Back to Journals » International Journal of Nanomedicine » Volume 15
The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry
Authors Yin IX , Zhang J, Zhao IS , Mei ML , Li Q, Chu CH
Received 21 January 2020
Accepted for publication 29 March 2020
Published 17 April 2020 Volume 2020:15 Pages 2555—2562
DOI https://doi.org/10.2147/IJN.S246764
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Iris Xiaoxue Yin,1,2 Jing Zhang,3 Irene Shuping Zhao,1 May Lei Mei,4 Quanli Li,3 Chun Hung Chu2
1School of Dentistry, Shenzhen University Health Science Center, Shenzhen, People’s Republic of China; 2Faculty of Dentistry, The University of Hong Kong, Hong Kong; 3College of Stomatology, Anhui Medical University, Hefei, People’s Republic of China; 4Faculty of Dentistry, University of Otago, Otago, New Zealand
Correspondence: Irene Shuping Zhao
School of Dentistry, Shenzhen University Health Science Center, Shenzhen, People’s Republic of China
Tel +86 18823781964
Email [email protected]
Chun Hung Chu
Faculty of Dentistry, The University of Hong Kong, Hong Kong SAR, People’s Republic of China
Tel +852 2859 0287
Fax +852 2858 2532
, Email [email protected]
Abstract: Nanotechnology has recently emerged as a rapidly growing field with numerous biomedical science applications. At the same time, silver has been adopted as an antimicrobial material and disinfectant that is relatively free of adverse effects. Silver nanoparticles possess a broad spectrum of antibacterial, antifungal and antiviral properties. Silver nanoparticles have the ability to penetrate bacterial cell walls, changing the structure of cell membranes and even resulting in cell death. Their efficacy is due not only to their nanoscale size but also to their large ratio of surface area to volume. They can increase the permeability of cell membranes, produce reactive oxygen species, and interrupt replication of deoxyribonucleic acid by releasing silver ions. Researchers have studied silver nanoparticles as antimicrobial agents in dentistry. For instance, silver nanoparticles can be incorporated into acrylic resins for fabrication of removable dentures in prosthetic treatment, composite resin in restorative treatment, irrigating solution and obturation material in endodontic treatment, adhesive materials in orthodontic treatment, membrane for guided tissue regeneration in periodontal treatment, and titanium coating in dental implant treatment. Although not all authorities have acknowledged the safety of silver nanoparticles, no systemic toxicity of ingested silver nanoparticles has been reported. A broad concern is their potential hazard if they are released into the environment. However, the interaction of nanoparticles with toxic materials and organic compounds can either increase or reduce their toxicity. This paper provides an overview of the antibacterial use of silver nanoparticles in dentistry, highlighting their antibacterial mechanism, potential applications and safety in clinical treatment.
Keywords: antibacterial, dentistry, nanoparticles, nanotechnology, silver
Introduction
Nanotechnology is defined as the design, characterization and application of structures, devices and systems by controlling shape and size at a nanometer scale (1 nm to 100 nm).1 It is an emerging field of research, with various applications in science and technology, particularly for developing new materials. Nanoparticles are developed with unique properties that make them desirable in materials science and biology.1 Among various nanoparticles, silver nanoparticles have been one of the most popular objects of study in recent decades.2 Silver nanoparticles contain 20 to 15,000 silver atoms, and their diameters are usually smaller than 100 nm. Due to a large surface-to-volume ratio, silver nanoparticles exhibit remarkable antimicrobial activity, even at a low concentration.3 In addition, they are low cost and have shown low cytotoxicity and immunological response.4 Therefore, silver nanoparticles have multiple potential biomedical applications. They are used for drug delivery, medical imaging and molecular diagnostics.5 They are also used in therapeutics, such as surgical mesh, fabrication of artificial joint replacements, wound dressing and medicament for promotion of wound healing.6
In dentistry, silver nanoparticles are used to develop antibacterial materials to improve the quality of the dental appliance for a better treatment outcome. They can be incorporated to acrylic resins for fabrication of removable dentures in prosthetic treatment, composite resin for direct restoration in restorative treatment, irrigating solution and obturation material in endodontic treatment, adhesive materials in orthodontic treatment, membrane for guided tissue regeneration in periodontal treatment, and titanium coating in dental implant treatment.7 This study provides an overview of the antibacterial use of silver nanoparticles in dentistry, highlighting their antimicrobial mechanism, applications and safety in clinical treatment.
