Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
The Anti-Obesity Potential of Cyperus rotundus Extract Containing Piceatannol, Scirpusin A and Scirpusin B from Rhizomes: Preclinical and Clinical Evaluations
Authors Majeed M, Nagabhushanam K , Bhat B, Ansari M, Pandey A, Bani S, Mundkur L
Received 13 November 2021
Accepted for publication 12 January 2022
Published 9 February 2022 Volume 2022:15 Pages 369—382
DOI https://doi.org/10.2147/DMSO.S348412
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Muhammed Majeed,1,2 Kalyanam Nagabhushanam,3 Beena Bhat,4 Mohammad Ansari,4 Anjali Pandey,5 Sarang Bani,5 Lakshmi Mundkur5
1Research and Development, Sami-Sabinsa Group Limited, Bangalore, India; 2Research and Development, Sabinsa Corporation, East Windsor, NJ, USA; 3Research and Development, Natural Product Chemistry Sabinsa Corporation, East Windsor, NJ, USA; 4Research and Development, Phytochemistry, Sami-Sabinsa Group Limited, Bangalore, India; 5Research and Development, Biological Research, Sami-Sabinsa Group Limited, Bangalore, India
Correspondence: Lakshmi Mundkur
Sami-Sabinsa Group Limited, 19/1, 19/2, 1st Main, 2nd Phase, Peenya Industrial Area Bangalore, Bengaluru, Karnataka, 560058, India
, Tel +80 2839 7973
, Email [email protected]
Purpose: Obesity is a complex medical problem that increases the risk of other diseases like diabetes, cardiovascular diseases, and fatty liver disease. The present study evaluated the efficacy and safety of Cyperus rotundus rhizome extract (CRE), standardized to contain Piceatannol, Scirpusin A, and Scirpusin B (5% total Stilbenoids) in overweight individuals. The mechanism of activity was evaluated in a diet-induced mice model of obesity and adipocytes in vitro.
Materials and Methods: The efficacy, safety, and tolerability of CRE were evaluated in 30 obese individuals with a BMI of 30 to 40 kg/m2 for 90 days in a randomized, double-blind, parallel-group, placebo-controlled study. In vitro studies were carried out in differentiated 3T3 L1 adipocytes, and the therapeutic efficacy was evaluated in high-fat diet-induced obese mice.
Results: The pilot clinical study showed a reduction in body weight with a significant decrease in waist circumference and BMI. The serum lipid profile showed a significant improvement in CRE-treated individuals. The extract was well tolerated, and no adverse effects were reported at the end of the study. CRE showed a dose-dependent adipogenesis reduction in vitro with an IC50 value of 9.39 μg/mL, while oral administration of CRE reduced weight gain in diet-induced obese mice. The efficacy in mice was associated with reduced levels of leptin, corticosteroids, and serum lipid levels, with no adverse effects.
Conclusion: CRE has anti-adipogenic properties, is safe for human consumption, and effectively manages weight and hypercholesterolemia in overweight individuals.
