Back to Journals » OncoTargets and Therapy » Volume 11
The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells
Authors Jiang H , Fan JJ, Cheng L, Hu P , Liu RB
Received 1 August 2018
Accepted for publication 3 October 2018
Published 14 November 2018 Volume 2018:11 Pages 8153—8163
DOI https://doi.org/10.2147/OTT.S182239
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Hua Jiang,1,* Jingjing Fan,2,* Lin Cheng,1 Pan Hu,1 Renbin Liu1
1Department of Breast and Thyroid Surgery, Breast Cancer Center, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong 510630, People’s Republic of China; 2Department of Breast and Neck Surgery, Xinjiang Medical University Affiliated Tumor Hospital, Urumqi, Xinjiang 830011, People’s Republic of China
*These authors contributed equally to this work
Background: Most breast cancers are estrogen dependent and were sensitive to endocrine therapy, and genistein (GEN) shows strong affinity with human oestrogen receptor beta (ERβ).
Purpose: The present study aimed to investigate the anticancer activity of GEN in breast cancer cell lines that constitutively expressing ERβ1 in vitro and in vivo.
Methods: MCF-7/ERβ1 and MDA-MB-231/ERβ1 cell sub-lines were established through lentiviral infection. Then, cells were treated with increasing concentrations of GEN (10-6 mol/l, 10-5 mol/l and 10-4 mol/l) for 48 h, and cell proliferation, cell cycle analyses were performed to investigate different biological characteristics of ERβ1-overexpressing cell lines. Studies in vivo were also performed to investigate the effects of dietary GEN on MCF-7/ERβ1 and MDA-MB-231/ERβ1 cells implanted mice.
Results: Results showed that compared to parental cells, GEN inhibited the proliferation ability of MCF-7/ERβ1 cells to a greater extent, especially at high concentrations. MDA-MB-231 cells were also inhibited by high doses of GEN, but the overexpressed ERβ1 did not enhance the anti-proliferative effect on MDA-MB-231 cells. ERβ1 arrested cells in G2/M phase, and GEN arrested cells in G0/G1, which led to a combinatorial effect on cell cycle blockade. Furthermore, ERβ1 increased the anti-tumour activity of dietary GEN in MCF-7/ERβ1 subcutaneous tumour models. Our data indicated that ERβ1 increased the anticancer efficacy of GEN in MCF-7 cells by affecting cell cycle transition.
Conclusion: As a result, GEN could be a potential therapeutic agent for ERβ1-positive cancer.
Keywords: breast cancer, estrogen receptor beta 1, genistein, MCF-7 cells, MDA-MB-231 cells, estrogen receptor alpha
Introduction
Breast cancer is one of the most frequently diagnosed malignant diseases in women. In spite of the achievements made in the past decades, breast cancer remains a major public health problem. The US National Cancer Institute has reported that almost one in eight American women will develop breast cancer during their lifetime.1,2 The increased incidence of breast cancer has been observed in recent years, possibly due to changes in diet and the environment. Most breast cancer cases (~70%) are estrogen dependent, which were sensitive to endocrine therapy.
Estrogen receptors alpha and beta (ERα and ERβ), two major estrogen receptors (ERs), are encoded by separate genes and have differential effects on breast tissues: ERα improves the growth and proliferation of cancer cells, whereas ERβ inhibits proliferation, differentiation, and promotes apoptosis. In most clinical trials, ERβ expression is correlated with small tumor size, node negativity, low histological grade, and increased disease-free survival (DFS) and overall survival (OS) in breast cancer.3–5 Because of the drug resistance and severe side effects of chemotherapy, it is urgent to explore effective antitumor drugs for the treatment of breast cancer.
Several epidemiological studies strongly support the relatively low incidence and recurrence rate of breast cancer in Asian populations, who consume a diet high in soy products.6–9 Based on this assumption, several studies have investigated the anticancer activities of isoflavones. Genistein (GEN), one of the most studied isoflavones enriched in soy products, was confirmed to be a potential treatment option against specific types of breast tumors. However, the mechanisms are still unclear.10–12 Previous studies have confirmed that GEN can bind to both α and β subtypes of ERs. Interestingly, GEN shows 9–10-fold increased affinity for ERβ, which counteracts the proliferative activity of ERα.13 However, there have been very few reports regarding the effect of GEN or the related mechanism on ERβ1-positive breast cancer cells.
Therefore, we hypothesized that upregulation of ERβ1 could promote the effectiveness of GEN in inhibiting breast cancer proliferation.
