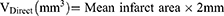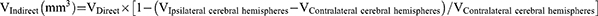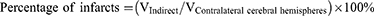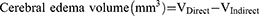Back to Journals » Drug Design, Development and Therapy » Volume 16
The Antithrombotic Effect of Recombinant Neorudin on Thrombi
Authors Liu YB , Zhang L, Zhou XC, Zhou Y, Liu Y, Zheng C, Xu X, Geng PP, Hao CH, Zhao ZY, Wu CT, Jin JD
Received 9 December 2021
Accepted for publication 19 May 2022
Published 2 June 2022 Volume 2022:16 Pages 1667—1678
DOI https://doi.org/10.2147/DDDT.S353088
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Tin Wui Wong
Yu-Bin Liu,1 Lin Zhang,1 Xing-Chen Zhou,1 Ying Zhou,1 Yun Liu,1 Can Zheng,1 Xiao Xu,1 Pan-Pan Geng,1 Chun-Hua Hao,2 Zhuan-You Zhao,2 Chu-Tse Wu,1 Ji-De Jin1
1Department of Experimental Hematology and Biochemistry, Beijing Institute of Radiation Medicine, Beijing, 100850, People’s Republic of China; 2Center for Pharmacodynamic Research, Tianjin Institute of Pharmaceutical Research, Tianjin, 300462, People’s Republic of China
Correspondence: Chu-Tse Wu; Ji-De Jin, Department of Experimental Hematology and Biochemistry, Beijing Institute of Radiation Medicine, No. 27 Taiping Road, Beijing, People’s Republic of China, Tel +86 1086-68158312 ; +86 1086-66931425, Email [email protected]; [email protected]
Introduction: Recombinant neorudin (EPR-hirudin, EH) was developed through the addition of an EPR (Glu-Pro-Arg) peptide to the amino terminus of hirudin, which can be recognized and cut by coagulation factors XIa (FXIa) and/or Xa (FXa). In this study, the low-bleeding antithrombotic effects of EH were evaluated utilizing experimental models of thrombosis in rabbits and rats to provide a test basis for clinical trials.
Methods: The bleeding risks of EH and hirudin were first compared in mice by the tail-clipping method, and then the antithrombotic activity of EH was investigated in a rabbit model of arteriovenous bypass thrombosis and a rat model of thrombotic cerebral infarction.
Results: In mice, intravenous administration of EH at 1.5 mg/kg and 3 mg/kg did not affect the bleeding time compared with normal saline, while the administration of hirudin at 1.5 mg/kg prolonged the bleeding time by over 3 times the administration of normal saline. Furthermore, intravenous administration of EH had a significant dose-dependent inhibitory effect on the formation and development of arteriovenous bypass thrombosis and thrombotic cerebral infarction. Compared with an equimolar dose of hirudin, the antithrombotic effect of EH was similar, while the bleeding side effects were significantly attenuated. Moreover, when the antithrombotic effects were similar, EH had a shorter bleeding time and was associated with less bleeding than low molecular weight heparin (LMWH). EH had a therapeutic effect on thrombotic cerebral infarction without increasing the occurrence of cerebral hemorrhage.
Conclusion: The findings from the preclinical animal models used in this study showed that EH could not only effectively inhibit thrombus formation but also reduce the risk of bleeding.
Keywords: recombinant neorudin, hirudin, antithrombotic effect, bleeding
Introduction
Anticoagulants are widely used for the prevention and treatment of venous or arterial thrombosis,1 but the interference of these drugs with physiological hemostasis and serious bleeding side effects result in many inconveniences and limitations in clinical use. Clinically, low molecular weight heparin (LMWH) is the most commonly used anticoagulant to treat various blood clot diseases. However, some troublesome side effects, including bleeding and heparin-induced thrombocytopenia, often occur with the use of LMWH.2,3 Warfarin, another commonly used anticoagulant, has many drawbacks, including the need for routine coagulation monitoring, frequent dose adjustments to maintain safe and effective levels, a narrow therapeutic window, a slow onset of action, and multiple food and drug interactions.4,5 Hirudin, a direct thrombin inhibitor, is more effective than heparin in preventing and treating arterial and venous thrombi,6,7 but the use of hirudin is often accompanied by major bleeding, even life-threatening bleeding, which limits its clinical use.8,9 In addition, two other direct thrombin inhibitors: Argatroban and Bivalirudin have been approved for the treatment of patients with heparin-induced thrombocytopenia (HIT) and undergoing percutaneous coronary intervention (PCI), respectively. However, some moderate bleeding often occurs accompanying their applications in clinic.10,11 The license of Rivaroxaban, a representative of direct oral anticoagulants (DOACs), brings more options to the anticoagulant market. Nevertheless, moderate or minor bleeding of rivaroxaban still puzzles doctors, especially for patients with high bleeding risk.12,13 Therefore, it is of scientific significance to develop new-generation anticoagulants with few or no bleeding side effects to improve the prevention and treatment of thrombotic diseases.