Antibacterial Mechanism of Silver Nanoparticles
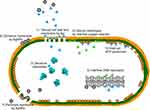
Although the exact mechanism of silver nanoparticles’ antibacterial effects has not been entirely clarified, various antibacterial actions have been proposed in Figure 1. Silver nanoparticles can continually release silver ions, which may be considered the mechanism of killing microbes.7 Owing to electrostatic attraction and affinity to sulfur proteins, silver ions can adhere to the cell wall and cytoplasmic membrane. The adhered ions can enhance the permeability of the cytoplasmic membrane and lead to disruption of the bacterial envelope.8 After the uptake of free silver ions into cells, respiratory enzymes can be deactivated, generating reactive oxygen species but interrupting adenosine triphosphate production.9 Reactive oxygen species can be a principal agent in the provocation of cell membrane disruption and deoxyribonucleic acid (DNA) modification. As sulfur and phosphorus are important components of DNA, the interaction of silver ions with the sulfur and phosphorus of DNA can cause problems in DNA replication, cell reproduction, or even result in termination of the microorganisms. Moreover, silver ions can inhibit the synthesis of proteins by denaturing ribosomes in the cytoplasm.10
In addition to being able to release silver ions, silver nanoparticles can themselves kill bacteria. Silver nanoparticles can accumulate in the pits that form on the cell wall after they anchor to the cell surface.11 The accumulated silver nanoparticles can cause cell membrane denaturation. Silver nanoparticles also have the ability to penetrate bacterial cell walls and subsequently change the structure of the cell membrane, because of their nanoscale size.11 The denaturation of cytoplasmic membrane can rupture organelles, and even result in cell lysis. In addition, silver nanoparticles can be involved in bacterial signal transduction. Bacterial signal transduction is affected by phosphorylation of protein substrates, and nanoparticles can dephosphorylate tyrosine residues on the peptide substrates. Disruption of the signal transduction can lead to cell apoptosis and termination of cell multiplication.12
The dissolution status of silver nanoparticles in exposure media strongly affects their antibacterial effect and mechanism. The dissolution efficiency depends on synthetic and processing factors, such as intrinsic silver nanoparticle characteristics and surrounding media.13 The influence of particle size and shape on the release of silver ions is described, in theoretical terms, by the Ostwald–Freundlich equation. Smaller silver nanoparticles with spherical or quasi-spherical format are more prone to silver release, due to their larger surface area.14 This also explains the lower silver release of aggregated nanoparticles, relative to isolated nanoparticles.13 Capping agents are used to modify the surfaces of silver nanoparticles, which can change their dissolution behavior.15 Apart from these intrinsic properties of silver nanoparticles, the surrounding media can influence the release of silver ions. The presence of organic or inorganic components in media can affect the dissolution of silver nanoparticles by aggregating with silver nanoparticles or complexing with silver ions. Researchers have also demonstrated that silver nanoparticles release silver ions faster in acidic solution than they do in neutral solution.16
Gram-negative bacteria are more susceptible to silver nanoparticles.17 The cellular wall of gram-negative bacteria is narrower than that of gram-positive strains. The thick cellular wall may reduce the penetration of nanoparticles into cells.17 The different antibacterial effects of silver nanoparticles on gram-negative and gram-positive bacteria suggest that uptake of silver nanoparticles is important to the antibacterial effect.13 It is commonly acknowledged that silver nanoparticles smaller than 10 nm can directly alter cell permeability, enter bacterial cells and cause cell damage.
