantly higher than those in healthy volunteers. Yuan et al44 reported that serum Apelin levels were higher in 34 septic patients (including 9 sepsis-related ARDS patients) compared with 13 healthy volunteers. Furthermore, patients with mild ARDS had lower Apelin levels than patients with severe ARDS, and survivors had higher Apelin levels than non-survivors (Figure 3B). Liu et al45 also confirmed significantly higher serum Apelin levels in 28 patients with sepsis-related ARDS compared with 20 healthy volunteers and a higher level in survivors compared with non-survivors.
Several studies have shown elevated Apelin levels in early septic patients, indicating that it may play a protective role.43,44 Clinical research has revealed that sepsis patients have higher blood Apelin content compared with healthy individuals. It’s noteworthy that David et al verified elevated levels of both Apelin and ELA in patients with sepsis46 (Figure 3C). Moreover, both Apelin and ELA are more prone to degradation in the septic environment.46 However, Apelin levels were markedly higher in septic shock patients compared with sepsis patients.47,48 The degree of increase was positively correlated with the severity of sepsis.41,42 Elderly survivors of sepsis also had lower plasma Apelin levels than non-survivors.49
This is stating that the mechanisms underlying these changes are unknown and need further elucidation. The amount of time it takes for the level of Apelin to increase after infection is unclear and needs further research. Overall, these studies suggest that Apelin may show utility in the diagnosis and prognostic prediction of sepsis (Table 1).
 |
Table 1 Summary of Serum Apelin Levels in Patients with Sepsis |
Protective Effects of Apelin on the Brain
Sepsis-associated encephalopathy (SAE) is a diffuse brain dysfunction that is secondary to sepsis and not caused by infection of the central nervous system.50 The pathophysiology of SAE is multifactorial, involving hemorrhage, blood-brain barrier (BBB) damage, changes in neuronal synaptic density, neurotransmitter dysregulation, neuroinflammation and ischemic injury.51,52
The Apelin/APJ system is a potential therapeutic target for neurological diseases and has been suggested to be protective against several neurological disorders.53 Intracerebroventricular injection of LPS is one of the methods used to construct a model of SAE.54 Therefore, examining the mechanisms of Apelin in LPS-induced neuroinflammation may provide insights into its effects on neuroprotection in SAE. In cultured mouse N9 microglia, Apelin-13 reduced the expression of proinflammatory cytokines IL-6 and iNOS and increased the expression of anti-inflammatory cytokines IL-10 and Arg-1 by inhibiting N9 microglial activation, thereby attenuating LPS-induced neuroinflammation.55 LPS has also been reported to be an activator of the NF-κB pathway in neuroinflammation. Apelin was shown to inhibit the NF-κB pathway, thereby reducing neuroinflammation in rats.56 In septic rats, Apelin-13 promoted the expression and nuclear translocation of the glucocorticoid receptor (GR) to ameliorate LPS-induced neuroinflammation and cognitive dysfunction in rats.57 There is also considerable evidence that LPS disrupts the BBB, causing SAE.58 Notably, Apelin has been proven to improve brain dysfunction by reducing the permeability of the BBB in rats.59 This shows that Apelin may alleviate SAE by improving the permeability of the BBB.
The above studies suggest that Apelin-13 may reduce LPS-induced neuroinflammation and cognitive impairment. Whether Apelin improves SAE still needs further investigation, especially in septic models.
Cardioprotective Effects of Apelin
During sepsis, the heart is one of the organs that are vulnerable to damage.60 Cardiac dysfunction is a frequent comorbidity in patients with sepsis and is linked to elevated mortality.61,62 Sepsis-induced myocardial dysfunction (SIMD) is a reversible cardiac dysfunction typically characterized by reduced myocardial contractility and ventricular dilation and is usually treated with positive inotropic drugs.63 Blockage of APJ further exacerbated cardiac dysfunction and mortality in septic rats,64 and down-regulation of cardiac Apelin expression was observed in non-surviving septic rats.65 These results demonstrated that the Apelin/APJ system may be involved in the amelioration of life-threatening septic cardiac dysfunction.
Currently, β-adrenoceptor agonists are recommended to improve cardiac dysfunction in sepsis, and the Surviving Sepsis Campaign guidelines recommend the use of dobutamine as the first-line cardiac medication.66 However, β-adrenoceptor agonists increase myocardial oxygen consumption and increase the incidence of atrial fibrillation.67 Furthermore, β-adrenergic receptor sensitivity to catecholamines decreases during sepsis, rendering them relatively ineffective.68 In this case, using even large amounts of exogenous β-adrenoceptor agonists (including dobutamine) to enhance myocardial contractility will not yield benefits but instead lead to worse outcomes. Thus, effective strategies to treat cardiac dysfunction in sepsis are required. Apelin is considered a potential candidate for cardiac dysfunction treatment in sepsis, and animal and human experiments have confirmed its positive inotropic effect. Crucially, although sepsis reduces the myocardial sensitivity to β-adrenergic receptor agonists, the effectiveness of Apelin in cardiac response is heightened during systemic inflammatory conditions or polymicrobial infection.64,69 Apelin’s inotropic and vasodilatory effects may provide significant therapeutic effects for low-output septic shock (ie, low output and high resistance).
Several studies have demonstrated positive inotropic effects of Apelin peptides in various animal models of heart failure.70–74 Systemic infusion of [Pyr1]-Apelin-13 in heart failure patients resulted in a decrease in blood pressure and systemic vascular resistance and an increase in cardiac index of approximately 10%.26 Cardiac output improved with increasing infusion time, with an ejection fraction increase of about 10%. Walley et al had proposed Apelin as a new treatment to improve SIMD.75 Some studies showed that the delivery of Apelin in septic rodents was effective in attenuating myocardial injury and improving cardiac function.43,46,76–78 Rat cardiomyocytes co-cultured with various inflammatory factors exhibited decreased contractility.79 Apelin has been shown to enhance myocardial contractility by reducing the production of inflammatory factors in rats43,80 (Figure 4C and D). One study reported that mild apoptosis of cardiomyocytes can cause severe structural and functional damage to the heart, leading to cardiac dysfunction.81 In addition to increasing myocardial contractility by reducing inflammatory factors, Apelin also reduces cardiomyocyte apoptosis in septic rats, thereby improving cardiac function43,64 (Figure 4B). The Apelin/ELA-APJ system showed a positive effect on hemodynamic stability in animal models of sepsis46,69,77 (Figure 4A). Apelin and ELA also significantly improved the left ventricular pressure-volume (P-V) relationship and reduced arterial elasticity (Ea) in experimental animal models of septic shock, which is beneficial for improving ventricular-arterial decoupling.69 Notably, in a rat model of sepsis, Apelin-13 showed superior performance compared with dobutamine (inotropes commonly used in sepsis), with higher responsiveness, significantly improved left ventricular function, and higher survival rate; dobutamine was associated with further myocardial damage and less responsiveness.64 These studies indicate that targeting the Apelin/APJ system may be a promising strategy to treat cardiac dysfunction in sepsis.