Recombinant neorudin (EPR-hirudin, EH)14,15 was developed as a prodrug of type II hirudin (hirudin) by introducing an EPR short peptide (Glu-Pro-Arg), which is recognized and cleaved by FXIa and/or FXa, into the N-terminus of hirudin.16 EH, a protein with a molecular weight of 7.28 Daltons consisting of 68 amino acid residues, is secreted by recombinant Pichia pastoris GS115. EH has received Chinese patents (ZL200780046340.6), European patents (EP2103630B1), United States patents (US8101379B2) and Japanese patents (5345069). The antithrombin activity of hirudin is completely inhibited by the EPR short peptide, and EH exerts its antithrombotic effects by releasing its active metabolite, hirudin, at the thrombus site via FXIa-mediated cleavage of the EPR peptide, resulting in direct inhibition of thrombin. EPR peptide was also used by Sheffield et al17 to link Hirudin variant III and human serum albumin to construct a fusion protein, which was proved to be an effective way to limit the hirudin’s bleeding side-effects. Hirudin, the most potent natural thrombin inhibitor, was first approved for clinical use by the European Medical Evaluation Agency (EMEA) for the treatment of HIT complicated by thrombosis and then by the US Food and Drug Administration (FDA) for thrombosis prophylaxis after major orthopedic surgery.18 However, the clinical application of hirudin is greatly limited because of its association with severe bleeding.19 By comparison, the bleeding associated with EH was evidently decreased, in that EH exhibits anticoagulant activity only after cleavage by the corresponding coagulation factors, which occurs when the coagulation system is activated and when the thrombus is present.18,19 On the basis of the structure and action mechanism, EH can not only effectively inhibit thrombosis but also reduce the risk of bleeding by increasing the specificity and efficiency of hirudin.
An animal thrombus model is an important method to study the mechanism of thrombosis formation. In general, the mechanism underlying the establishment of a thrombus model is that the endothelium of the vessel wall is destroyed, and the subcutaneous matrix is exposed to blood flow, which activates platelets and the clotting cascade and then the thrombi form.20 There are many ways to induce blood vessel damage; for example, Pierangeli et al21 used a microsurgical tool to damage the femoral vein, Rosen et al22 used high-intensity short-pulse laser lamps to induce blood vessel damage, Kikuchi et al23 used induced mouse endothelial injury by photochemical methods and Huttinger et al24 used FeCl3 to induce carotid artery injury in canines. FeCl3 can cause lipid peroxidation and endothelial cell damage; thus, the FeCl3-induced arteriovenous thrombosis model can clearly replicate the initial stage of thrombosis and play an important role in the evaluation of new antithrombotic drugs and their mechanisms. Furthermore, an arteriovenous bypass thrombosis animal model is sometimes used to evaluate the effects of anticoagulants or antiplatelet drugs.25,26
In this study, the bleeding risks of EH and hirudin were first compared in Kunming mice (Swiss mice) by the tail-clipping method. Thereafter, we investigated the anticoagulating activity of EH in vitro in a rabbit model of arteriovenous bypass thrombosis and a rat model of thrombotic cerebral infarction to provide experimental evidence for a further clinical study.
Materials and Methods
Materials
EH (20 mg/bottle) and hirudin (9 mg/bottle) were obtained from the Beijing Institute of Radiation Medicine (Beijing, China). LMWH sodium injection (FLUXUM) manufactured by Alfa Wassermann was purchased from No. 307 Hospital (Beijing, China). Determination reagents for the thrombin time (TT), prothrombin time (PT) and activated partial thromboplastin time (APTT) were purchased from MDC/TECO MEDICAL (Germany). The fibrinogen (FG) content determination kit was purchased from Beijing Shidi Scientific Instrument Co., Ltd (Beijing, China). A micro-free hemoglobin (FHb) determination kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
The RM6300 Multichannel Physiological Recorder and Mfv-3200 electromagnetic blood flowmeter were purchased from NIHON KOHDEN CORP (Japan). The MP100 multichannel biological signal acquisition system was purchased from Biopac Systems Inc (Santa Barbara CA, USA). The PARBER Coagulation Factor Analyzer was purchased from Beijing Shidi Scientific Instrument Co., Ltd (Beijing, China). A 722 grating spectrophotometer was purchased from Shanghai Third Analytical Instrument Factory (Shanghai, China). The PK121R cryogenic centrifuge was purchased from ALC International (Milan, Italy).
Kunming mice (Swiss mice) and Sprague Dawley (SD) rats were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). Male rabbits were provided by the Beijing Longan Experimental Animal Breeding Center (Beijing, China). The animal experiment in this paper was approved by the Animal Ethics Committee of Tianjin Institute of Pharmaceutical Research (2018010901). Animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Methods
The Tail Bleeding Time Experiment in Mice
Kunming mice were randomly divided into the following 4 groups with 10 mice in each group, half male and half female: a normal saline group, a hirudin (1.5 mg/kg) group and an EH (1.5, 3.0 mg/kg) group. Animals were administered 0.2 mL saline or the corresponding drugs through the tail vein. Twenty minutes later, the mouse was anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg), and approximately 6 mm of the mouse tail tip was quickly cut off with a sharp blade, and the tail was immediately put into saline at room temperature to record the bleeding time of the mouse tail.