Biofilm rapidly forms in the oral environment and protects bacteria from both silver ions and nanoparticles by hindering their transport. Researchers have found that 100% viability loss of bacteria did not occur in biofilm when silver nanoparticles with the same concentration kill all planktonic bacteria.18 Therefore, the biofilm is tolerant of the silver nanoparticles, due to its complicated architecture. Silver nanoparticles’ diffusion coefficients, which are generally related to size and physicochemical characteristics, determine their mobility and bioavailability in biofilm. First, these coefficients decrease with increasing molar mass, which means that it is harder for larger silver nanoparticles to penetrate biofilm.19 Transport through biofilm can be greatly obstructed for particles larger than 50 nm. Second, the nanoparticles’ chemical composition can arouse adsorption and accumulation of silver nanoparticles in the biofilm, thereby reducing their diffusion. Third, electrostatic interaction between bacteria and silver nanoparticles can influence charged nanoparticles’ penetration through biofilm.20
Clinical Applications of Silver Nanoparticles in Dentistry
Research on silver nanoparticles has become an emerging field in recent years. The first reason for this is that silver nanoparticles can be synthesized by mediating their nucleation and growth process, using different synthetic reagents. Second, silver nanoparticles can be specifically functionalized with molecular capping agents, such as proteins and chemical groups. Third, silver nanoparticles possess a strong antibacterial effect that improves clinical treatment outcomes.13 Therefore, homogenous silver nanoparticles with controlled size, morphology and function can be regarded as multifunctional building blocks in diverse dental materials. Silver nanoparticles can be added to acrylic resins for fabrication of removable dentures in prosthetic treatment, composite resin for direct restoration, irrigating solution and obturation material in endodontic treatment, adhesive materials in orthodontic treatment, membrane for guided tissue regeneration in periodontal treatment, and titanium coating in dental implant treatment (Figure 2).
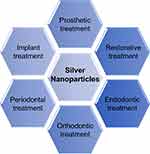 |
Figure 2 The antibacterial application of silver nanoparticles in dentistry. |
Prosthetic Treatment
Acrylic resin is commonly used to fabricate removable dentures. Opportunistic oral pathogens can colonize acrylic materials, causing dental infections, such as denture stomatitis. Silver nanoparticles can be added to acrylic resin to inhibit the growth of such bacteria as Streptococcus mutans, Escherichia coli and Staphylococcus aureus.21 In addition to the antibacterial effects, acrylic resin incorporated with silver nanoparticles has also displayed antifungal properties against the adhesion of Candida albicans, which is one of the key opportunistic pathogens on a denture base.22
Silver nanoparticles can also enhance the mechanical properties of acrylic resin. The effect of silver nanoparticles on the mechanical properties is influenced by the concentration of silver nanoparticles, the type of acrylic resin and the polar interactions between the polymethyl methacrylate chains and silver nanoparticles.23 Silver nanoparticles can increase the flexural strength and elastic modulus of acryl resin. Moreover, they can increase the thermal conductivity and compressive strength of the acrylic base.24
Restoration Treatment
Residual bacteria in the prepared tooth cavity and colonized bacteria in microleakages along tooth–restoration interfaces may result in secondary caries of the dental restoration. Silver nanoparticles can be added to adhesive systems and composite resins. They can prevent secondary caries by bringing notable antimicrobial properties to bear at low concentration.25 Restorative adhesives containing silver nanoparticles can interfere with biofilm formation, due to the widespread inhibitory action of cariogenic bacteria. Composite resin with silver nanoparticles had no significant adverse effect on fibroblasts, because the silver ions released per day are very low.26 In addition, researchers immobilized silver nanoparticles in a biocompatible film of polymers to obtain a maximum lethal effect on microbial cells without killing human cells.
Silver nanoparticles can also be added to porcelain to enhance its mechanical properties. Porcelain’s reinforced mechanical properties, such as hardness and fracture toughness, can be attributed to the residual compressive stress generated by an ion-exchange reaction and differential thermal expansion of silver nanoparticles.27
Endodontic Treatment
Silver nanoparticles can be used as a substitute of sodium hypochlorite in intracanal irrigation for endodontic treatment. Gutta-percha coated with silver nanoparticles has been developed as an antimicrobial obturator for root canal obturation. Silver nanoparticles are also incorporated as antibacterial material into mineral trioxide aggregate to enhance the success of pulp-capping, apexification and sealing perforations in teeth.