Protective Effect of Apelin on Blood Vessels
During sepsis and septic shock, endothelial cells undergo severe damage and the vascular integrity and tone are disrupted,82 which causes and enhance vascular leakage. Therefore, maintenance of the vascular barrier is critical to improve the prognosis of sepsis patients.83–85
Damage to the major component vascular endothelial calmodulin (VE-Cad) may disrupt adherens junctions (AJ) and lead to loss of the endothelial barrier.86,87 LPS decreases VE-Cad expression in pulmonary vessels and increases its phosphorylation, which leads to an increase in vascular permeability.88 One study showed that the increase of ROS promotes the phosphorylation of VE-Cad.89 Another report in mice demonstrated that Apelin-13 restored the expression of VE-Cad and reduced the phosphorylation of VE-Cad, thereby reducing LPS-induced pulmonary vascular leakage.90 Apelin activates the AMPK pathway to promote mitochondrial biogenesis, reduce ROS production and inhibit VE-Cad phosphorylation, thereby reducing pulmonary vascular leakage. In addition to reducing VE-Cad phosphorylation, Apelin has been reported to reduce NF-κB p65 entry into the nucleus in cultured human umbilical vein endothelial cells (HUVECs), thereby attenuating LPS-induced permeability.91 Additionally, Apelin and ELA were recently reported to reduce vascular endothelial growth factor (VEGF) and inflammatory factor production, thereby reducing sepsis-induced increased vascular permeability in rats.69 Notably, the effect of Apelin/ELA-system to relieve vascular leakage further contributes to the maintenance of plasma volume and hemodynamic stability.69
Protective Effect of Apelin on the Lungs
The large amounts of inflammatory factors released in sepsis damage the pulmonary capillary endothelial cells and type II alveolar epithelial cells, inducing pulmonary edema and alveolar atrophy. This cascade of events ultimately leads to ALI/ARDS. The main manifestations are increased pulmonary vascular permeability, hypoxemia, and respiratory distress.92 LPS-induced ALI/ARDS and pulmonary fibrosis mimic sepsis-related pulmonary ALI and pulmonary fibrosis.93
Exogenous Apelin-13 was reported to attenuate the inflammatory response by reducing NF-κB and NLRP3 inflammasome, thereby improving ALI in mice94 (Figure 5D). Apelin-13 suppressed pulmonary inflammatory responses by inhibiting the upregulation of NADPH oxidase 4 (NOX4) and reducing ROS production and ROS-activated fructose-2,6-bisphosphate kinase 3-mediated glycolysis, thereby attenuating ALI in septic mice44 (Figure 5A–C). Previous studies have shown that endothelial-mesenchymal transition (EndMT) is a common disease-causing mechanism leading to lung fibrosis and inhibition of EndMT may be beneficial in the alleviation of lung fibrosis.95 The Apelin/APJ system can alleviate the fibrotic process in multiple organs; for example, Apelin/APJ reduces myocardial fibrosis by inhibiting the activation of cardiac fibroblasts and blocking TGF-β signaling to reduce fibrosis in the renal interstitium.96,97 Studies have shown that Apelin may be able to alleviate sepsis-induced lung fibrosis through inhibition of the TGF-β/Smad signaling pathway in mice.45 In recent times, there has been significant attention on angiotensin-converting enzyme 2 (ACE2) as the cellular receptor for the causative virus of the SARS-CoV-2 coronavirus disease pandemic.98 ACE2 competes with angiotensin-converting enzyme 2 in the conversion of Angiotensin II to Angiotensin-(1–7), leading to anti-inflammatory, anti-vasoconstrictive, and anti-fibrotic effects,99,100 (ACE2) can effectively alleviate ALI and pulmonary fibrosis.101 Apelin was shown to promote ACE2 expression, thereby reducing endothelial-mesenchymal transition and preventing sepsis-associated pulmonary fibrosis in mice.93 Additionally, inhibition of APJ exacerbated pulmonary fibrosis. This is further evidence of the protective role of the Apelin/APJ system against organ damage in sepsis. Together, these investigations indicate that activation of the Apelin/APJ system may have a protective effect against septic ALI and pulmonary fibrosis (Figure 5D).
Protective Effects of Apelin in the Liver
Inflammation and oxidative stress are the main mechanisms leading to liver injury in sepsis.102 In animal models of sepsis, liver injury can be attenuated by inhibiting the inflammatory response and scavenging ROS.103 Several studies have demonstrated the anti-inflammatory and antioxidant effects of Apelin.104,105 Zhou et al106 explored the protective effects of a novel long-acting Fc-Apelin fusion protein on LPS-induced liver injury in septic mice (Figure 6). The authors found that Fc-Apelin significantly reduced the level of alanine aminotransferase (ALT) in septic mice (Figure 6A) and reduced hepatic cell apoptosis and ROS generation (Figure 6C). Additionally, Fc-Apelin alleviated the infiltration of hepatic macrophages and decreased the expressions of IL-6 and TNF-α in the liver (Figure 6B). However, systemic IL-6 was not significantly reduced as in the liver, and the reasons still need clarification. Thus, Apelin has great potential in the treatment of liver injury caused by sepsis, although the mechanisms remain to be elucidated.