Materials and methods
Cell culture and reagents
The human breast cancer MCF-7 and MDA-MB-231 cell lines were purchased from State Key Laboratory of Oncology in Southern China (Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China). Cells were cultured in DMEM containing glucose (4.5 g/L; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific) at 37°C in 5% CO2.
GEN and 17β-estradiol (E2) were obtained from Sigma-Aldrich (St Louis, MO, USA). The selective ERβ agonist diaryl propionitrile (DPN) and the selective ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]-pyrimidin-3-yl]phenol (PHTPP) were purchased from Tocris Bioscience (Bristol, UK).
Establishment of ERβ-positive cell lines
We established breast cancer cells with stable expression of human ERβ1 using the Lentiviral-Packaging HIV Expression System (GeneCopoeia, Rockville, MD, USA). Plasmids containing human ERβ1 and control plasmid only containing enhanced green fluorescence protein (eGFP) were provided by GeneCopoeia, and both of them encode eGFP and puromycin (Puro) reporter proteins (containing a CMV-eGFP-Puro fragment). The lentiviral transfer vectors were cotransfected into 293 T cells (GeneCopoeia) to obtain lentivirus containing ERβ1, and the titer of the virus was determined. The lentivirus particles were purified and stored at −80°C.
The constructed lentivirus containing ERβ1 was applied to infect parental MCF-7 and MDA-MB-231 cells at an MOI of 20. The cells were incubated at 37°C, with 5% CO2 for 24 hours, and then, stable cell lines were selected by treatment with 0.5 μg/mL Puro for 1 week. The expression of the eGFP reporter was confirmed with a fluorescence microscope after infection.
Real-time quantitative PCR
The MCF-7/ERβ1 cells, negative control cells (MCF-7/eGFP), and parental cells (MCF-7) were seeded in 6-well plates. When cells achieved 100% confluence, total RNA was isolated with TRIzol (KeyGen Biotech, Nanjing, People’s Republic of China) and was reverse transcribed using an iScript kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. MDA-MB-231 cells were dealt with in the same way. The sequences of primers for ERβ1 and ERα were obtained from the published literature (Table 1).14 PCR reactions were performed using an ABI Prism 7700 Sequence Detection System (Perkin-Elmer Applied Biosystems). The analysis was carried out in three replicates.
  | Table 1 Primers for ERβ1 and ERα |
Western blot analyses
Cells were seeded in 6-well plates and treated with reagent or vehicle for 48 hours. Then the proteins were extracted using KeyGen Whole Cell Lysis Assays (KeyGen Biotech). Western blotting was performed using standard procedures with antibodies to ERα and ERβ1 (1:1,000 diluted; Abcam, Cambridge, MA, USA) or antibodies to cyclin D1, p21, and β-actin (1:1,000 diluted; Cell Signaling Technology, Danvers, MA, USA). The secondary antibody was HRP-goat anti-rabbit IgG (1:5,000 diluted; Thermo Fisher Scientific). Proteins were detected using ECL kits (Amersham Life Science, Arlington Heights, IL, USA). The analysis was carried out in three replicates.
Cell proliferation assay
ERβ1-positive cells, negative control cells, and parental cells were seeded in 96-well plates (2.0×103 cells/well). Complete medium containing ligands was added the following day, and the control group was treated with an equal volume of 0.1% ethanol. For the treatment group, the dose of GEN was increased exponentially (10−6 mol/L, 10−5 mol/L, and 10−4 mol/L). After 48 hours, cell proliferation was measured using a Cell Counting Kit-8 assay (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. The analysis was carried out in three replicates.
Flow cytometry
MCF-7 and MDA-MB-231 cells were seeded in a 6-well plate (5.0×105 cells/well) and cultured with increasing concentrations of GEN (10−5 mol/L and 10−4 mol/L) or vehicle (0.1% ethanol). After 48 hours, each group of cells was harvested and washed with PBS. After centrifugation, the sedimented cells were resuspended and fixed in 70% ethanol overnight. A cell cycle detection kit (KeyGen Biotech) was used to analyze the cell cycle phase distribution. The cells were centrifuged and resuspended in a cell cycle mix of PBS, 400 μL of PI (50 mg/mL), and 20 μL of Rnase (10 mg/mL). Cell cycle distribution was analyzed with flow cytometry software (BD LSR II; BD Biosciences, San Jose, CA, USA). The analysis was carried out in three replicates.