Sodium hypochlorite is regarded as the gold standard for chemical disinfection of root canals in endodontic treatment.28 However, sodium hypochlorite reduces the elastic modulus and flexural strength of dentine and causes toxic damage to the periapical tissues. A study found that root canal irrigation with silver nanoparticle solution did not significantly affect the mechanical properties of dentine.29 Another study found that a low concentration of silver nanoparticles had superior biocompatibility, relative to sodium hypochlorite. In addition, silver nanoparticle-based irrigation solution was as potent as sodium hypochlorite in the elimination of both Enterococcus faecalis and Staphylococcus aureus. Therefore, some have suggested using silver nanoparticle solution for root canal irrigation during endodontic treatment.
Gutta-percha is commonly used in root canal obturation. Although it is desirable that endodontic obturators have antibacterial properties, gutta-percha’s antibacterial properties are limited. A study found gutta-percha coated with silver nanoparticles had antibacterial and antifungal properties and was as effective as conventional gutta-percha in preventing bacterial leakage.30 In addition, there is no difference in the cytotoxicity of fibroblasts and subcutaneous tissue reaction or inflammation between gutta-percha-coated silver nanoparticles and conventional gutta-percha.31
Mineral trioxide aggregate has extensive applications in dentistry, such as pulp-capping, apexification and sealing perforations. It is essential to add antimicrobial agents into mineral trioxide aggregate to enhance its antimicrobial properties. Mineral trioxide aggregate, combined with silver nanoparticles, can enhance antibacterial activity against anaerobic endodontic pathogens. The antifungal ability against Candida albicans was also improved by the addition of silver nanoparticles to mineral trioxide aggregate. Moreover, research has reported that mineral trioxide aggregate, incorporated with silver nanoparticles, can increase pH value and compressive strength with proper radiopacity and shorter setting time.32 In addition, researchers have revealed that mineral trioxide aggregate with silver nanoparticles has good biocompatibility without inducing inflammatory reaction.33
Orthodontic Treatment
Silver nanoparticles can be used to prevent enamel caries (white spot lesion), which is a common complication for patients with orthodontic treatment. Silver nanoparticles can be added to adhesive materials, such as resin-modified glass ionomers and composite adhesives, to inhibit biofilm growth. Orthodontic elastomeric modules, such as ligatures, can be allied with silver nanoparticles to prevent enamel caries.34 They can affect the adhesion of Streptococcus mutans to the materials by contact with bacteria and inhibit bacterial action around brackets and wires by the release of silver ions.35 In addition, the materials can release silver ions for 4 months to exert a long-term antibacterial effect. Materials with silver nanoparticles are biocompatible and have no significant cytotoxic or mutagenic effects. Moreover, the materials have negligible irritation and delayed-type hypersensitivity potential. There are no adverse effects on their mechanical properties, such as shear bond strength.36
Dental Implant Treatment
Peri-implant infection is a major threat for implant treatment. Conventional antibacterial methods are insufficient, due to constant risk of infection from peri-operation, which persists until later in life.37 Modifying implant surfaces with silver nanoparticles, using various doped methods, is currently an area of intensive interest, due to the safety and strong antibacterial properties of silver nanoparticles.38
Researchers have demonstrated the long-term antibacterial effect of titanium implants coated with silver nanoparticles by steadily controlling silver ion release with various coating methods.39 The antibacterial effect can also be prolonged by immobilizing silver nanoparticles on implant surfaces. The embedded silver nanoparticles inhibit bacteria without consumption through continuous direct contact with bacterial cells. Although decreasing the size of silver nanoparticles generally increases their antimicrobial ability, larger-sized silver nanoparticles embedded in titanium had a better antibacterial effect, due to the consumption of more protons.40 Silver nanoparticles can kill Staphylococcus aureus and Pseudomonas aeruginosa at a low concentration, which has no significant cytotoxic effects on osteoblastic cells.41 In addition, titanium embedded with silver nanoparticles can enhance bone mineral density, bone formation and trabecular pattern, with no harm to tissues adjacent to dental implants.40