Protective Effect of Apelin on the Kidney
Acute kidney injury (AKI) is very common in sepsis and is often associated with more severe hemodynamic disturbance, cardio-renal syndrome, and an increased need for mechanical ventilation and vasopressors.107–109 AKI in sepsis is characterized by a sudden decline in renal function, indicated by increased serum creatinine and oliguria.110,111
Apelin-13 improved renal function in sheep with septic shock in a dose-dependent manner, restoring the kidney’s ability to excrete urine and creatinine.46 Apelin and ELA were both shown to exert protective effects in multiple kidney disease models,36 and the effects of ELA may be superior.112 In a septic rat model, both Apelin-13 and ELA improved water intake and urine output. However, ELA significantly reduced AKI and renal inflammation, whereas Apelin-13 did not appear to be effective in preventing renal injury.69 Apelin-13 also improved AKI in septic rats by reducing renal artery resistance and increasing creatinine clearance through countering the renin-angiotensin system (RAS).113 ELA was fused with the Fc domain of human immunoglobulin IgG to generate Fc-ELA-21. Fc-ELA-21 was examined for treatment of LPS-induced AKI in septic mice and showed a longer half-life compared with ELA (Figure 7); it reduced renal tubular apoptosis, macrophage infiltration and inflammatory cytokine expression and improved AKI.114
Body Fluid Homeostasis Regulation by Apelin
The Apelin/APJ system and AVP co-localize in hypothalamic supraoptic and paraventricular nuclei and exhibit a reciprocal regulatory relationship.115,116 These findings are in line with clinical research results in healthy individuals, showing plasma osmolality changes paralleling reciprocal vasopressin and Apelin changes. Blood AVP level increases and Apelin level decreases under hypertonic saline stimulation. Conversely, blood AVP levels decrease and Apelin levels increase after water loading reduces osmotic pressure.117 The cross-modulation of osmotic stimulation by Apelin and AVP has important physiological significance; it can prevent renal water excretion after dehydration and promote water excretion after water load to maintain body fluid homeostasis. This has very important clinical implications for the management of fluid resuscitation in patients with septic shock.
Disordered fluid homeostasis is common in septic progression and the resuscitation process.118 Multiple studies have shown that the Apelin/APJ system may be involved in maintaining fluid homeostasis.34,115,119–121 An increase in water intake and urine output in septic rats in response to both Apelin-13 and ELA was observed. ELA prevents plasma volume loss without altering AVP level, whereas Apelin-13 reduces plasma AVP level and changes urine balance toward unwanted aquaresis, which results in a loss of plasma volume69 (Figure 7F). Apelin-13 and ELA reduced vascular permeability in several major organs, which also played a role in maintaining fluid homeostasis.69 These findings suggest that ELA and Apelin-13 have contrasting effects on the cardio-renal axis primarily through counter-regulation in the vasopressinergic system. Additionally, Apelin prevents the reduction of plasma volume by regulating fluid homeostasis, which facilitates the amelioration of hemodynamic disturbances.69 These effects further support its potential as a therapeutic target for sepsis.
Summary of the Protective Effects of the Apelin/APJ System Against Sepsis
Much effort has been invested into exploring the protective mechanism of the Apelin/APJ system in sepsis, and multiple mechanisms have been implicated in its protective effects, including reducing inflammatory factors and ROS, enhancing myocardial contractility, and reducing vascular permeability (Table 2). It is important to mention that most studies have been conducted in animal models and human studies are rare. The protective effect of ELA on sepsis has also been reported. Apelin levels in the plasma of septic patients have been reported to be higher than those of healthy individuals and to predict prognostic outcome. Some evidence also shows that Apelin can increase ACE2 expression and inhibit TGF-β/Smad signaling to reduce sepsis-associated pulmonary fibrosis. Notably, Apelin can alleviate LPS-induced neuroinflammation, which provides a valuable basis for further study of Apelin in the treatment of SAE. ELA has also been proven to attenuate septic kidney injury. Together these findings indicate that the Apelin-ELA/APJ system may be a potential therapeutic target in sepsis.
 |
Table 2 Summary of the Animal Experiments |
Agonists and Antagonists That Target the Apelin/APJ System
In spite of the close association of the Apelin/APJ system with several physiological processes and diseases, no drugs have been identified that directly activate or inhibit APJ. Because the half-life of Apelin in the body is several minutes,122 the bioavailability is low. Therefore, the identification of peptide analogs and small molecules with high biological activity and long half-lives is very important. Additionally, from a clinical perspective, ideal agonists and antagonists should not only be degradation resistant but also should be biased towards G protein signaling to avoid receptor desensitization caused by activation of the β-arrestin signaling pathway.
To prolong the half-life of Apelin peptides and increase their biological activities, extensive research has been performed and several Apelin analogues have been developed. M007 is a cyclic peptidomimetic with a longer half-life than endogenous peptides such as Apelin-13, −36 and −17. Compared with [Pyr 1]-Apelin-13, the effects of M007 in enhancing myocardial contractility, dilating blood vessels and lowering blood pressure in rats are stronger.123 M007 also significantly increased cardiac output in human volunteers.123 A longer half-life is observed for another Apelin mimetic peptide that binds anti-serum albumin domain antibodies in rats.124 In vivo, it lowers blood pressure and increases myocardial contractility, stroke volume, and cardiac output. Encapsulating [Pyr1]-Apelin-13 in lipid nanocarriers significantly prolonged the half-life, and the protective effect of the encapsulated form on ischemia and perfusion injury is better than that of non-encapsulated [Pyr1]-Apelin-13 in rats.125 E339-3D6 is the first reported non-peptide APJ agonist that induces the production of intracellular cAMP and the internalization of APJ.126 Furthermore, E339-3D6 induces vasodilation in isolated rat aortas. However, the relatively large molecular weight of E339-3D6 and the difficulty of its synthesis and isolation make it difficult to apply to clinical practice.126 A new small-molecule agonist ML233 was later developed using high-throughput screening technology, but its stability in human plasma was shown to be very poor and it exhibited hepatotoxicity.127,128 Another non-peptide APJ agonist, CMF-019, was developed with a high affinity for APJ and biased activation of the G protein signaling pathway; it has been proven to enhance myocardial contractility in rats.129 AMG-986 and BMS-986224, two oral small molecule agonists, have also been shown to increase cardiac output in rats.130–132 Substantial evidence suggests that these two small molecule agonists have high therapeutic potential in the treatment of a variety of heart diseases.132–134 In a recent clinical trial, AMG-986 demonstrated good tolerability in healthy individuals but it had no pharmacodynamic effects in patients with HF.134
ALX40-4C is the first reported peptidic antagonist of APJ. In an in vitro study, ALX40-4C was shown to inhibit APJ-mediated membrane fusion and intracellular Ca2+ elevation in a dose-dependent manner.135 The F13A peptide antagonist of APJ was obtained by mutating the C-terminal phenylalanine of Apelin-13 to alanine; F13A inhibited many Apelin-13-induced physiological effects in animal models.136 ML221 is the first non-peptide APJ antagonist reported to prevent pathologic retinal angiogenesis in ischemic retinopathy in mice and is expected to be a drug candidate for the treatment of ischemic retinopathy.137 With further in-depth study of the Apelin/APJ system, more APJ agonists and antagonists will be developed. APJ agonists and antagonists are safe and well-targeted, and thus drugs that target Apelin/APJ system will have clinical value. The feasibility of Apelin as a new drug for chronic HF was indicated by the results of the first human trial. Most APJ agonists and antagonists are currently still in preclinical research and further research will be required to explore their utility in the treatment of sepsis.