Efficacy of Arteriovenous Bypass Thrombosis in a Rabbit Model
Surgical Procedure
Arteriovenous bypass thrombosis was established in a rabbit model as previously reported.27–29 Briefly, white male rabbits weighing 2.3 ± 0.19 kg were injected intravenously with sodium pentobarbital (35 mg/kg) for anesthesia, the common carotid artery and vena jugularis externa were separated and a polyethylene tube (length 7 cm, inner diameter 1.5 mm) was inserted. Arteriovenous bypass was established by connecting both ends of the pipe with a polyethylene tube (length 16 cm, inner diameter 3 mm) filled with heparin (50 U/L) and a 6 cm filament thread. The arterial clamp was opened to allow blood flow from the common carotid artery to the polyethylene tube and then back to the external jugular vein for 60 minutes. After that, the common carotid artery was clamped, and the thrombus was removed for analysis. The experimental design and timeline are showed in Figure 1A.
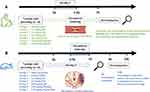 |
Figure 1 Antithrombotic experiment design. (A) Experimental process in rabbit model of arteriovenous bypass thrombosis. (B) Experimental process in rat model of thrombotic cerebral infarction. |
Grouping and Dosing Schedule
After the operation, 70 rabbits were randomly divided into 7 groups (n = 10). The four test groups were given EH at doses of 0.3, 1.0, 3.0 and 10.0 mg/kg; the two positive groups were given hirudin at doses of 2.88 mg/kg (equimolar dose with 3 mg/kg EH) or LMWH at 150 IU/kg; and the negative group was injected with the corresponding amount of saline. Except for LMWH, the other drugs or saline were administered immediately by marginal auricular vein injection after the bypass was opened. A total of 1/3 of the drug volume was injected first as a bolus, the other 2/3 was given by constant speed infusion for 30 min, and the volume of administration was 1 mL/kg. LMWH was administered by subcutaneous injection 0.5 h before surgery.
Determination of Thrombus Size and Thrombus Fibrin Mass
After the thrombus was removed, the blood on the surface was removed with filter paper. The thrombus was divided into two parts: for one part, the wet weight was recorded first, and then the dry weight was determined after baking to a constant weight in an oven at 50°C for three days; the other part was digested with 1% NaOH to determine the amount of thrombus matrix fibrin. The result was corrected according to the proportion of the two parts initially divided, and the dry weight of the whole thrombus and the fibrin content of the thrombus were calculated.
Determination of Bleeding Time and Whole Blood Coagulation Time
After being anesthetized by sodium pentobarbital (35mg/kg), a fixed length wound (avoiding large blood vessels) was cut at the ear edge of the rabbit, and the bleeding time was recorded. A drop of whole blood was taken in a glass dish, and the coagulation time was recorded by needle picking.
Anticoagulant Monitoring
After the thrombus was removed, peripheral blood was collected with 3.8% sodium citrate (the volume ratio of whole blood to anticoagulant was 9:1) and centrifuged at 4°C and 3000 rpm for 10 min. Plasma was collected, and the corresponding reagents were added to determine the TT, PT, and APTT with a PARBER Coagulation Factor Analyzer and to determine the FG content with a 722 grating spectrophotometer following the kit instructions.
Efficacy of EH on Thrombotic Cerebral Infarction in a Rat Model
Model Preparation and Surgical Procedure
Middle cerebral artery occlusion (MCAO) was induced by the method previously reported with a few modifications.30,31 Male rats weighing 258.0 ± 11.0 g were fasted overnight but were allowed to drink freely and were anesthetized by sodium pentobarbital (50 mg/kg). The zygomatic arch and temporal bone were exposed by cutting the skin at the midpoint between the external auditory canal and the eye canthus. Under a surgical microscope, the zygomatic arch was reamed. A 2 mm cranial window was drilled with a dental drill approximately 2 mm below the anterior junction of the zygomatic arch and the temporal bone to expose the middle cerebral artery. A small piece of filter paper was applied to the middle cerebral artery, 8 μL of FeCl3 solution (50%) was dropped onto the filter paper, and the filter paper with FeCl3 solution remained in place for 30 min. The anal temperature of the animal was monitored to ensure the consistency of strokes during the whole surgery. Thereafter, the filter paper was removed, the middle artery was cleaned with saline, and 2~3 drops of penicillin solution (1.6 × 105 U/mL) were added to prevent infection. After suturing layer by layer, the rats were put back into cages for breeding. In the sham group, the same operations were performed except that FeCl3 was not applied. The experimental design and timeline are showed in Figure 1B.
Grouping and Dosing Schedule
After the operation, 70 rats (n = 10) were randomly divided into 7 groups. The three test groups were given EH at doses of 1.0, 3.0 and 10.0 mg/kg; the two positive groups were given hirudin at a dose of 2.88 mg/kg or Argatroban at a dose of 5.39 mg/kg; and the negative group and the sham operation group were injected with the corresponding amount of saline. Thirty minutes before FeCl3 application, 2 mL of drugs or saline were infused through the caudal vein for 60 min.