Periodontal Treatment
Periodontitis is a prevalent chronic inflammatory disease caused by various types of microorganisms. Adequate infection control is essential for periodontal treatment through disruption of the biofilm and suppression of inflammation.42 Compared to traditional antibiotics, silver nanoparticles possess antibacterial properties without the generation of bacterial resistance.43 Silver nanoparticles significantly enhance bactericidal properties when combined with antibiotics, including cefotaxime, ceftazidime, meropenem and ciprofloxacin. Importantly, inactive antibiotics restore strong antibacterial activity against multi-resistant bacterial strains when they are combined with silver nanoparticles.43 Silver nanoparticles synthesized with an appropriated capping agent can promote the inhibition effect against gram-negative bacteria that predominantly cause periodontal infections. Smaller-sized silver nanoparticles presented a higher antibacterial property against oral anaerobic pathogenic bacteria. Because guided tissue regeneration membrane with silver nanoparticles reduced adherence and penetration of bacteria, treatment of intra-bony defects using guided tissue regeneration membrane with silver nanoparticles can improve clinical success.44
Silver nanoparticles were reported to possess an anti-inflammatory effect, through modulation of the level of inflammatory cytokines and growth factors.45 Periodontal dressing coated with silver nanoparticles can be used to treat gingival wounds. This treatment accelerates the early phases of wound healing, with apparently new collagen synthesis and neovascularization. However, some silver nanoparticles can provoke inflammation and foreign body reaction in periodontal dressing.46
Safety of Silver Nanoparticles in Dental Applications
Some researchers are concerned about the toxicity of silver nanoparticles. The toxicity of silver nanoparticles is directly associated with the free silver ions.13 Because of the nanoscale size of silver nanoparticles, they can readily disturb biological molecules, cells and human organs. Some laboratory studies have reported that silver nanoparticles can induce oxidative stress and impair mitochondrial function of human cells.47 Moreover, silver can be detected in organs after administration of massive silver nanoparticle doses, especially in the liver and spleen. Researchers are also concerned about the ability of silver nanoparticles to cross the blood-brain barrier, via trans-synaptic transport, and accumulate in the brain.48
However, even at low concentration, small-size silver nanoparticles decrease some inflammatory cytokines and angiogenesis parameters. Silver nanoparticles can express anti-inflammatory properties. They are biocompatible with fibroblasts and keratinocyte. Silver accumulated in organs can mostly be cleared after 8 weeks.19 An animal study using rats found no observable adverse effect with the oral administration of silver nanoparticles. Another clinical study did not find clinically important observable toxic effects on patients taking commercial colloidal silver products.11 Researchers discovered mineral trioxide aggregate with silver nanoparticles did not induce a significant inflammatory reaction on subcutaneous tissues of rats. It has also been proven that periodontal dressing with a high concentration of silver nanoparticles is biocompatible with gingival wound healing.49 Although authorities have not fully acknowledged the safety of silver nanoparticles, clinicians often apply silver nanoparticle-based wound dressings to their patients’ wounded skin.50 Silver nanoparticles can enter into the body through a wound because the strict barrier is lost. These silver nanoparticles are phagocytosed by macrophages and may cause immune system perturbations. Silver nanoparticles also enter other organs, such as the liver and spleen, via circulation. Nevertheless, the systemic toxicity of ingested silver nanoparticles has not been reported. Another concern is their potential hazard to the marine life if they are released into the environment. However, the toxicity of silver nanoparticles may be increased or reduced when they interact with materials and organic compounds. Hence, nanoparticles can have a harmful or helpful effect on the environment. It is essential to perform a nanomaterial-specific evaluation to ensure their use is safe for humans and the environment.