Keywords: Cyperus rotundus, piceatannol, Scirpusin A, Scirpusin B, obesity, adipogenesis, lipid levels, leptin, randomized clinical trial
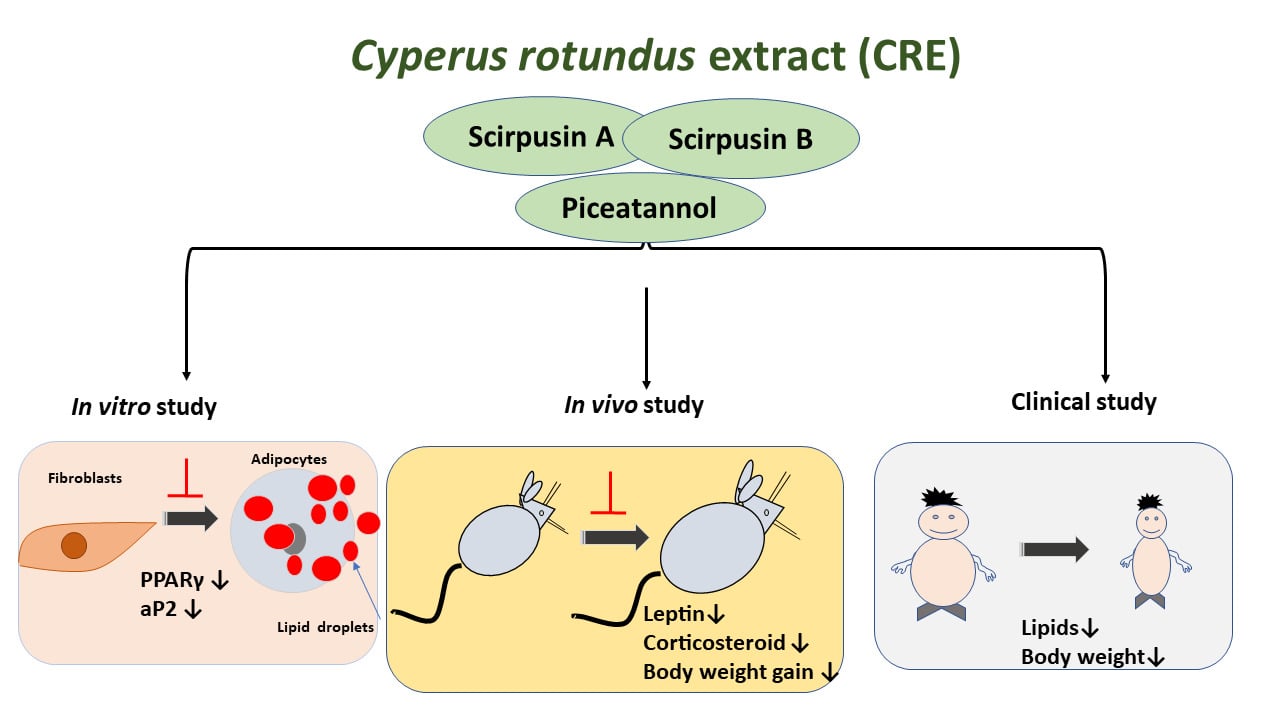
Graphical Abstract:
Plain Language Summary
The present study shows the benefits of a standardized extract of Cyperus rotundus rhizomes in the management of obesity in humans. Obesity is a global health problem that increases the risk of several chronic diseases. Obese individuals are more susceptible to infections and have lower immunity to pathogens. Pharmaceutical drugs have not been very successful in the treatment of obesity. Supplements derived from plants possess several polyphenolic compounds, which can help in reducing lipid accumulation and fat storage. We developed a standardized extract of C. rotundus rhizomes to contain novel compounds Scirpusin A and Scirpusin B. This extract was safe and effective in reducing body weight, BMI, waist circumference, and lipid levels in obese individuals when given as a supplement for 90 days. The extract could reduce weight gain and lower stress hormone levels (corticosterone) and the satiety hormone (leptin) in animals. The extract prevented the conversion of preadipocyte cells to adipocytes in vitro. These results suggest that Cyperus rotundus extract may be a safe and effective supplement in the management of obesity in human
Introduction
Obesity is one of the most predominant public health problems and significantly contributes to the global burden of chronic diseases.1 According to the World Health Organization, the prevalence of global obesity has tripled since 1975, with nearly 40% of the adult population being overweight.2 It is a risk factor for various comorbidities, including diabetes mellitus, heart and liver diseases, cancer, and a serious concern for health and life expectancy.3
The imbalance between energy intake and expenditure gives rise to excessive growth and expansion of adipose tissue.4, A dysfunctional adipose tissue causes inflammation, hypoxia, fibrosis, and disrupted mitochondrial function.5 Diet control, exercise, and lifestyle management are the primary options available to control obesity, as pharmaceutical treatments have proved insufficient in providing a long-term solution, mainly due to adverse side effects. Natural products can help regulate various mechanisms related to obesity, such as appetite suppression, modulating adipogenesis, and regulating lipid hydrolyzing enzymes such as pancreatic lipase and lipoprotein lipase.6 Phenolic compounds such as flavonoids are the key ingredients in natural products, purported to have several health benefits.7 Among the polyphenolic compounds, stilbenes like Resveratrol are known for their anti-obesity activity.8 Many plant stilbenes are monomeric units like the famed trans-Resveratrol (trans-3,4ˈ,5- trihydroxystilbene) and are reported to have phytoalexin activity.9 Piceatannol (trans-3’,4’,3,5-tetrahydroxystilbene), commonly found in berries, grapes, rhubarb (Rheum species), and white tea, is very similar in structure to Resveratrol, except