Neurological Evaluation After MCAO in Rats
The degree of neurological damage in rats was scored with a blinded method at 4 h and 24 h after drug administration according to the evaluation criteria in Table 1, and the total possible score was 16.
 |
Table 1 Neurological Evaluation Criterion After Middle Cerebral Artery Occlusion (MCAO) in Rats |
Measurement of Infarct Size and Edema Volume
The whole rat's brain was removed 24 h after the operation and frozen rapidly at −80°C for 4 min. Brain slices were cut 2 mm along the coronal plane of the brain and then put into 2% triphenyltetrazole chloride (TTC) dye solution to incubate at 37°C for 5 min away from the light. Digital images of the slices were recorded using a COOLPIX955 digital camera before and after staining, and were analyzed using pathological image acquisition and analysis system v4.0 software (Image Center of Beihang University, Beijing, China). The area of the ipsilateral and contralateral cerebral hemispheres of infarcts was measured using the images before staining, and the area of infarcts was measured with the stained image. The volume of cerebral edema and the percentage of infarct area were calculated according to the following formulas:
Anticoagulant Monitoring
The rats were anesthetized with 3% sodium pentobarbital (50 mg/kg) 24 h after surgery, and 2 mL of blood was collected from the abdominal aorta in tubes with 3.8% sodium citrate (the ratio of whole blood to anticoagulant was 9:1). The blood was centrifuged at 4°C and 3000 rpm for 10 min, and the plasma was taken to determine the TT, PT, APTT and FG by the coagulation method.
Determination of Intracerebral Hemorrhage
After TTC staining, rat brain sections were homogenized to 2.5 mL of saline. Thereafter, the upper suspension was collected after the centrifugation of the homogenate at 3000 rpm for 10 min, and cerebral hemorrhage was determined by the quantity of trace FHb.
Statistical Analysis
Data are expressed as the means ± standard deviation, and statistical analysis of the data was performed using one-way ANOVA and the difference between groups was analysed with Tukey’s post-hoc test, with P < 0.05 indicating a significant difference.
Results
Effect of EH on the Bleeding Time of Kunming Mice with Cut Tails
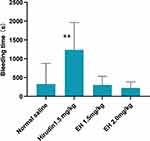
In Kunming mice, intravenous administration of hirudin 1.5 mg/kg prolonged the bleeding time by over 3 times compared with the administration of normal saline, while EH 1.5 mg/kg and 3 mg/kg did not affect the bleeding time (Figure 2).
Effect of EH on Arteriovenous Bypass Thrombosis in a Rabbit Model
Effect on Thrombus Weight
After blood was refluxed by an arteriovenous bypass for 1 h, an obvious thrombus was formed in the bypass tube. As shown in Figure 3A, after EH was given intravenously to rabbits at 0.3, 1.0, 3.0 and 10 mg/kg, the wet weight and dry weight of the thrombus were obviously reduced in a dose-dependent manner. Compared with the normal saline group, the wet weight of thrombi in the 0.3, 1.0, 3.0 and 10 mg/kg EH treatment groups was reduced by 19.7% (P > 0.05), 27.6% (P < 0.05), 34.1% (P < 0.01), 51.1% (P < 0.001), respectively; at the same time, the dry weight of thrombi decreased by 9.3% (P > 0.05), 14.4% (P > 0.05), 22.4% (P < 0.05) and 36.5% (P < 0.01), respectively. After treatment with EH at 0.3, 1.0, 3.0 and 10 mg/kg, the amount of thrombus matrix fibrin also decreased in a dose-dependent manner by 25.0% (P < 0.01), 29.4% (P < 0.05), 32.4% (P < 0.001) and 39.7% (P < 0.001), respectively, compared with the normal saline. In addition, both hirudin and LMWH significantly reduced the wet weight and dry weight of thrombi and the amount of thrombus matrix fibrin compared with the normal saline (Figure 3B). Hirudin has a stronger antithrombotic effect compared with equimolar EH (Figure 3A and B).
Influence on Wound Bleeding Time and Coagulation Time of Whole Blood
The administration of EH 0.3, 1.0 and 3.0 mg/kg had no significant effects on auricle bleeding time or whole-blood coagulation time in rabbits. However, the administration of 10.0 mg/kg EH led to a longer bleeding time than that of the normal saline (P < 0.05). The auricle bleeding time and whole-blood coagulation time in the hirudin group were prolonged significantly compared with those in the normal saline group (p < 0.05). Compared with hirudin, an equal molar dose of EH led to a shorter bleeding time and shorter whole-blood coagulation time (P < 0.05). Moreover, the coagulation time of whole blood was significantly prolonged after the administration of LMWH compared with the administration of the normal saline (P < 0.05) (Figure 3C).