Potential for Improvements of APJ Agonists for Sepsis
Over the past 20 years, the physiological structures of Apelin and APJ have been revealed and APJ agonists have been developed. Typically, GPCR activation activates both G protein–dependent and –independent signaling pathways, regardless of the structure of the ligand. However, some ligands selectively activate or inhibit certain signaling pathways, resulting in biased signaling.138 In this case, the strategic design of drugs may help ensure specific and desired results with minimized side effects. A biased APJ agonist MM07 was previously developed for application in heart failure. This agonist selectively activates the G protein pathway and avoids activation of the β-arrestin pathway.123
Studies have indicated that APJ receptor signaling involves activation of Gαi, triggering adenylyl cyclase inhibition, resulting in cAMP inhibition and subsequent physiological effects.139,140 APJ also binds other G protein trimers, especially Gq, thereby activating the phospholipase C (PLC) and AMP-activated protein kinase (AMPK) signaling pathways.23,141 Several endogenous APJ ligands (such as Apelin-17, Apelin-36, ELA-32, and ELA-21) have been shown to produce a certain bias in the conduction of β-arrestin signaling.13,25 Overexpression of β-arrestin exacerbates immunosuppression, cardiac dysfunction, and mortality in septic mice.142 The development of APJ receptor agonists that preferentially activate the G protein signaling pathway is crucial for the targeted treatment of sepsis, and these agonists may show reduced adverse effects.
Summary
Sepsis is a global public health problem and has a high mortality rate. Apelin has shown promising outcomes in pre-clinical studies of sepsis and heart failure clinical research. In this review, we presented a brief overview of the functions of the Apelin/APJ system and discussed the potential of Apelin and ELA to treat sepsis. The Apelin/APJ system may be a promising new target for the treatment of sepsis, some candidate agonists and antagonists have been developed, with promising preclinical research results. Apelin has been proven to have inotropic and vasodilatory effects in healthy individuals and patients with chronic heart failure; however, whether Apelin exerts these effects in septic patients remains unclear. Studies on the treatment of sepsis with Apelin and ELA have been pursued in animal models and cell lines; clinical research results are currently lacking. Thus, further studies are required to determine the applicability of Apelin as a potential drug for the treatment of sepsis.
Acknowledgment
L-SW would like to acknowledge the support from the Dalian City Dengfeng Program (DF2022043).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–386. doi:10.1016/s2213-2600(14)70061-x
2. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
3. Liu R, Luo X, Li J, et al. Melatonin: a window into the organ-protective effects of sepsis. Biomed Pharmacother. 2022;154:113556. doi:10.1016/j.biopha.2022.113556
4. O’Dowd BF, Heiber M, Chan A, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136(1–2):355–360. doi:10.1016/0378-1119(93)90495-o
5. Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–476. doi:10.1006/bbrc.1998.9489
6. Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell. 2013;27(6):672–680. doi:10.1016/j.devcel.2013.11.002
7. Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension. 2009;54(3):598–604. doi:10.1161/hypertensionaha.109.134619
8. Zhen EY, Higgs RE, Gutierrez JA. Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal Biochem. 2013;442(1):1–9. doi:10.1016/j.ab.2013.07.006
9. Read C, Nyimanu D, Williams TL, et al. International union of basic and clinical pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that elabela/toddler is a second endogenous peptide ligand. Pharmacol Rev. 2019;71(4):467–502. doi:10.1124/pr.119.017533
10. Cheng J, Luo X, Huang Z, Chen L. Apelin/APJ system: a potential therapeutic target for endothelial dysfunction-related diseases. J Cell Physiol. 2019;234(8):12149–12160. doi:10.1002/jcp.27942
11. Zhang Y, Wang Y, Lou Y, et al. Elabela, a newly discovered APJ ligand: similarities and differences with apelin. Peptides. 2018;109:23–32. doi:10.1016/j.peptides.2018.09.006
12. Freyer L, Hsu CW, Nowotschin S, et al. Loss of apela peptide in mice causes low penetrance embryonic lethality and defects in early mesodermal derivatives. Cell Rep. 2017;20(9):2116–2130. doi:10.1016/j.celrep.2017.08.014
13. Yang P, Read C, Kuc RE, et al. Elabela/toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation. 2017;135(12):1160–1173. doi:10.1161/circulationaha.116.023218
14. El Messari S, Iturrioz X, Fassot C, De Mota N, Roesch D, Llorens-Cortes C. Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. J Neurochem. 2004;90(6):1290–1301. doi:10.1111/j.1471-4159.2004.02591.x
15. Ishida J, Hashimoto T, Hashimoto Y, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279(25):26274–26279. doi:10.1074/jbc.M404149200
16. Hashimoto T, Kihara M, Ishida J, et al. Apelin stimulates myosin light chain phosphorylation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006;26(6):1267–1272. doi:10.1161/01.Atv.0000218841.39828.91
17. Wang Z, Yu D, Wang M, et al. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci Rep. 2015;5:8170. doi:10.1038/srep08170
18. Perjés Á, Kilpiö T, Ulvila J, et al. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic Res Cardiol. 2016;111(1):2. doi:10.1007/s00395-015-0521-6
19. Kidoya H, Ueno M, Yamada Y, et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27(3):522–534. doi:10.1038/sj.emboj.7601982