for an additional hydroxyl group.10 It was reported to inhibit adipogenesis and lower triglycerides and lipid droplet accumulation in vitro and in vivo models. Scirpusins are dimerized stilbenes that were earlier reported to possess anti-cancer11 and vasodilation12 effects. Scirpusin A is a dimerized Resveratrol isolated for the first time from the rhizome of Scirpus fluviatilis in 1978 along with Resveratrol and 3, 3’, 4, 5’-tetrahydroxystilbene.13 Scirpusin B is one of the many possible dimeric structures of Piceatannol. Even though these Scirpusins are the dimers of well-studied Resveratrol and Piceatannol, their biological activity is much less explored. For the first time, Majeed et al investigated the components in ethyl acetate extract of Cyperus rotundus rhizomes and isolated Scirpusin A and Scirpusin B as the pharmacologically active molecules.14
Cyperus rotundus Lin., belonging to the Cyperaceae family, grows naturally in tropical and temperate regions and is traditionally used to treat stomach disorders and emotional disturbances.15,16 It has also been used as an analgesic, antimalarial, anti-inflammatory, antidiarrheal, antidiabetic, wound healing, and antioxidant activities.17 The plant finds its mention in ancient ayurvedic literature as a drug capable of “de-fatting” adipose or muscular tissues.18 Crude aqueous extract of C. rotundus was reported to have an anti-obesity activity in rat models earlier.19 The methanol extract of the rhizome was reported to contain Methyl 3,4-dihydroxybenzoate, Ipolamiide, 6-β-Hydroxyipolamiide, and Rutin.20 Other studies have reported the presence of phenolic glycosides (Rotundusides) in the n-BuOH fraction,21 while the essential oil from the rhizome of C. rotundus was reported to contain α-Cyperone (38.46%), Cyperene (12.84%), and α-Selinene.22
In the current study, the extract containing Scirpusin A, Scirpusin B, and Piceatannol (standardized for 5% total stilbenoids) from the rhizomes of C. rotundus was evaluated for anti-adipogenic potential. The extract was evaluated in differentiated 3T3 L1 adipocytes in vitro, in an animal model of diet-induced obesity and a clinical study in overweight individuals.
Materials and Methods
Extraction
Coarse powder from the dried, pulverized rhizomes of Cyperus rotundus was extracted as per the protocol described earlier.14 Briefly, the pulverized powder was extracted with three volumes of heptane/hexane followed by heating, reflux for 3 hours, and filtering to obtain the hydrocarbon soluble fraction and spent material. The spent material was further extracted with three volumes of methanol followed by heating, reflux for 3 hours, and filtering to obtain the methanol soluble active fraction and spent material. The methanol soluble fraction was solubilized in aqueous methanol and successively partitioned with ethyl acetate.
The ethyl acetate fraction containing 5% total Stilbenoids (Scirpusin A & B and Piceatannol), termed CRE, was used for the in vitro in vivo and human clinical study. The marketing trade name for the product is CIRPUSINS®.