Conclusions
Incorporating silver nanoparticles into dental materials may enhance the mechanical features and antibacterial properties of the dental materials. Although the mechanism of silver nanoparticles’ antibacterial effects is not yet fully understood, many researchers believe that silver nanoparticles can continually release silver ions to kill microbes. An increasing number of dental materials with silver nanoparticles are being developed for prosthetic, restorative, endodontic, orthodontic, periodontal and implant treatment. Some laboratory studies reported that silver nanoparticles have cytotoxic effects on human cells. However, the clinical significance of the potential toxicity of silver nanoparticles remains unknown. Further studies are essential because clinical evidence is still limited.
Acknowledgments
This study is supported by the Hong Kong University Grant Council General Research Fund (No. 17100218) and the National Natural Science Foundation of China (NSFC) – General Program (2018) No. 8187082.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Kesharwani P, Gorain B, Low SY, et al. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res Clin Pract. 2018;136:52–77. doi:10.1016/j.diabres.2017.11.018
2. Saravanan M, Barik SK, Mubarakali D, Prakash P, Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog. 2018;116:221–226. doi:10.1016/j.micpath.2018.01.038
3. Oves M, Aslam M, Rauf MA, et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Materials Science and Engineering: C. 2018;89:429–443. doi:10.1016/j.msec.2018.03.035
4. Samuel MS, Jose S, Selvarajan E, Mathimani T, Pugazhendhi A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J Photochem Photobiol. 2020;202:111642.
5. Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(1–2):104–111. doi:10.1016/j.ijpharm.2018.01.034
6. Shanmuganathan R, Karuppusamy I, Saravanan M, et al. Synthesis of Silver nanoparticles and their biomedical applications - A comprehensive review. Curr Pharm Des. 2019;25(24):2650–2660. doi:10.2174/1381612825666190708185506
7. Bapat RA, Chaubal TV, Joshi CP, et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng C. 2018;91:881–898. doi:10.1016/j.msec.2018.05.069
8. Khorrami S, Zarrabi A, Khaleghi M, Danaei M, Mozafari M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int J Nanomedicine. 2018;13:8013–8024. doi:10.2147/IJN.S189295
9. Ramkumar VS, Pugazhendhi A, Gopalakrishnan K, et al. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol Rep. 2017;14:1–7. doi:10.1016/j.btre.2017.02.001
10. Durán N, Nakazato G, Seabra A. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: an overview and comments. Appl Microbiol Biotechnol. 2016;100(15):6555–6570. doi:10.1007/s00253-016-7657-7
11. Liao C, Li Y, Tjong SC. Bactericidal and cytotoxic properties of silver nanoparticles. Int J Mol Sci. 2019;20(2):449. doi:10.3390/ijms20020449
12. Li L, Li L, Zhou X, et al. Silver nanoparticles induce protective autophagy via Ca 2+ /CaMKKβ/AMPK/mTOR pathway in SH-SY5Y cells and rat brains. Nanotoxicology. 2019;13(3):369–391. doi:10.1080/17435390.2018.1550226
13. Noronha VT, Paula AJ, Durán G, et al. Silver nanoparticles in dentistry. Dent Mater. 2017;33(10):1110–1126. doi:10.1016/j.dental.2017.07.002
14. Shanmuganathan R, MubarakAli D, Prabakar D, et al. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res Int. 2018;25(11):10362–10370. doi:10.1007/s11356-017-9367-9
15. Khorrami S, Jafari F, Zarrabi A, Zarepour A. Is Astragalus gossypinus honey a natural antibacterial and cytotoxic agent? An investigation on A. gossypinus honey biological activity and its green synthesized silver nanoparticles. Bionanosci. 2018;9(3):603–10.
16. Jacob JM, John MS, Jacob A, et al. Bactericidal coating of paper towels via sustainable biosynthesis of silver nanoparticles using ocimum sanctum leaf extract. Bactericidal Coat Pap Towels Sustainable Biosynth Silver Nanopart Ocimum Sanctum Leaf Extr. 2019;6(4):045401.