In vivo experiments
Female 4-week-old athymic nude mice (BALB/c) were purchased from Vital Rival Laboratories (Beijing, People’s Republic of China). Mice were acclimated for 7 days and then were ovariectomized. The animals were maintained in a climate-controlled room and provided food and water ad libitum according to the animal experimental guideline set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental operations performed on mice were approved by the Institutional Animal Care and Use Committee of The Third Affiliated Hospital of Sun Yat-sen University.
The mice were then randomly assigned to four groups (n=10): MCF-7/ERβ1 group, MCF-7/eGFP group, MDA-MB-231/ERβ1 group, and MDA-MB-231/eGFP group. The MCF-7 cells (1×107/site) or MDA-MB-231 cells (1×106/site) were injected into the left flank on the back of each mice. In the MCF-7 cell group, a sustained release pellet (obtained from Innovative Research of America) containing 0.36 mg of estrogen was implanted in the mice. Once the tumor volume reached 40 mm3, mice were divided into three treatment subgroups: control; 100 ppm (parts per million) GEN; and 1,000 ppm GEN. It is the same in MDA-MB-231 cell group. AIN-93G (American Institute of Nutrition 93 growth, Dyets Inc.) diet was selected as the basic diet for control mice; mice in the GEN treatment groups were fed with basic diet containing GEN that was reprocessed by Guangdong Medical Laboratory Animal Center (Foshan, People’s Republic of China).
Tumor growth and body weight were monitored every 3 days, and tumor size was calculated using the following formula: tumor size=width2×length/2. Observation was terminated on the 30th day after treatment or when the tumor size reached 1.5 cm3 or if the mouse was visibly in pain or unable to ambulate. Then, they were sacrificed in a CO2 chamber, and all tumors were removed. H&E staining and immunohistochemical staining (Histostain-plus kit; ZSGB-BIO, Beijing, People’s Republic of China) with ERα and ERβ1 antibodies were performed. For ERα and ERβ1 analyses, fields in five nonnecrotic areas of each section were randomly selected and examined using light microscopy at 200-fold magnification.
Statistical analyses
All statistical analyses were performed using SPSS 22.0 software (IBM, Armonk, NY, USA). Experimental data are presented as the mean±SE. A paired-sample t-test or one-way ANOVA was used to evaluate the difference between groups. P<0.05 was considered statistically significant.
Results
Construction of MCF-7 and MDA-MB-231 cell lines with increased expression of ERβ1
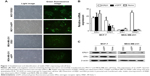
To study the possible roles that ERβ1 may play in breast cancer proliferation, we established breast cancer cell lines that constitutively express human ERβ1 through lentiviral infection. The expression of an eGFP reporter was confirmed using a fluorescence microscope. Strong green fluorescence was observed in the transfected cells, and more than 90% of the cells were eGFP-positive (Figure 1A).
ERβ1 and ERα RNA expression levels of parental and transfected cells were detected using real-time PCR. Stable overexpression of ERβ1 was detected in MCF-7/ERβ1 and MDA-MB-231/ERβ1 cells. MCF-7 was considered as high ERα/ERβ ratio cell line and MDA-MB-231 as ERα-negative, and the ERβ level in MDA-MB-231 was low. The levels of ERα were consistent with the previous research ignoring the PCR background in Figure 1B.15 The mRNA levels of ERβ1 in MCF-7/ERβ1 and MDA-MB-231/ERβ1 cells were increased by 3.3 and 12.9 times, respectively, compared with parental cells (Figure 1B).
Western blot analyses also revealed that the expression of ERβ1 was significantly increased in MCF-7/ERβ1 and MDA-MB-231/ERβ1 cells compared to MCF-7/eGFP and MDA-MB-231/eGFP cells or parental cells, which was consistent with the results shown in Figure 1B and C. In summary, we successfully created subcell lines of MCF-7 and MDA-MB-231 cells that stably overexpress ERβ1.
Ligand-induced effects on ERβ1
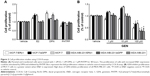
To investigate whether the ERβ1 stably expressed in MCF-7/ERβ1 and MDA-MB-231/ERβ1 cells was functional, these cells and control cells of both cell lines were treated with 1 nM E2 (an ERβ-selective antagonist), 1 nM DPN (an ERβ-selective agonist), and 1 μM PHTPP (an ERβ-selective antagonist), respectively. We noted that the proliferation of cells with increased ERβ1 expression could be stimulated by DPN and inhibited by PHTPP. However, E2 only inhibited the proliferation of MCF-7/ERβ1 cells and had no influence on MDA-MB-231/ERβ1 cells, which may be the reason of different activity or affinity in both the cell lines. (Figure 2A). Overall, these data indicated that ERβ1 in transfected cells was functionally responsive to ligand.