Effects on Anticoagulation Parameters
One hour after administration, compared with rabbits administered the normal saline, the PTs of rabbits administered EH at 0.3, 1.0, and 3.0 mg/kg were significantly prolonged, and the PT, TT and APTT of the animals that received EH at 10.0 mg/kg were evidently prolonged (p < 0.05–0.01). In comparison, the PT, TT and APTT of the animals treated with Hirudin were significantly longer than those of the animals administered the normal saline (p < 0.01–0.001), and the TT and APTT were longer than those of rabbits administered an equimolar dose of EH (p < 0.05). Moreover, the PT and APTT of the rabbits treated with LMWH were longer than those of the normal saline (p < 0.05–0.01). In contrast, the FG concentration was not influenced by the administration of EH, hirudin or LMWH. The results are shown in Table 2.
 |
Table 2 Effect of EH on Coagulation Parameters in Rabbits |
Effect on Cerebral Infarction in Rats
Effects on Neurological Impairment in Rats
When cerebral infarction was successfully induced by the FeCl3 method, the apoptosis or necrosis of neurons occurred due to ischemia, resulting in the impairment of nerve function in animals,32,33 and the typical symptoms of impaired nerve function were obviously demonstrated in the rat model control group. However, when anticoagulant was applied, the formation of clots induced by endothelial damage was attenuated, and then the impairment of nerve function was alleviated. The impaired neurological function could be improved in a dose-dependent manner by the administration of EH at 1.0, 3.0, and 10.0 mg/kg, and the neurological disorder score in each dose group decreased by 5.9% (P > 0.05), 12.6% (P < 0.05) and 16.8% (P < 0.05) 4 h after administration and 11.9% (P > 0.05), 19.8% (P < 0.05), and 21.8% (P < 0.05) 24 h after administration, respectively. The effects of EH (3 mg/kg) and hirudin (equal molar dose with EH) on neurological dysfunction improvement were similar 4 h after administration (16.8% vs 21.8%, P > 0.05) and 24 h after administration (21.8% vs 26.7%, P > 0.05). In addition, the nerve damage to the rats administered Argatroban also improved (Figure 4).
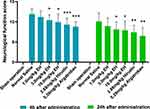 |
Figure 4 The effect of EH on neurological impairment scores at 4 h and 24 h after operation in rat with cerebral infarction. Thirty minutes after drug administration, the cerebral infarction in rats was induced with FeCl3, and the degree of neurological damage was scored with a blinded method at 4 h and 24 h after drug treatment according to the evaluation criteria in Table 1. *P < 0.05, **P < 0.01, ***P < 0.001, in comparison with normal saline. |
Effect on Cerebral Infarction Extent and Brain Edema Volume in Rats
Brain tissue necrosis and edema were induced by cerebral infarction, which was demonstrated by the TTC dye method (Figure 5A). Normal brain tissue, but not ischemic necrotic tissue, was dyed red by TTC, and the cerebral infarction extent and brain edema volume could be calculated by the TTC dye method. The normal saline group had obvious cerebral infarction and cerebral edema. However, as shown in Figure 5B and C, after the administration of EH at 1.0, 3.0 and 10 mg/kg, the cerebral infarction extent and brain edema volume of the rats were reduced in a dose-dependent manner; the cerebral infarction extent in each dose group was reduced by 6.5% (P > 0.05), 25.9% (P < 0.05), and 35.7% (P < 0.01); and the brain edema volume in each dose group was reduced by 9.2% (P > 0.05), 22.3% (P < 0.05), and 30.9% (P < 0.05), respectively. EH 3 mg/kg had a similar effect as equimolar hirudin on the cerebral infarction extent (35.7% vs 43.8%) and cerebral edema volume (30.9% vs 35.0%). Moreover, Argatroban also had an effective therapy for cerebral infarction.
Influence on Anticoagulation Parameters
There were no significant influences on the TT, PT, APTT, or FG 24 h after treatment, and the negative results were not listed.
The Effect on Cerebral Hemorrhage
An increase in the content of free hemoglobin in brain homogenate can indicate intracerebral hemorrhage. The results of the analysis of free hemoglobin showed that compared with the normal saline, neither EH nor hirudin had an obvious effect on the content of free hemoglobin in brain homogenate, suggesting that none of EH and hirudin increased cerebral hemorrhage. However, the content of free hemoglobin in the brain tissue homogenates of the Argatroban group increased by 8.3% (P < 0.05), suggesting that 5.39 mg/kg Argatroban significantly increased cerebral hemorrhage, although it had an effective treatment better anticoagulant effect (Figure 5D).
Discussion
Anticoagulant is one of the most common medical interventions for the treatment and prevention of thrombosis. At the same time, bleeding, the primary complication of anticoagulant therapy, often accompanies all currently used anticoagulants such as LMWH, Argatroban, Bivalirudin and Rivaroxaban, even when maintained within their therapeutic dose range.13,34 The mechanism of action of the above anticoagulants inhibiting one or several coagulation factors in the whole blood determines that their clinical application for treating or preventing blood clots will inevitably result in bleeding risks. However, as a prodrug of hirudin, the antithrombin activity of EH is totally blocked by the introduced EPR oligopeptide, and is released after incubation with activated coagulation factor X or XI (FXa or FIXa).35 Our previous study showed that the concentration of EH and hirudin in local thrombus is higher than in circulating blood,36 and the FXa and FIXa mainly enrich in the thrombus local,; thus, the antithrombin action of EH also limit the region of clots resulting in a significant decrease in the bleeding risk. In a previous study,37 we reported that EH not only effectively inhibited the formation of blood clots in a rat model of carotid artery thrombosis and posterior vena cava thrombosis but also was associated with less bleeding than hirudin or LMWH at similar levels of antithrombotic activity, which results from its unique thrombus focal activity. When local thrombosis is formed in vivo and the coagulation system is activated, the enzymatic hydrolysis of the activated coagulation factors Xa and XIa transforms the inactive form of EH into hirudin, which locally inhibits thrombin activity in thrombi.