Clinical Study
A pilot, single-center, randomized, double-blind, parallel-group, placebo-controlled study was conducted to investigate the efficacy, safety, and tolerability of CRE in 30 obese individuals for 90 days. The institutional ethics committee of Government Ayurveda Medical College (GAMS) Mysore approved the study protocol in May 2014, which functioned independently of Sami Labs Limited or Syncretic Clinical Research Services (SMO) or ClinWorld Private Limited (CRO). The trial was registered under the Clinical Trials Registry – India (CTRI/2014/05/004633). The study was performed at GAMS Mysore, India, following clinical research guidelines established by the Drugs and Cosmetics Act, 1940 of India, Drugs and Cosmetics Rules, 1945 of India, Ethical Guidelines for Biomedical Research on Human Participants, 2006 of Indian Council of Medical Research (ICMR) in India, the principles enunciated in the Declaration of Helsinki (Edinburgh, 2000) and the ICH-harmonized tripartite guideline regarding Good Clinical Practice (GCP). Each volunteer was informed by the investigator, prior to the screening, of the purpose of this clinical trial, including possible risks and benefits, and documented the informed consent process in the patient’s file. Prior to entry into the study or initiation of any study-related procedures, the patient/volunteer read, signed, and dated the IEC-approved informed consent form. The study was conducted between May 2014 and December 2014. All the data pertaining to the clinical trial are included in the main manuscript and the details in supplementary section. The study details will be made available on request to the corresponding author.
Participants
Subjects in the age between 20 and 65 years, with elevated levels of serum cholesterol >200 mg/dL), triglycerides more than >150 mg/dL), LDL > 130 mg/dL), VLDL > 40 mg/dL), BMI between ≥30 to 40 kg/m2 and willingness to come for regular follow-up visits were recruited for the study. Participants who had taken any weight loss agents or centrally acting appetite suppressants in the previous six months and those under prolonged medication or with any congenital diseases or malignancies were excluded. Participants with poorly controlled diabetes (HbA1c >10%), hypertension >160/100 mm Hg), suffering from systemic illness, and those with a history of cardiovascular diseases, hepatic diseases, history of HIV, or other viral diseases were excluded from the study. Alcoholics and drug abusers, participants with bariatric surgeries, pregnant and lactating women were also excluded from the study.
Study Design
The subjects were randomized to receive 525 mg of CRE (n = 15) or placebo (n = 15) 30 minutes before food twice a day, in a 1:1 ratio. Since the study was double-blinded, both CRE and placebo capsules were identical in all aspects, including their size, shape, and weight. The active study material used in the study constituted 525 mg of CRE, 30 mg dibasic calcium phosphate, and 5 mg of magnesium stearate, while the placebo contained 560 mg of microcrystalline cellulose, administered twice daily at least 30 minutes before food. The study progress was monitored on days 15, 30, 60, and 90, and a telephonic follow-up 15 days from the last visit to assess the wellbeing of the subjects. The formulation details and schedule of participant visit are given in Tables S1 and S2.
Outcomes
Primary endpoints included (i) change in body weight and body mass index, (ii) change in waist circumference and waist: hip ratio (anthropometric measurements), (iii) changes in cholesterol, triglycerides, LDL, and VLDL values from baseline, (iv) change in HDL values from baseline. The secondary endpoints studied included assessing the tolerability of the study material in terms of adverse events and other physical signs or symptoms in the study subjects. A daily diet, physical activity, and compliance with study supplements were assessed in all the subjects for holistic management. No interim analysis was done during the duration of the study. All the participants were provided a daily record diary to capture the food intake and physical activity. The calorie intake was not found to be different between the baseline and the end of the study. All the participants followed regular exercise but no rigorous physical activity.
Assessments and Safety Evaluation
All the efficacy and safety assessments were carried out in the accredited hospital laboratories.
Vital signs, namely blood pressure, respiratory rate, pulse rate, and any abnormal lab/diagnostic parameters, were considered for safety evaluations. The safety assessment was based mainly on the intensity and relationship of adverse events.