20. Kasai A, Shintani N, Kato H, et al. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28(10):1717–1722. doi:10.1161/atvbaha.108.163402
21. Eyries M, Siegfried G, Ciumas M, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103(4):432–440. doi:10.1161/circresaha.108.179333
22. Ronkainen VP, Ronkainen JJ, Hänninen SL, et al. Hypoxia inducible factor regulates the cardiac expression and secretion of apelin. FASEB J. 2007;21(8):1821–1830. doi:10.1096/fj.06-7294com
23. Szokodi I, Tavi P, Földes G, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002;91(5):434–440. doi:10.1161/01.res.0000033522.37861.69
24. Barnes GD, Alam S, Carter G, et al. Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circulation. 2013;6(3):482–491. doi:10.1161/circheartfailure.111.000077
25. Yang P, Kuc RE, Brame AL, et al. [Pyr(1)]apelin-13((1–12)) is a biologically active ACE2 metabolite of the endogenous cardiovascular peptide [Pyr(1)]apelin-13. Front Neurosci. 2017;11:92. doi:10.3389/fnins.2017.00092
26. Japp AG, Cruden NL, Barnes G, et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121(16):1818–1827. doi:10.1161/circulationaha.109.911339
27. Kuba K, Zhang L, Imai Y, et al. Impaired heart contractility in apelin gene-deficient mice associated with aging and pressure overload. Circ Res. 2007;101(4):e32–e42. doi:10.1161/circresaha.107.158659
28. Wang C, Du JF, Wu F, Wang HC. Apelin decreases the SR Ca2+ content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2008;294(6):H2540. doi:10.1152/ajpheart.00046.2008
29. Ashley EA, Powers J, Chen M, et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res. 2005;65(1):73–82. doi:10.1016/j.cardiores.2004.08.018
30. Chatterjee P, Gheblawi M, Wang K, Vu J, Kondaiah P, Oudit GY. Interaction between the apelinergic system and ACE2 in the cardiovascular system: therapeutic implications. Clin Sci. 2020;134(17):2319–2336. doi:10.1042/cs20200479
31. Sato T, Kadowaki A, Suzuki T, et al. Loss of apelin augments angiotensin II-induced cardiac dysfunction and pathological remodeling. Int J Mol Sci. 2019;20(2):239. doi:10.3390/ijms20020239
32. Zhang G, Xiang B, Dong A, et al. Biphasic roles for soluble guanylyl cyclase (sGC) in platelet activation. Blood. 2011;118(13):3670–3679. doi:10.1182/blood-2011-03-341107
33. Adam F, Khatib AM, Lopez JJ, et al. Apelin: an antithrombotic factor that inhibits platelet function. Blood. 2016;127(7):908–920. doi:10.1182/blood-2014-05-578781
34. Hus-Citharel A, Bouby N, Frugière A, Bodineau L, Gasc JM, Llorens-Cortes C. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 2008;74(4):486–494. doi:10.1038/ki.2008.199
35. Hus-Citharel A, Bodineau L, Frugière A, Joubert F, Bouby N, Llorens-Cortes C. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology. 2014;155(11):4483–4493. doi:10.1210/en.2014-1257
36. Huang Z, Wu L, Chen L. Apelin/APJ system: a novel potential therapy target for kidney disease. J Cell Physiol. 2018;233(5):3892–3900. doi:10.1002/jcp.26144
37. Nishida M, Okumura Y, Oka T, et al. The role of apelin on the alleviative effect of angiotensin receptor blocker in unilateral ureteral obstruction-induced renal fibrosis. Nephron extra. 2012;2(1):39–47. doi:10.1159/000337091
38. Bircan B, Çakır M, Kırbağ S, Gül HF. Effect of apelin hormone on renal ischemia/reperfusion induced oxidative damage in rats. Renal Failure. 2016;38(7):1122–1128. doi:10.1080/0886022x.2016.1184957
39. Han X, Wang LY, Diao ZL, Liu WH. Apelin: a novel inhibitor of vascular calcification in chronic kidney disease. Atherosclerosis. 2016;244:1–8. doi:10.1016/j.atherosclerosis.2015.10.102
40. Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50(1):23–36. doi:10.3109/10408363.2013.764490
41. Saen EL, Bagoury I, Mohamed KES. Role of serum apelin in the diagnosis of early-onset neonatal sepsis. Turkish Archives of Pediatrics. 2021;56(6):563–568. doi:10.5152/TurkArchPediatr.2021.21108
42. Gad GI, Ismail RI, El-Masry SA, Gouda HR. Serum apelin in early-onset neonatal sepsis: is it diagnostic? J Neonatal Perinatal Med. 2014;7(3):207–212. doi:10.3233/npm-14814014
43. Luo Q, Liu G, Chen G, et al. Apelin protects against sepsis‑induced cardiomyopathy by inhibiting the TLR4 and NLRP3 signaling pathways. IntJ Mol Med. 2018;42(2):1161–1167. doi:10.3892/ijmm.2018.3665
44. Yuan Y, Wang W, Zhang Y, et al. Apelin-13 attenuates lipopolysaccharide-induced inflammatory responses and acute lung injury by regulating PFKFB3-driven glycolysis induced by NOX4-dependent ROS. J Inflamm Res. 2022;15:2121–2139. doi:10.2147/jir.S348850
45. Liu H, Shi Q, Tang L, Wang H, Wang D. Apelin-13 ameliorates LPS-induced endothelial-to-mesenchymal transition and post-acute lung injury pulmonary fibrosis by suppressing transforming growth factor-Β1 signaling. Shock Augusta Ga. 2023;59(1):108–117. doi:10.1097/shk.0000000000002046
46. Coquerel D, Lamoureux J, Chagnon F, et al. Apelin-13 in septic shock: effective in supporting hemodynamics in sheep but compromised by enzymatic breakdown in patients. Sci Rep. 2021;11(1):22770. doi:10.1038/s41598-021-02087-4
47. Chen XY, Liu XM, Feng LL, Tang CS. Changes and clinical significance of serum Apelin in patients with severe sepsis and septic shock. Acta Academiae Medicinae Sinicae. 2008;30(2):131–135. Chinese.
48. Lesur O, Roussy JF, Chagnon F, et al. Proven infection-related sepsis induces a differential stress response early after ICU admission. Critical Care. 2010;14(4):R131. doi:10.1186/cc9102
49. Wang Y, Zheng Y, Yan F, Ding NJCJo G. The changes and the clinical significance of plasma apelin in elderly patients with sepsis. J Geriatr. 2013;32(8):861–863.
50. Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–566. doi:10.1038/nrneurol.2012.183
51. Catarina AV, Branchini G, Bettoni L, De Oliveira JR, Nunes FB. Sepsis-associated encephalopathy: from pathophysiology to progress in experimental studies. Mol Neurobiol. 2021;58(6):2770–2779. doi:10.1007/s12035-021-02303-2
52. Tauber SC, Djukic M, Gossner J, Eiffert H, Brück W, Nau R. Sepsis-associated encephalopathy and septic encephalitis: an update. Expert Rev Anti Infect Ther. 2021;19(2):215–231. doi:10.1080/14787210.2020.1812384
53. Zhou JX, Shuai NN, Wang B, Jin X, Kuang X, Tian SW. Neuroprotective gain of apelin/APJ system. Neuropeptides. 2021;87:102131. doi:10.1016/j.npep.2021.102131
54. Savi FF, de Oliveira A, de Medeiros GF, et al. What animal models can tell us about long-term cognitive dysfunction following sepsis: a systematic review. Neurosci Biobehav Rev. 2021;124:386–404. doi:10.1016/j.neubiorev.2020.12.005
55. Zhou S, Guo X, Chen S, Xu Z, Duan W, Zeng B. Apelin-13 regulates LPS-induced N9 microglia polarization involving STAT3 signaling pathway. Neuropeptides. 2019;76:101938. doi:10.1016/j.npep.2019.101938
56. Zhang ZX, Li E, Yan JP, et al. Apelin attenuates depressive-like behavior and neuroinflammation in rats co-treated with chronic stress and lipopolysaccharide. Neuropeptides. 2019;77:101959. doi:10.1016/j.npep.2019.101959
57. Hu S, Shen P, Chen B, Tian SW, You Y. Apelin-13 reduces lipopolysaccharide-induced neuroinflammation and cognitive impairment via promoting glucocorticoid receptor expression and nuclear translocation. Neurosci Lett. 2022;788:136850. doi:10.1016/j.neulet.2022.136850
58. Peng X, Luo Z, He S, Zhang L, Li Y. Blood-brain barrier disruption by lipopolysaccharide and sepsis-associated encephalopathy. Front Cell Infect Microbiol. 2021;11:768108. doi:10.3389/fcimb.2021.768108
59. Xu W, Gao L, Li T, Zheng J, Shao A, Zhang J. Apelin-13 alleviates early brain injury after subarachnoid hemorrhage via suppression of endoplasmic reticulum stress-mediated apoptosis and blood-brain barrier disruption: possible involvement of ATF6/CHOP pathway. Neuroscience. 2018;388:284–296. doi:10.1016/j.neuroscience.2018.07.023
60. Schuler A, Wulf DA, Lu Y, et al. The impact of acute organ dysfunction on long-term survival in sepsis. Crit Care Med. 2018;46(6):843–849. doi:10.1097/ccm.0000000000003023
61. Landesberg G, Gilon D, Meroz Y, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012;33(7):895–903. doi:10.1093/eurheartj/ehr351
62. Sanfilippo F, Corredor C, Fletcher N, et al. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med. 2015;41(6):1004–1013. doi:10.1007/s00134-015-3748-7
63. Lin Y, Xu Y, Zhang Z. Sepsis-Induced Myocardial Dysfunction (SIMD): the pathophysiological mechanisms and therapeutic strategies targeting mitochondria. Inflammation. 2020;43(4):1184–1200. doi:10.1007/s10753-020-01233-w
64. Chagnon F, Coquerel D, Salvail D, et al. Apelin compared with dobutamine exerts cardioprotection and extends survival in a rat model of endotoxin-induced myocardial dysfunction. Crit Care Med. 2017;45(4):e391–e398. doi:10.1097/ccm.0000000000002097
65. Rudiger A, Dyson A, Felsmann K, et al. Early functional and transcriptomic changes in the myocardium predict outcome in a long-term rat model of sepsis. Clin Sci. 2013;124(6):391–401. doi:10.1042/cs20120334
66. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi:10.1007/s00134-021-06506-y
67. Schmittinger CA, Torgersen C, Luckner G, Schröder DC, Lorenz I, Dünser MW. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. 2012;38(6):950–958. doi:10.1007/s00134-012-2531-2
68. Silverman HJ, Penaranda R, Orens JB, Lee NH. Impaired beta-adrenergic receptor stimulation of cyclic adenosine monophosphate in human septic shock: association with myocardial hyporesponsiveness to catecholamines. Crit Care Med. 1993;21(1):31–39. doi:10.1097/00003246-199301000-00010
69. Coquerel D, Chagnon F, Sainsily X, et al. ELABELA improves cardio-renal outcome in fatal experimental septic shock. Crit Care Med. 2017;45(11):e1139–e1148. doi:10.1097/ccm.0000000000002639
70. Koguchi W, Kobayashi N, Takeshima H, Ishikawa M, Sugiyama F, Ishimitsu T. Cardioprotective effect of apelin-13 on cardiac performance and remodeling in end-stage heart failure. Circ J. 2012;76(1):137–144. doi:10.1253/circj.cj-11-0689
71. Boal F, Roumegoux J, Alfarano C, et al. Apelin regulates FoxO3 translocation to mediate cardioprotective responses to myocardial injury and obesity. Sci Rep. 2015;5:16104. doi:10.1038/srep16104
72. Wang M, Gupta RC, Rastogi S, et al. Effects of acute intravenous infusion of apelin on left ventricular function in dogs with advanced heart failure. J Card Fail. 2013;19(7):509–516. doi:10.1016/j.cardfail.2013.05.004
73. Ouyang Q, You T, Guo J, et al. Effects of apelin on left ventricular-arterial coupling and mechanical efficiency in rats with ischemic heart failure. Dis. Markers. 2019;2019:4823156. doi:10.1155/2019/4823156
74. Pang H, Han B, Yu T, Zong Z. Effect of apelin on the cardiac hemodynamics in hypertensive rats with heart failure. IntJ Mol Med. 2014;34(3):756–764. doi:10.3892/ijmm.2014.1829
75. Walley KR. New approaches to modifying inotropy in sepsis-induced myocardial dysfunction. Crit Care Med. 2017;45(4):754–756. doi:10.1097/ccm.0000000000002126
76. Luo K, Long H, Xu B, Luo Y. Apelin attenuates postburn sepsis via a phosphatidylinositol 3-kinase/protein kinase B dependent mechanism: a randomized animal study. Int J Surg. 2015;21:22–27. doi:10.1016/j.ijsu.2015.06.072
77. Simard É, Morin C, Coquerel D, et al. Hemodynamic impacts of apelin-13 in a neonatal lamb model of septic peritonitis. Pediatr Res. 2022. doi:10.1038/s41390-022-02407-y
78. Hu J, Huo S, Dou S, Jiang W, Li Y. Protective effect of Apelin/APJ system on lipopolysaccharide-related cardiac dysfunction. Gen Physiol Biophys. 2021;40(3):161–171. doi:10.4149/gpb_2021006