In this work, the blood clot inhibition effects and low-bleeding features were further evaluated in a rabbit model of arteriovenous bypass thrombosis and a rat cerebral infarction model induced by FeCl3. The blood clots formed mainly as a result of platelets adhering to silk thread, and then the clotting cascade was activated in the rabbit model of arteriovenous bypass thrombosis.38 The results of this model showed that compared with the control, EH dose-dependently inhibited the formation of arteriovenous thrombus in rabbits, which demonstrated that EH, similar to hirudin, can inhibit the blood clots induced by platelet adhesion and aggregation. At the same time, the thrombus inhibition effects of EH did not increase the bleeding risk compared with those of hirudin and LMWH. Although the TT and APTT were slightly elongated after administration, EH had less influence on the TT and APTT (P < 0.05) than the equimolar dose of hirudin. Compared with EH, the thrombotic inhibition rate [(Wnormal saline group - Wtreatment group)/Wnormal saline group × 100%, W: the weight of thrombus] of equimolar hirudin increased (hirudin 59.6% vs EH 34.1%), but the bleeding time was prolonged 1.4-fold, and the whole blood coagulation time was prolonged 2.8-fold. The possible reason is that the cut of EH by FXIa and/or FXa is time-consuming and some EH is not turned into hirudin. Approximate 44% of EH incubated with FXIa for 24 hours in vitro were converted into hirudin.18
The rat cerebral infarction model induced by FeCl3 mainly simulated the formation of cerebral blood clots resulting from the vascular endothelial injury and the activation of the endogenous coagulation pathway. There was evidence that intracerebral injection of the thrombin inhibitor hirudin attenuated tissue damage and neurological deficits after transient focal cerebral ischemia.39,40 The results of the rat model in this study showed that EH could also dose-dependently improve the impaired neurological function of rats resulting from cerebral ischemia and reduce the scope of cerebral infarction and the volume of cerebral edema in rats with cerebral ischemia. The effect of EH 3.0 mg/kg was equivalent to that of hirudin at an equimolar dose, suggesting that EH was similar to hirudin in the treatment of acute cerebral infarction. Administration of EH did not increase the risk of cerebral hemorrhage and had no obvious effect on the TT, PT, APTT or FG at the therapeutic dose, mainly resulting from thrombin focal inhibition of EH to prevent fibrinogen from producing fibrin. This study showed that Argatroban had a positive anticoagulant effect, but the elevated free hemoglobin content in brain tissue homogenate indicated a higher risk of bleeding, which can sometimes result in severe consequences, especially for intracerebral hemorrhage.41,42 In the current study, it was demonstrated that EH had a strong neuroprotective effect on acute cerebral infarction by focally inhibiting the formation of clots and was a potential low-bleeding agent for the treatment of acute cerebral infarction by inhibiting the generation of thrombi.
As the population ages, the incidence of myocardial and cerebral infarction increases because of the higher adhesion and aggregation of platelets and the vulnerability of the endothelium, a serious threat to patient life and health.43,44 The application of anticoagulants is the main therapeutic measure for all thrombus disease types, including myocardial and cerebral infarction. Given the low-bleeding and anticoagulation characteristics of EH, it is expected that EH could be used in clinic to effectively treat cerebral infarction to improve the neurological function of patients, with fewer side effects of bleeding than LMWH. Although effective therapies for EH were demonstrated in these animal blood clot models, the safety and efficacy of EH still need to be further confirmed in clinical study.
Conclusion
In the present study, we further investigated the pharmacodynamics of EH using a rabbit model of arteriovenous bypass thrombosis and a rat model of thrombotic cerebral infarction, and the results demonstrated that EH dose-dependently inhibited thrombus formation in both models and showed a trend of less bleeding than hirudin and LMWH.
Abbreviations
EH, EPR-hirudin, recombinant neorudin; FXIa, coagulation factor xia; FXa, coagulation factor xa; LMWH, low molecular weight heparin; HIT, heparin-induced thrombocytopenia; PCI, percutaneous coronary interventions; DOACs, direct oral anticoagulants; EMEA, European Medical Evaluation Agency; FDA, Food and Drug Administration; TT, thrombin time; PT, prothrombin time; APTT, activated partial thromboplastin time; FG, fibrinogen; FHB, micro-free hemoglobin; MCAO, middle cerebral artery occlusion; TTC, triphenyltetrazole chloride; ATIII, antithrombin III.