Cell Culture
3T3-L1 mouse adipocytes (American Type Culture Collection (ATCC, Rockville, MD, USA)) were cultured in DMEM media. Cells were seeded at a density of 5×103 cells per well (96-well plates) and allowed to reach confluency. Two days post-confluent state, differentiation was initiated by supplementing with 1 µM dexamethasone, 0.5 mM methyl isobutyl xanthine, and insulin (10 μg/mL) in the presence of different concentrations of CRE (3.12–50 µg/mL). The culture supernatants were checked by the lactate dehydrogenase (LDH) method to confirm cell viability. The cells were fixed with 100 μL of 10% formalin and stained with Oil Red O (ORO) to estimate the intracellular triglyceride accumulation. The mechanism of action was assessed by the change in expression of PPARγ & aP2 adipogenesis-related genes compared to β-Actin as the housekeeping gene.
The primer sequence used in the study were
β-Actin
F-GAAGTCCCTCACCCTCCCAA
R-GGCATGGACGCGACCA
PPARγ
F- TCGCTGATGCACTGCCTATG
R- GAGAGGTCCACAGAGCTGATT
aP2
F-CAAAATGTGTGATGCCTTTGTG
R-CTCTTCCTTTGGCTCATGCC
In vivo Anti-Obesity Efficacy Study in Mice
Animals
The animal experiments were approved by the Institutional Animal Ethics Committee of LIVEON Biolabs (Registration Number: 1610/ROBiBt/S/2012/CPCSEA) in compliance with Government of India guidelines and conformance to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). C57/BL6 mice (N = 60, 8–10-week-old) weighing 20–27 g were housed under standard laboratory conditions at room temperature 22±3°C and relative humidity 30–70% with 12 hours light and 12 hours dark cycle.
Experimental Design
The mice were randomly allocated into five groups containing six animals/group. The control group (G1) animals were given (D12450B diet with 10 kcal% fat, Research Diet Inc, USA) throughout the study duration. Animals in other groups (G2-G5) were fed with a high-fat diet (HFD) (D12492 containing 60 kcal% fat, Research Diet Inc, USA) for 27 days to induce obesity, followed by a treatment regimen (Table S3). CRE was administered by oral gavage at 50, 100, and 200 mg/kg doses to obese animals along with a high-fat diet for the next 27 consecutive days. The volume of dosage per animal was maintained at 10 mL/kg body weight.
Analysis
The average weekly feed consumption and weekly body weights were recorded, and the animals were observed for clinical signs during the study. After the study period, blood samples were collected by retro-orbital plexus. Serum levels of leptin and corticosteroids were evaluated using ELISA kits as per the manufacturer’s specifications (R&D systems, USA). Lipid levels were recorded as efficacy parameters, while the hematological parameters (using Sysmex, KX-21, Transasia Bio-Medicals Ltd., India), clinical chemistry parameters (using Erba Mannheim Chem Touch analyzer, Transasia Bio-Medicals Ltd., India), and organ weight were recorded to assess the safety.
Statistical Analysis
Data are presented as mean ± standard deviation. One-way ANOVA and Bonferroni post-test were used to assess differences between untreated and treated animals and in vitro studies. P < 0.05 was considered as statistical significance.
The clinical study data were analyzed by Statistical Analysis Software (SAS) of version 9.2. By considering Last Observation Carry Forward (intent to treat analysis), 27 subjects’ data were considered for efficacy analysis. Whereas for safety analysis, 26 subjects’ data were considered. Paired t-test, Analysis of Covariance (ANCOVA), and Wilcoxon signed-rank sum test were used for appropriate data set variables to reach the best possible statistical conclusion between the active and placebo receiving groups. The baseline descriptors were summarized as means and standard deviations for continuous variables and as frequencies and percentages for categorical variables. The last Observation Carry Forward (LOCF) method was followed for efficacy evaluations of subjects whose data were not available in the last/final visit. All the analyses and comparisons were evaluated at the 95% confidence level, with a P-value <0.05 being considered statistically significant. A detailed description of the methodology is given in the supplementary section.