17. Meikle T, Dyett BP, Strachan JB, White J, Drummond CJ, Conn CE. Preparation, characterization, and antimicrobial activity of cubosome encapsulated metal nanocrystals. ACS Appl Mater Interfaces. 2020;12(6):6944–6954. doi:10.1021/acsami.9b21783
18. Saravanan M, Arokiyaraj S, Lakshmi T, Pugazhendhi A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog. 2018;117:68–72. doi:10.1016/j.micpath.2018.02.008
19. Yin IX, Yu OY, Zhao IS, et al. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch Oral Biol. 2019;102:106–112. doi:10.1016/j.archoralbio.2019.03.022
20. Pugazhendhi A, Prabakar D, Jacob JM, Karuppusamy I, Saratale RG. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb Pathog. 2018;114:41–45. doi:10.1016/j.micpath.2017.11.013
21. de Castro DT, Do Nascimento C, Alves OL, de Souza Santos E, Agnelli JAM, Dos Reis AC. Analysis of the oral microbiome on the surface of modified dental polymers. Arch Oral Biol. 2018;93:107–114. doi:10.1016/j.archoralbio.2018.06.005
22. Li Z, Sun J, Lan J, Qi Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology. 2016;33(2):209–216. doi:10.1111/ger.12142
23. Gad MM, Fouda SM, Al-Harbi FA, Napankangas R, Raustia A. PMMA denture base material enhancement: a review of fiber, filler, and nanofiller addition. Int J Nanomedicine. 2017;12:3801–3812. doi:10.2147/IJN.S130722
24. Bacali C, Baldea I, Moldovan M, et al. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin Oral Investig. 2019. doi:10.1007/s00784-019-03133-2
25. Dias HB, Bernardi MIB, Marangoni VS, de Abreu Bernardi AC, de Souza Rastelli AN, Hernandes AC. Synthesis, characterization and application of Ag doped ZnO nanoparticles in a composite resin. Mater Sci Eng C. 2019;96:391–401. doi:10.1016/j.msec.2018.10.063
26. Ai M, Du Z, Zhu S, et al. Composite resin reinforced with silver nanoparticles–laden hydroxyapatite nanowires for dental application. Dent Mater. 2017;33(1):12–22. doi:10.1016/j.dental.2016.09.038
27. Meng M, Li XC, Guo JW, et al. Improving the wear performance of feldspathic veneering porcelain by ion-exchange strengthening. J Dent. 2019;90:103210.
28. Ioannidis K, Niazi S, Mylonas P, Mannocci F, Deb S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent Mater. 2019;35(11):1614–1629. doi:10.1016/j.dental.2019.08.105
29. Suzuki TYU, Gallego J, Assunção WG, Briso ALF, Dos Santos PH. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS One. 2019;14(6):e0217750. doi:10.1371/journal.pone.0217750
30. Mishra P, Tyagi S. Surface analysis of gutta percha after disinfecting with sodium hypochlorite and silver nanoparticles by atomic force microscopy: an in vitro study. Dent Res J (Isfahan). 2018;15(4):242–247.
31. Shantiaee Y, Dianat O, Mohammad Khani H, Mozayani M, Paymanpour P. Subcutaneous reaction of rat tissues to nanosilver coated gutta-percha. Iran Endod J. 2017;12(2):157–161. doi:10.22037/iej.2017.31
32. Vazquez-Garcia F, Tanomaru-Filho M, Chávez-Andrade GM, Bosso-Martelo R, Basso-Bernardi MI, Guerreiro-Tanomaru JM. Effect of silver nanoparticles on physicochemical and antibacterial properties of calcium silicate cements. Braz Dent J. 2016;27(5):508–514. doi:10.1590/0103-6440201600689
33. Samiei M, Ghasemi N, Asl-Aminabadi N, Divband B, Golparvar-Dashti Y, Shirazi S. Zeolite-silver-zinc nanoparticles: biocompatibility and their effect on the compressive strength of mineral trioxide aggregate. J Clin Exp Dent. 2017;9(3):e356–e360. doi:10.4317/jced.53392
34. Hernandez-Gomora AE, Lara-Carrillo E, Robles-Navarro JB, et al. Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: evaluation of mechanical and antibacterial properties. Molecules. 2017;22(9):1407. doi:10.3390/molecules22091407
35. Espinosa-Cristóbal LF, López-Ruiz N, Cabada-Tarín D, et al. Antiadherence and antimicrobial properties of silver nanoparticles against streptococcus mutants on brackets and wires used for orthodontic treatments. J Nanomater. 2018;2018.