ERβ1-overexpressing cell lines were more sensitive to GEN
We treated MCF-7/ERβ1, MDA-MB-231/ERβ1, negative control, and parental cells with increasing concentrations of GEN, and cell proliferation was analyzed after 48 hours. A high dose of GEN inhibited the proliferation of all sublines of MDA-MB-231 and MCF-7 cells (Figure 2B). Significant inhibition of cell proliferation was also observed in MCF-7/ERβ1 cells even at low concentration. Besides, a high concentration of GEN could suppress proliferation of MCF-7 cells to a greater extent when ERβ1 was overexpressed (Figure 2B). Thus, overexpressed ERβ1 increased the antiproliferative activity of GEN.
Underlying mechanism of antiproliferative activity of GEN
To explore the mechanism whereby ERβ1 enhances the antiproliferative activity of GEN, we analyzed the cell cycle distribution and relative protein expression. Increased cell cycle arrest in G2/M phase was observed in MDA-MB-231/ERβ1 cells (Figure 3A). As expected, a high dose of GEN arrested MDA-MB-231 cells in G0/G1, which increased the percentage of cells in G0/G1 compared with cells of the vehicle group (Figure 3A). Similar effects were observed in MCF-7 cell lines, and a high dose of GEN arrested MCF-7 cells in G0/G1 (Figure 3B). Thus, the combinatorial effect of GEN and overexpressed ERβ1 resulted in an active blockade of cell cycle progression and a dramatic inhibition of proliferation.
Western blot analyses confirmed that GEN downregulated the expression of cyclin D1 in both ERβ1-expressing and parental cells (Figure 4). In addition, cyclin D1 expression was further downregulated when ERβ1 was overexpressed. p21, also known as cyclin-dependent kinase inhibitor 1A (CDKN1A), has been shown to be upregulated when ERβ is expressed,16 but this was not observed in our model system. However, Western blot analyses showed that GEN could decrease the expression of cyclin D1 and increase the expression of p21, in accordance with results of previous reports.17,18
Effect of dietary GEN in vivo
The animal models of MCF-7/ERβ1 and MCF-7/eGFP were successfully established using female 5-week-old nude ovariectomized BALB/c mice. We could observe the tumor size under different treatment of GEN in mice before sacrifice (Figure 5A and B). The growth curves of MCF-7/eGFP tumors revealed that GEN suppressed tumor growth remarkably in a dose-dependent and time-dependent manner (Figure 5C). After observation for 30 days, the average tumor sizes of the 1,000 ppm GEN group were significantly smaller than either the 100 ppm GEN group or the vehicle group (P<0.05). In addition, we observed that GEN was more effective in ERβ1-overexpressing cell models. There was a statistical difference in tumor size between the MCF-7/ERβ1 group and the MCF-7/eGFP group. The median tumor volume of the 1,000 ppm GEN group without ERβ1 expression was 650.4±91.21 mm3, whereas in the ERβ1 expression group, it was 368.9±73.96 mm3 (P<0.001).
We found that 1,000 ppm GEN suppressed the tumor growth of both the MDA-MB-231/ERβ1 and the MDA-MB-231/eGFP groups (P<0.001, compared to the vehicle group; Figure 5D). However, a statistically significant difference was not observed between the high ERβ1 expression and low ERβ1 expression groups. None of the mice died during the observation time. Immunohistochemical staining revealed that the expression of ERβ1 was significantly increased in MCF-7/ERβ1 and MDA-MB-231/ERβ1 groups, while there was no obvious difference of the expression of ERα between each group (Figure 6).
Discussion
To investigate the interaction between ERβ1 and GEN, we upregulated the expression of ERβ1 in MCF-7 and MDA-MB-231 cells. We demonstrated that GEN was more effective in ERβ1-overexpressing cell lines. Our data indicated that ERβ1 and GEN could cooperate in their antiproliferative effect by blocking cell cycle transition.