Results
Analysis of the CRE
The LC-MS analysis of CRE and the molecular structures of the compounds are represented in Figure 1. The chromatogram shows the presence of Piceatannol, Scirpusin A, and B in CRE. The total Stilbenoids content in the extract was standardized to 5% w/w.
 |
Figure 1 Analysis of the CRE.(A) LC-MS analysis of Cyperus rotundus extract (CRE) enriched with Piceatannol, Scirpusin A and Scirpusin B, (B) Structures of Piceatannol, Scirpusin A, and Scirpusin B. |
Clinical Study on Weight Management
Demographic Details
A total of 30 subjects were included in the study, of which 8 (27%) were men, and 22 (73%) were women. Baseline demographics were comparable in both CRE and placebo arms (Table 1). Out of 30 overweight subjects recruited for the study, four dropped out (3 from placebo and 1 from CRE) during the follow-up, and 26 subjects completed the study. The treatment compliance was 100% in 19 subjects, more than 90% in 6 subjects, and 87.2% in 1 subject. The consort diagram is given in Figure 2.
 |
Table 1 Demographics of Subjects in the Pilot Clinical Study |
Effect on Body Weight
The subjects’ body weight, BMI, and waist circumference were measured during each visit. Treatment with CRE showed a statistically significant reduction (P < 0.05) in the body weight (from 88.3±9.70 to 78.8±8.16 Kg), while no significant change was observed in the placebo (92.8±21.3 to 92.6 ±22.2). The BMI (34.7±2.73 to 31.5±3.51) and waist circumference (101.5±10.66 to 97.6±9.83) also showed a significant decrease from baseline to end of the study in subjects supplemented with CRE but not in the placebo group (Table 2). The reduction in body weight could be observed as early as 30 days, but the difference in these parameters was significant between the CRE and the placebo group at the final visit after 90 days (Figure 3A–C). However, the hip circumference did not decrease significantly in the CRE-treated subjects, and hence there was no reduction in the waist-hip ratio. The total calorie intake did not differ significantly between the CRE and the placebo group (Table 2).
 |
Table 2 Effect on Body Weight and Related Parameters |
Effect on Lipid Profile
The lipid profile parameters, such as TC, TG, LDL, HDL, VLDL, were assessed as secondary efficacy parameters. The TC and TG levels decreased from 219.4±16.3 to 182.4±10.3 mg/dL and 182.3±30.8 to 171.2±24.9 mg/dL, while the LDL (173.5±19.9 to 159.3±19.6 mg/dL) and VLDL (47.9±11.4 to 33.9±7.21 mg/dL) showed a decrease with a significance of P < 0.01 between the two groups. Interestingly, the HDL levels increased from 40.9 ± 3.70 to 64.4±3.65 mg/dL in the subjects supplemented with CRE (p < 0.01), suggesting a healthy lipid profile in these subjects (Table 3).
 |
Table 3 Effect on Lipid Levels |
Safety Analysis
Cyperus rotundus extract was well tolerated by all the subjects during the three months study period. The participants were not subjected to any rigorous physical activity during the study. No statistically significant changes in vitals and laboratory values were observed between the treatment groups in any of the study visits (Figure S1 and Table S4). The biochemical and hematological parameters were found to be in the normal range for all the participating subjects. One adverse event was reported in the entire study duration, which was found to be unrelated to the study product. Other than that, no serious adverse events were noticed during the study duration (Table S3).
In-vitro Anti-Adipogenesis Activity of CRE
To understand the mechanism of the anti-obesity effect of CRE, we studied the anti-adipogenic activity of the extract in vitro. The 3T3-L1 fibroblasts differentiation is one of the most common models used to study the biology of adipogenesis.23 CRE treatment resulted in a dose-dependent reduction in lipid accumulation in 3T3 L1 cells with an IC50 value of 9.39 μg/mL without affecting cell viability (Figure 4A–C). The reduction in adipogenesis was associated with reduced levels of transcripts for peroxisome proliferator-activated receptor (PPARγ) and adipocyte protein 2/adipocyte fatty acid-binding protein (aP2) considered as the master regulators of adipogenesis (Figure 4D).