36. Mazumder JA, Khatoon N, Batra P, Sardar M. Biosynthesized silver nanoparticles for orthodontic applications. Adv Sci Eng Med. 2018;10(12):1169–1173. doi:10.1166/asem.2018.2289
37. Pokrowiecki R, Zaręba T, Szaraniec B, et al. In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int J Nanomedicine. 2017;12:4285–4297. doi:10.2147/IJN.S131163
38. Gunputh UF, Le H, Lawton K, Besinis A, Tredwin C, Handy RD. Antibacterial properties of silver nanoparticles grown in situ and anchored to titanium dioxide nanotubes on titanium implant against Staphylococcus aureus. Nanotoxicology. 2020;14(1):97–110. doi:10.1080/17435390.2019.1665727
39. Lampe I, Beke D, Biri S, et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int J Nanomedicine. 2019;14:4709–4721. doi:10.2147/IJN.S197782
40. Zhou W, Jia Z, Xiong P, et al. Bioinspired and biomimetic AgNPs/gentamicin-embedded silk fibroin coatings for robust antibacterial and osteogenetic applications. ACS Appl Mater Interfaces. 2017;9(31):25830–25846. doi:10.1021/acsami.7b06757
41. Salaie RN, Besinis A, Le H, Tredwin C, Handy RD. The biocompatibility of silver and nanohydroxyapatite coatings on titanium dental implants with human primary osteoblast cells. Materials Science and Engineering: C. 2020;107:110210. doi:10.1016/j.msec.2019.110210
42. Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai RS. Biosynthesis, characterization and antibacterial efficacy of silver nanoparticles derived from endophytic fungi against P. Gingivalis. J Clin Diagn Res. 2017;11(9):ZC92. doi:10.7860/JCDR/2017/24731.9963
43. Panáček A, Smékalová M, Večeřová R, et al. Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae. Colloids Surf B Biointerfaces. 2016;142:392–399. doi:10.1016/j.colsurfb.2016.03.007
44. Chi M, Qi M, Wang P, et al. Novel Bioactive and therapeutic dental polymeric materials to inhibit periodontal pathogens and biofilms. Int J Mol Sci. 2019;20(2):278. doi:10.3390/ijms20020278
45. Singh P, Ahn S, Kang JP, et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: a green synthetic approach. Artif Cells Nanomed Biotechnol. 2018;46(8):2022–2032. doi:10.1080/21691401.2017.1408117
46. Sugiharti R, Widyasar E, Rusminah N, Mustika I. Evaluation of silver nanoparticles addition in periodontal dressing for wound tissue healing by 99mTc-ciprofloxacin. J Young Pharm. 2019;11(1):17–20.
47. Palacios-Hernandez T, Diaz-Diestra DM, Nguyen AK, et al. Cytotoxicity, cellular uptake and apoptotic responses in human coronary artery endothelial cells exposed to ultrasmall superparamagnetic iron oxide nanoparticles. J Appl Toxicol. 2020. doi:10.1002/jat.3953
48. Lebda MA, Sadek KM, Tohamy HG, et al. Potential role of α-lipoic acid and Ginkgo biloba against silver nanoparticles-induced neuronal apoptosis and blood-brain barrier impairments in rats. Life Sci. 2018;212:251–260. doi:10.1016/j.lfs.2018.10.011
49. Lee SJ, Heo DN, Lee D, et al. One-step fabrication of AgNPs embedded hybrid dual nanofibrous oral wound dressings. J Biomed Nanotechnol. 2016;12(11):2041–2050. doi:10.1166/jbn.2016.2304
50. Mehrabani MG, Karimian R, Mehramouz B, Rahimi M, Kafil HS. Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int J Biol Macromol. 2018;114:961–971. doi:10.1016/j.ijbiomac.2018.03.128
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.