Isoflavones are abundant in soy-containing foods that are consumed daily by Asian populations, and they have weak estrogen-like activity.6–9 As breast cancer survival improves, whether to continue the consumption of soybean foods has become a question of concern for patients with breast cancer. GEN is one of the most important isoflavones, and it has both agonistic and antagonistic effects on ERs.19,20 Previous studies21 have revealed that GEN has a biphasic effect on MCF-7 cell proliferation, inhibiting breast cancer cell growth at high concentrations and stimulating proliferation at low concentrations. In our study, GEN inhibited MCF-7 cell growth in a dose-dependent manner. In addition, we observed that GEN did not have a promotive effect on MDA-MB-231/ERβ1 cell growth at low concentrations and inhibited MDA-MB-231/ERβ1 cell proliferation at high concentrations. It is well known that phytoestrogen activity is mediated by the dimerization of ERβ with ERα.21 Therefore, we presume that the action of GEN varies with the ratio of ERα and ERβ, consistent with Pons et al studies.22,23 Furthermore, we also observed that oral GEN significantly suppressed the tumor growth of mouse xenograft models in vivo, which is inconsistent with results reported by Du et al,24 and a possible reason may be the higher concentration of GEN used in this study.
Since the report of full-length human ERβ (also named ERβ1) by Kuiper et al in 1996,25 at least five ERβ splice variants have been discovered. ERβ1 and ERβ2 (also known as ERβcx) are the best characterized isoforms in breast tissue.26,27 Although ERα has been well established as an important mediator of proliferation in breast cancer, the role of ERβ remains controversial. Hartman et al28 found that ERβ-positive breast cancer had a higher histological grade than ERβ-negative breast cancer. Another study reported that compared with single ERα-positive breast cancer patients, the prognosis of ERβ-positive patients is poor.29 These studies apparently suggested that ERβ is a poor prognosis factor for breast cancer patients. However, most of these studies were performed with nonspecific antibodies that detect all ERβ isoforms, including ERβ2. The conclusions of recent studies using specific ERβ antibodies have been completely different. Rosin et al30 found that ERβ1 was negatively correlated with higher grade and stage of breast tumors. Similarly, Huang et al31 found that ERβ1-positivity in ERα-positive patients was associated with smaller tumor size and longer DFS, whereas ERβ2 expression was associated with shorter DFS. In our study, we analyzed ERβ expression with a well-validated C-terminus-targeted monoclonal antibody specific for ERβ1. We observed that ERβ1 could arrest cells in G2/M phase and enhance the antiproliferative effect of GEN in vitro and in vivo.
The MDA-MB-231 cell line is a representative TNBC without ERα expression. It has been reported that low doses (<10 μM) of GEN may stimulate ERα-positive cell growth, but there is no need to worry about the proliferative effects on TNBC cells.32 In this study, we observed the same phenomenon: GEN did not have a promotive effect on MDA-MB-231 cells at low concentration, whereas it intensely inhibited proliferation at a high dose, which indicated that antiproliferative effect of ERβ1 was mediated by ERα-induced transcription of downstream targets.
In this study, we tried to explore a possible mechanism to explain how ERβ1 and GEN cooperate with each other to inhibit cell proliferation. Previous studies have reported that isoflavones can inhibit cell cycle transition in breast cancer cells.17 Based on this hypothesis, we analyzed the cell cycle distribution. Potential mechanisms may involve cooperativity between GEN and ERβ1 in blocking cell cycle progression.
In this study, we confirmed that ERβ1 expression increased the anticancer efficacy of GEN in MCF-7 cells in vitro and in vivo, suggesting that GEN could be a potential therapeutic agent for ERβ1-positive cancer, which merits further clinical research in the future.
Acknowledgments
We thank GeneCopoeia company for providing the ERβ1-Lv201 plasmid. We also thank American Journal Experts for the English language editing service.