79. Hobai IA, Morse JC, Siwik DA, Colucci WS. Lipopolysaccharide and cytokines inhibit rat cardiomyocyte contractility in vitro. J Surg Res. 2015;193(2):888–901. doi:10.1016/j.jss.2014.09.015
80. Pan CS, Teng X, Zhang J, et al. Apelin antagonizes myocardial impairment in sepsis. J Card Fail. 2010;16(7):609–617. doi:10.1016/j.cardfail.2010.02.002
81. Boal F, Timotin A, Roumegoux J, et al. Apelin-13 administration protects against ischaemia/reperfusion-mediated apoptosis through the FoxO1 pathway in high-fat diet-induced obesity. Br J Pharmacol. 2016;173(11):1850–1863. doi:10.1111/bph.13485
82. Geven C, Bergmann A, Kox M, Pickkers P. Vascular effects of adrenomedullin and the anti-adrenomedullin antibody adrecizumab in sepsis. Shock. 2018;50(2):132–140. doi:10.1097/shk.0000000000001103
83. Castanares-Zapatero D, Bouleti C, Sommereyns C, et al. Connection between cardiac vascular permeability, myocardial edema, and inflammation during sepsis: role of the α1AMP-activated protein kinase isoform. Crit Care Med. 2013;41(12):e411–e422. doi:10.1097/CCM.0b013e31829866dc
84. Filewod NC, Lee WL. Inflammation without vascular leakage. science fiction no longer? Am J Respir Crit Care Med. 2019;200(12):1472–1476. doi:10.1164/rccm.201905-1011CP
85. Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;305(11):L844–L855. doi:10.1152/ajplung.00173.2013
86. Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689–691. doi:10.1056/NEJMcibr1007320
87. Yang K, Fan M, Wang X, et al. Lactate induces vascular permeability via disruption of VE-cadherin in endothelial cells during sepsis. Sci Adv. 2022;8(17):eabm8965. doi:10.1126/sciadv.abm8965
88. Chan YH, Harith HH, Israf DA, Tham CL. Differential regulation of LPS-mediated VE-cadherin disruption in human endothelial cells and the underlying signaling pathways: a mini review. Front Cell Develop Biol. 2019;7:280. doi:10.3389/fcell.2019.00280
89. Chen S, Wang Y, Zhang H, et al. The antioxidant MitoQ protects against CSE-induced endothelial barrier injury and inflammation by inhibiting ROS and autophagy in human umbilical vein endothelial cells. Int J Biol Sci. 2019;15(7):1440–1451. doi:10.7150/ijbs.30193
90. Kong X, Lin D, Lu L, Lin L, Zhang H, Zhang H. Apelin-13-mediated AMPK ameliorates endothelial barrier dysfunction in acute lung injury mice via improvement of mitochondrial function and autophagy. Int Immunopharmacol. 2021;101(Pt B):108230. doi:10.1016/j.intimp.2021.108230
91. Lin DP, Chen S, Lu LL, et al. Effects of apelin-13 on barrier injury of human umbilical vein endothelial cells induced by LPS. Chinese journal of applied physiology . 2020;36(5):390–393. Chinese. doi:10.12047/j.cjap.5896.2020.083
92. Li W, Li D, Chen Y, et al. Classic signaling pathways in alveolar injury and repair involved in sepsis-induced ALI/ARDS: new research progress and prospect. Dis Markers. 2022;2022:6362344. doi:10.1155/2022/6362344
93. Wang H, Cong L, Yin X, et al. The apelin-APJ axis alleviates LPS-induced pulmonary fibrosis and endothelial mesenchymal transformation in mice by promoting angiotensin-converting enzyme 2. Cell Signal. 2022;98:110418. doi:10.1016/j.cellsig.2022.110418
94. Zhang H, Chen S, Zeng M, et al. Apelin-13 administration protects against LPS-induced acute lung injury by inhibiting NF-κB pathway and NLRP3 inflammasome activation. Cell Physiol Biochem. 2018;49(5):1918–1932. doi:10.1159/000493653
95. Hashimoto N, Phan SH, Imaizumi K, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43(2):161–172. doi:10.1165/rcmb.2009-0031OC
96. Lv W, Zhang L, Cheng X, et al. Apelin inhibits angiotensin II-induced atrial fibrosis and atrial fibrillation via TGF-β1/Smad2/α-SMA pathway. Front Physiol. 2020;11:583570. doi:10.3389/fphys.2020.583570
97. Huang S, Chen L, Lu L, Li L. The apelin-APJ axis: a novel potential therapeutic target for organ fibrosis. Clin Chim Acta. 2016;456:81–88. doi:10.1016/j.cca.2016.02.025
98. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi:10.1016/j.cell.2020.02.052
99. Shen H, Zhang J, Wang C, et al. MDM2-mediated ubiquitination of angiotensin-converting enzyme 2 contributes to the development of pulmonary arterial hypertension. Circulation. 2020;142(12):1190–1204. doi:10.1161/circulationaha.120.048191
100. Wang L, Wang Y, Yang T, Guo Y, Sun T. Angiotensin-converting enzyme 2 attenuates bleomycin-induced lung fibrosis in mice. Cell Physiol Biochem. 2015;36(2):697–711. doi:10.1159/000430131
101. Sato T, Suzuki T, Watanabe H, et al. Apelin is a positive regulator of ACE2 in failing hearts. J Clin Invest. 2013;123(12):5203–5211. doi:10.1172/jci69608
102. Hamesch K, Borkham-Kamphorst E, Strnad P, Weiskirchen R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab Anim. 2015;49(1 Suppl):37–46. doi:10.1177/0023677215570087
103. Bautista AP, Mészáros K, Bojta J, Spitzer JJ. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J Leukoc Biol. 1990;48(2):123–128. doi:10.1002/jlb.48.2.123
104. Wang X, Zhang L, Li P, Zheng Y, Yang Y, Ji S. Apelin/APJ system in inflammation. Int Immunopharmacol. 2022;109:108822. doi:10.1016/j.intimp.2022.108822
105. Zhou Q, Cao J, Chen L. Apelin/APJ system: a novel therapeutic target for oxidative stress-related inflammatory diseases (Review). IntJ Mol Med. 2016;37(5):1159–1169. doi:10.3892/ijmm.2016.2544
106. Zhou H, Yang R, Wang W, et al. Fc-apelin fusion protein attenuates lipopolysaccharide-induced liver injury in mice. Sci Rep. 2018;8(1):11428. doi:10.1038/s41598-018-29491-7
107. Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439. doi:10.2215/cjn.03681106
108. Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187(5):509–517. doi:10.1164/rccm.201211-1983OC
109. Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169. doi:10.1056/NEJMra032401
110. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12. doi:10.1186/cc2872
111. Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828. doi:10.1007/s00134-017-4755-7