Funding
This study was partially supported by a grant from the Beijing Science and Technology Plan (No. Z181100002218004).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Germain M, Chasman DI, de Haan H, et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet. 2015;96(4):532–542. doi:10.1016/j.ajhg.2015.01.019
2. Weitz JI, Wood AJJ. Low-molecular-weight heparins. N Engl J Med. 1997;337(10):688–698. doi:10.1056/NEJM199709043371007
3. Pineo GF, Hull RD. Heparin and low-molecular-weight heparin in the treatment of venous thromboembolism. Baillieres Clin Haematol. 1998;11(3):621–637. doi:10.1016/s0950-3536(98)80086-3
4. Nafee T, Aslam A, Chi G, et al. Andexanet alfa for the reversal of anticoagulant activity in patients treated with direct and indirect factor Xa inhibitors. Expert Rev Cardiovasc Ther. 2017;15(4):237–245. doi:10.1080/14779072.2017.1305889
5. Amato B, Compagna R, Rocca A, et al. Fondaparinux vs warfarin for the treatment of unsuspected pulmonary embolism in cancer patients. Drug Des Devel Ther. 2016;10:2041–2046. doi:10.2147/DDDT.S106153
6. Greinacher A, Lubenow N. Recombinant hirudin in clinical practice: focus on lepirudin. Circulation. 2001;103(10):1479–1484. doi:10.1161/01.cir.103.10.1479
7. Markwardt F. The development of hirudin as an antithrombotic drug. Thromb Res. 1994;74(1):1–23. doi:10.1016/0049-3848(94)90032-9
8. Zhang J, Lan N. Hirudin variants production by genetic engineered microbial factory. Biotechnol Genet Eng Rev. 2018;34(2):261–280. doi:10.1080/02648725.2018.1506898
9. Fox KA. r-Hirudin in unstable angina pectoris. Rationale and preliminary data from the APT pilot study. Eur Heart J. 1995;16(Suppl D):28–32. doi:10.1093/eurheartj/16.suppl_d.28
10. Klingele M, Enkel J, Speer T, Bomberg H, Baerens L, Schäfers HJ. Bleeding complications after cardiac surgery, before anticoagulation start and then with argatroban or heparin in the early postoperative setting. J Cardiothorac Surg. 2020;15(1):27. doi:10.1186/s13019-020-1059-8
11. Mahat KC, Sedhai YR, Krishnan P. Argatroban. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. PMID: 32310431.
12. Ajmal M, Friedman J, Sipra QUAR, Lassar T, Garcia V. Rivaroxaban: expanded role in cardiovascular disease management-a literature review. Cardiovasc Ther. 2021;2021:8886210. doi:10.1155/2021/8886210
13. Fitchett DH. Potential role of rivaroxaban in patients with acute coronary syndrome. Drug Des Devel Ther. 2012;6:349–357. doi:10.2147/DDDT.S30342
14. Wu Z, Guo Y, Liu Y, et al. Production technology of recombinant neorudin expressed in E. coli. Beijing Univ Technol. 2016;42:302–308.
15. Wu Z, Yu A, Zhang C, et al. Preparation of low bleeding anticoagulant fusion protein and its use. US Patent 20100113345A1. PCT No. PCT/CN2007/ 03526. WO 2008/071081 A1. Filed December 11, 2007; issued Jan 24, 2012.
16. Zhang C, Yu A, Yuan B, et al. Construction and functional evaluation of hirudin derivatives with low bleeding risk. Thromb Haemost. 2008;99(2):324–330. doi:10.1160/TH07-07-0453
17. Sheffield WP, Eltringham-Smith LJ, Bhakta V. A factor XIa-activatable hirudin-albumin fusion protein reduces thrombosis in mice without promoting blood loss. BMC Biotechnol. 2018;18(1):21. doi:10.1186/s12896-018-0431-4
18. Dong X, Meng Z, Gu R, et al. Predicting the metabolic characteristics of neorudin, a novel anticoagulant fusion protein, in patients with deep vein thrombosis. Thromb Res. 2020;194:121–134. doi:10.1016/j.thromres.2020.05.048
19. Fareed J, Walenga JM, Iyer L, Hoppensteadt D, Pifarre R. An objective perspective on recombinant hirudin: a new anticoagulant and antithrombotic agent. Blood Coagul Fibrinolysis. 1991;2(1):135–147. doi:10.1097/00001721-199102000-00021
20. Sachs UJ, Nieswandt B. In vivo thrombus formation in murine models. Circ Res. 2007;100(7):979–991. doi:10.1161/01.RES.0000261936.85776.5f
21. Pierangeli SS, Liu XW, Barker JH, Anderson G, Harris EN. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thromb Haemost. 1995;74(5):1361–1367. PMID: 8607123. doi:10.1055/s-0038-1649940
22. Rosen ED, Raymond S, Zollman A, et al. Laser-induced noninvasive vascular injury models in mice generate platelet- and coagulation-dependent thrombi. Am J Pathol. 2001;158(5):1613–1622. doi:10.1016/S0002-9440(10)64117-X
23. Kikuchi S, Umemura K, Kondo K, Saniabadi AR, Nakashima M. Photochemically induced endothelial injury in the mouse as a screening model for inhibitors of vascular intimal thickening. Arterioscler Thromb Vasc Biol. 1998;18(7):1069–1078. doi:10.1161/01.atv.18.7.1069
24. Huttinger AL, Wheeler DG, Gnyawali S, et al. Ferric chloride-induced canine carotid artery thrombosis: a large animal model of vascular injury. J Vis Exp. 2018;(139):57981. doi:10.3791/57981
25. Wong AG, Gunn AC, Ku P, Hollenbach SJ, Sinha U. Relative efficacy of active site-blocked factors IXa, Xa in models of rabbit venous and arterio-venous thrombosis. Thromb Haemost. 1997;77(6):1143–1147. [PMID: 9241747]. doi:10.1055/s-0038-1656127
26. Valentin JP, Vieu S, Bertolino F, Fauré P, John GW. Differential involvement of serotonin 2A/C and thromboxane A2/prostanoid receptors in high- vs. low-shear rate arterial thrombosis in rabbits. J Pharmacol Exp Ther. 1997;280(2):761–769. PMID: 9023289.
27. Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6(222):222ra17. doi:10.1126/scitranslmed.3006804
28. May F, Krupka J, Fries M, et al. FXIIa inhibitor rHA-Infestin-4: safe thromboprotection in experimental venous, arterial and foreign surface-induced thrombosis. Br J Haematol. 2016;173(5):769–778. doi:10.1111/bjh.13990
29. Wong PC, Quan ML, Crain EJ, Watson CA, Wexler RR, Knabb RM. Nonpeptide factor Xa inhibitors: i. Studies with SF303 and SK549, a new class of potent antithrombotics. J Pharmacol Exp Ther. 2000;292(1):351–357. PMID: 10604970.
30. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi:10.1161/01.str.20.1.84
31. Liu XG, Xu LN. [A rat middle cerebral artery thrombosis model for evaluation of thrombolytic and antithrombotic agents]. Yao Xue Xue Bao. 1995;30(9):662–667. Chinese. PMID: 8701741.
32. Liu D, Wang H, Zhang Y, Zhang Z. Protective effects of chlorogenic acid on cerebral ischemia/reperfusion injury rats by regulating oxidative stress-related Nrf2 pathway. Drug Des Devel Ther. 2020;14:51–60. doi:10.2147/DDDT.S228751
33. Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int Rev Cytol. 2002;221:93–148. doi:10.1016/s0074-7696(02)21011-6
34. Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol. 2013;26(2):191–202. doi:10.1016/j.beha.2013.07.001
35. Qin XY, Yu AP, Wang WW, et al. The antithrombin activity detection of EH in vitro. China Biotechnol. 2011;31(5):108–112. doi:10.13523/j.cb.20110518
36. Dong X, Gu R, Zhu X, et al. Evaluating prodrug characteristics of a novel anticoagulant fusion protein neorudin, a prodrug targeting release of hirudin variant 2-Lys47 at the thrombosis site, by means of in vitro pharmacokinetics. Eur J Pharm Sci. 2018;121:166–177. doi:10.1016/j.ejps.2018.05.025
37. Wang W, Xu X, Zhao Z, et al. Anti-thrombus activity of a novel anti-thrombus protein EPR- hirudin. Chin Pharm J. 2013;48(2):111–115.
38. Shinozawa E, Kawamura M. Anti-thrombotic effect of a factor Xa inhibitor TAK-442 in a rabbit model of arteriovenous shunt thrombosis stimulated with tissue factor. BMC Res Notes. 2018;11(1):776. doi:10.1186/s13104-018-3886-4
39. Karabiyikoglu M, Hua Y, Keep RF, Ennis SR, Xi G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cereb Blood Flow Metab. 2004;24(2):159–166. doi:10.1097/01.WCB.0000100062.36077.84
40. Wu W, Qiu C, Feng X, et al. Protective effect of paeoniflorin on acute cerebral infarction in rats. Curr Pharm Biotechnol. 2020;21(8):702–709. doi:10.2174/1389201021666191224151634
41. Barreto AD, Ford GA, Shen L, et al. Randomized, multicenter trial of ARTSS-2 (argatroban with recombinant tissue plasminogen activator for acute stroke). Stroke. 2017;48(6):1608–1616. doi:10.1161/STROKEAHA.117.016720
42. Sun Z, Lan X, Li S, Zhao H, Tang Z, Xi Y. Comparisons of argatroban to lepirudin and bivalirudin in the treatment of heparin-induced thrombocytopenia: a systematic review and meta-analysis. Int J Hematol. 2017;106(4):476–483. doi:10.1007/s12185-017-2271-8
43. Liu YX, Cao QM, Ma BC. Pathogens distribution and drug resistance in patients with acute cerebral infarction complicated with diabetes and nosocomial pulmonary infection. BMC Infect Dis. 2019;19(1):603. doi:10.1186/s12879-019-4142-9
44. Liang Y, Wu J, Liu J, Liu H, Chen J. The clinical implications of thrombelastography in the diagnosis of acute cerebral infarction. Clin Lab. 2018;64(1):147–152. doi:10.7754/Clin.Lab.2017.170803
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.