Author contributions
Conceived and designed the experiments: RL, HJ. Performed the experiments: HJ, JF. Analyzed the data: LC, PH. Wrote the paper: All authors. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Jonsson P, Katchy A, Williams C. Support of a bi-faceted role of estrogen receptor β (ERβ) in ERα-positive breast cancer cells. Endocr Relat Cancer. 2014;21(2):143–160. | ||
Guo L, Zhu Q, Yilamu D, Jakulin A, Liu S, Liang T. Expression and prognostic value of estrogen receptor beta in breast cancer patients. Int J Clin Exp Med. 2014;7(10):3730–3736. | ||
Rizza P, Barone I, Zito D, et al. Estrogen receptor beta as a novel target of androgen receptor action in breast cancer cell lines. Breast Cancer Res. 2014;16(1):R21. | ||
Kucuk O. Soy foods, isoflavones, and breast cancer. Cancer. 2017;123(11):1901–1903. | ||
Maskarinec G, Ju D, Morimoto Y, Franke AA, Stanczyk FZ. Soy food intake and biomarkers of breast cancer risk: possible difference in Asian women? Nutr Cancer. 2017;69(1):146–153. | ||
Farina HG, Pomies M, Alonso DF, Gomez DE. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol Rep. 2006;16(4):885–891. | ||
Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125(2):315–323. | ||
Pan H, Zhou W, He W, et al. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int J Mol Med. 2012;30(2):337–343. | ||
Bilal I, Chowdhury A, Davidson J, Whitehead S. Phytoestrogens and prevention of breast cancer: the contentious debate. World J Clin Oncol. 2014;5(4):705–712. | ||
Fellegara G, Basciu M, Pagni F, Oriana S. Phytoestrogens and breast carcinoma: a word of caution. Eur J Cancer Prev. 2014;23(5):491–492. | ||
Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. | ||
Horne AW, King AE, Shaw E, et al. Attenuated sex steroid receptor expression in fallopian tube of women with ectopic pregnancy. J Clin Endocrinol Metab. 2009;94(12):5146–5154. | ||
Pons DG, Nadal-Serrano M, Blanquer-Rossello MM, Sastre-Serra J, Oliver J, Roca P. Genistein modulates proliferation and mitochondrial functionality in breast cancer cell depending on ERalpha/ERbeta ratio. J Cell Biochem. 2014;115:9. | ||
Hodges-Gallagher L, Valentine CD, El Bader S, Kushner PJ. Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res Treat. 2008;109(2):241–250. | ||
Jiang X, Patterson NM, Ling Y, Xie J, Helferich WG, Shapiro DJ. Low concentrations of the soy phytoestrogen genistein induce proteinase inhibitor 9 and block killing of breast cancer cells by immune cells. Endocrinology. 2008;149(11):5366–5373. 18.Fan P, Fan S, Wang H, Mao J. Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res Ther. 2013;4(6):10. | ||
Fritz H, Seely D, Flower G, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013;8(11):e81968. | ||
Kwon Y. Effect of soy isoflavones on the growth of human breast tumors: findings from preclinical studies. Food Sci Nutr. 2014;2(6):613–622. | ||
Choi EJ, Kim GH. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol Med Rep. 2013;7(3):781–784. | ||
Pons DG, Nadal-Serrano M, Blanquer-Rossello MM, Sastre-Serra J, Oliver J, Roca P. Genistein modulates proliferation and mitochondrial functionality in breast cancer cells depending on ERalpha/ERbeta ratio. J Cell Biochem. 2014;115(5):949–958. | ||
Pons DG, Nadal-Serrano M, Torrens-Mas M, Oliver J, Roca P. The phytoestrogen genistein affects breast cancer cells treatment depending on the ERα/ERβ ratio. J Cell Biochem. 2016;117(1):218–229. | ||
du M, Yang X, Hartman JA, et al. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis. 2012;33(4):895–901. | ||
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. | ||
Shanle EK, Onitilo AA, Huang W, et al. Prognostic significance of full-length estrogen receptor beta expression in stage I-III triple negative breast cancer. Am J Transl Res. 2015;7(7):1246–1259. | ||
Song W, Tang L, Xu Y, Sun Q, Yang F, Guan X. ERβ1 inhibits metastasis of androgen receptor-positive triple-negative breast cancer by suppressing ZEB1. J Exp Clin Cancer Res. 2017;36(1):75. | ||
Hartman J, Ström A, Gustafsson JA. Estrogen receptor beta in breast cancer – diagnostic and therapeutic implications. Steroids. 2009;74(8):635–641. | ||
Dhimolea E, Tiniakos DG, Chantzi NI, et al. Estrogen receptors β1 and β2 are associated with distinct responses of estrogen receptor α-positive breast carcinoma to adjuvant endocrine therapy. Cancer Lett. 2015;358(1):37–42. | ||
Rosin G, de Boniface J, Karthik GM, Frisell J, Bergh J, Hartman J. Oestrogen receptors β1 and βcx have divergent roles in breast cancer survival and lymph node metastasis. Br J Cancer. 2014;111(5):918–926. | ||
Huang B, Omoto Y, Iwase H, et al. Differential expression of estrogen receptor α, β1, and β2 in lobular and ductal breast cancer. Proc Natl Acad Sci U S A. 2014;111(5):1933–1938. | ||
Hamilton N, Márquez-Garbán D, Mah V, et al. Biologic roles of estrogen receptor-β and insulin-like growth factor-2 in triple-negative breast cancer. Biomed Res Int. 2015;2015:925703–925715. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.