112. Chen H, Wang L, Wang W, et al. ELABELA and an ELABELA fragment protect against AKI. J Am Soc Nephrol. 2017;28(9):2694–2707. doi:10.1681/asn.2016111210
113. Sabry MM, Ahmed MM, Maksoud OMA, et al. Carnitine, apelin and resveratrol regulate mitochondrial quality control (QC) related proteins and ameliorate acute kidney injury: role of hydrogen peroxide. Arch Physiol Biochem. 2022;128(5):1391–1400. doi:10.1080/13813455.2020.1773504
114. Xu F, Zhou H, Wu M, et al. Fc-Elabela fusion protein attenuates lipopolysaccharide-induced kidney injury in mice. Biosci Rep. 2020;40(9). doi:10.1042/BSR20192397
115. De Mota N, Reaux-Le Goazigo A, El Messari S, et al. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci USA. 2004;101(28):10464–10469. doi:10.1073/pnas.0403518101
116. Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology. 2004;145(9):4392–4400. doi:10.1210/en.2004-0384
117. Azizi M, Iturrioz X, Blanchard A, et al. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J Am Soc Nephrol. 2008;19(5):1015–1024. doi:10.1681/asn.2007070816
118. Wiedermann CJ. Phases of fluid management and the roles of human albumin solution in perioperative and critically ill patients. Curr Med Res Opin. 2020;36(12):1961–1973. doi:10.1080/03007995.2020.1840970
119. Kadekaro M, Summy-Long JY, Freeman S, Harris JS, Terrell ML, Eisenberg HM. Cerebral metabolic responses and vasopressin and oxytocin secretions during progressive water deprivation in rats. Am J Physiol. 1992;262(2 Pt 2):R310–R317. doi:10.1152/ajpregu.1992.262.2.R310
120. Deng C, Chen H, Yang N, Feng Y, Hsueh AJ. Apela regulates fluid homeostasis by binding to the APJ receptor to activate gi signaling. J Biol Chem. 2015;290(30):18261–18268. doi:10.1074/jbc.M115.648238
121. Reaux A, De Mota N, Skultetyova I, et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77(4):1085–1096. doi:10.1046/j.1471-4159.2001.00320.x
122. Japp AG, Cruden NL, Amer DA, et al. Vascular effects of apelin in vivo in man. J Am Coll Cardiol. 2008;52(11):908–913. doi:10.1016/j.jacc.2008.06.013
123. Brame AL, Maguire JJ, Yang P, et al. Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension. 2015;65(4):834–840. doi:10.1161/hypertensionaha.114.05099
124. Read C, Yang P, Kuc RE, et al. Apelin peptides linked to anti-serum albumin domain antibodies retain affinity in vitro and are efficacious receptor agonists in vivo. Basic Clin Pharmacol Toxicol. 2020;126 Suppl 6:96–103. doi:10.1111/bcpt.13227
125. Serpooshan V, Sivanesan S, Huang X, et al. [Pyr1]-Apelin-13 delivery via nano-liposomal encapsulation attenuates pressure overload-induced cardiac dysfunction. Biomaterials. 2015;37:289–298. doi:10.1016/j.biomaterials.2014.08.045
126. Iturrioz X, Alvear-Perez R, De Mota N, et al. Identification and pharmacological properties of E339-3D6, the first nonpeptidic apelin receptor agonist. FASEB J. 2010;24(5):1506–1517. doi:10.1096/fj.09-140715
127. National Center for Biotechnology Information (US). Probe Reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information (US); 2010.
128. Fischer C. A patent review of apelin receptor (APJR) modulators (2014–2019). Expert Opin Ther Pat. 2020;30(4):251–261. doi:10.1080/13543776.2020.1731473
129. Read C, Fitzpatrick CM, Yang P, et al. Cardiac action of the first G protein biased small molecule apelin agonist. Biochem Pharmacol. 2016;116:63–72. doi:10.1016/j.bcp.2016.07.018
130. Ason B, Chen Y, Guo Q, et al. Cardiovascular response to small-molecule APJ activation. JCI Insight. 2020;5(8). doi:10.1172/jci.insight.132898
131. Narayanan S, Dai D, Vyas Devambatla RK, et al. Synthesis and characterization of an orally bioavailable small molecule agonist of the apelin receptor. Bioorg Med Chem. 2022;66:116789. doi:10.1016/j.bmc.2022.116789
132. Pi Z, Johnson JA, Meng W, et al. Identification of 6-hydroxypyrimidin-4(1H)-one-3-carboxamides as potent and orally active APJ receptor agonists. ACS Med Chem Lett. 2021;12(11):1766–1772. doi:10.1021/acsmedchemlett.1c00385
133. Gargalovic P, Wong P, Onorato J, et al. In vitro and in vivo evaluation of a small-molecule APJ (apelin receptor) agonist, BMS-986224, as a potential treatment for heart failure. Circ Heart Fail. 2021;14(3):e007351. doi:10.1161/circheartfailure.120.007351
134. Tora G, Jiang J, Bostwick JS, et al. Identification of 6-hydroxy-5-phenyl sulfonylpyrimidin-4(1H)-one APJ receptor agonists. Bioorg Med Chem Lett. 2021;50:128325. doi:10.1016/j.bmcl.2021.128325
135. Zhou N, Fang J, Acheampong E, Mukhtar M, Pomerantz RJ. Binding of ALX40-4C to APJ, a CNS-based receptor, inhibits its utilization as a co-receptor by HIV-1. Virology. 2003;312(1):196–203. doi:10.1016/s0042-6822(03)00185-5
136. Lee DK, Saldivia VR, Nguyen T, Cheng R, George SR, O’Dowd BF. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146(1):231–236. doi:10.1210/en.2004-0359
137. Ishimaru Y, Shibagaki F, Yamamuro A, Yoshioka Y, Maeda S. An apelin receptor antagonist prevents pathological retinal angiogenesis with ischemic retinopathy in mice. Sci Rep. 2017;7(1):15062. doi:10.1038/s41598-017-15602-3
138. Pupo AS, Duarte DA, Lima V, Teixeira LB, Parreiras ESLT, Costa-Neto CM. Recent updates on GPCR biased agonism. Pharmacol Res. 2016;112:49–57. doi:10.1016/j.phrs.2016.01.031
139. Masri B, Morin N, Pedebernade L, Knibiehler B, Audigier Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J Biol Chem. 2006;281(27):18317–18326. doi:10.1074/jbc.M600606200
140. Hashimoto Y, Ishida J, Yamamoto R, et al. G protein-coupled APJ receptor signaling induces focal adhesion formation and cell motility. IntJ Mol Med. 2005;16(5):787–792.
141. Dray C, Knauf C, Daviaud D, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8(5):437–445. doi:10.1016/j.cmet.2008.10.003
142. Zhou Y, Song Y, Shaikh Z, et al. MicroRNA-155 attenuates late sepsis-induced cardiac dysfunction through JNK and β-arrestin 2. Oncotarget. 2017;8(29):47317–47329. doi:10.18632/oncotarget.17636
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.