Anti-Obesity Effect of CRE in High-Fat Diet-Induced Obese Mice
Next, to understand the effect of CRE in reducing body weight gain in obese mice, the animals were first fed with a high-fat diet (HFD) for 27 days to induce obesity before supplementation with CRE for another 27 days. At the end of 55 days, animals were sacrificed and analyzed. Animals in the untreated HFD group gained 7.84 grams of weight in the last four weeks. In comparison, the animals treated with CRE gained 5.14, 3.95, and 4.34 grams, at 50, 100, and 200 mg/kg, respectively, leading to 30.1%, 46.3%, and 40.9% reduction in weight gain, respectively. The weight gain was comparable to control animals receiving a chow diet for the higher doses of CRE (Figure 5A). Feed consumption was reduced in HFD fed animals compared to control but was statistically insignificant (Figure 5B). The time-course of body weight reduction in the five different groups from Day 27 (start of the treatment) to the end of the treatment (Day 55) is represented in Figure 5C. The trend obtained by CRE treatment at 100 mg/kg was comparable with the control diet-fed mice. Since there was no significant difference in food consumption among the animals upon treatment, we could rule out satiety as a contributory factor for weight reduction.
Leptin and corticosterone are the two hormones that affect the regulation of energy balance, related to food intake and thermogenesis. CRE treatment showed a dose-dependent reduction in both leptin and corticosterone levels in the serum (Figure 5E).
Effect on Serum Lipid Profile and Liver Function
The effect of CRE on serum lipid levels showed a significant reduction in triglycerides, total cholesterol, HDL, and VLDL levels. Surprisingly, the reduction in LDL cholesterol levels was not significant. Although the enzymes were reduced in CRE-treated animals, it did not reach statistical significance (Table 4). The gonadal fat reduction was also not found to be significant (Table S5). The weight of vital organs and hematological and clinical parameters were found to be comparable to control animals (Tables S5 and S6). No mortality or adverse effects were observed in the entire study, suggesting that CRE was well tolerated in rodents.
 |
Table 4 Effect of CRE Lipid, and Liver Enzymes, in C57 Mice |
Discussion
In the present study, we demonstrate the anti-obesity activity of Cyperus rotundus extract standardized for 5% total Stilbenoids (Piceatannol, Scirpusin A, and Scirpusin B). The extract was effective in reducing body weight and BMI in overweight individuals. CRE showed anti-adipogenic activity in vitro in differentiated 3T3 L1 adipocytes and could reduce body weight gain in obese mice. Although stilbenes have been shown to have anti-obesity activity in vitro and in animal models, this is the first observation of the activity of an extract containing Piceatannol and dimeric Stilbenoids, namely Scirpusin A and Scirpusin B.
Scirpusin A (a mixed dimer of Resveratrol and Piceatannol) and Scirpusin B (dimer of Piceatannol) are dimers of hydroxystilbenes known to have a biological activity such as inhibition of amyloid peptide aggregation and vasorelaxation. Piceatannol, a Phase 1 metabolite of Resveratrol, showed higher metabolic stability and could inhibit adipogenesis in vitro in 3T3-L1 cells.24,25 It was reported to modify the gut microbiota by increasing Firmicutes and Lactobacillus and decreasing Bacteroidetes in mice,26 thus favorably modifying obesity in animals.
Participants supplemented with CRE showed a significant reduction in body weight, BMI, and waist circumference in the present clinical study. According to Brown et al, the reduction in body weight by >5% is associated with the improvements in metabolic and cardiovascular health.27 We observed an average of 18% bodyweight reduction after 90 days of CRE supplementation. BMI is the marker of global adiposity as well as a reliable marker of visceral adiposity. An increase in BMI can be observed in a quasi-linear manner with the increase in visceral adipose tissue.28 Also, waist circumference is commonly used as a surrogate marker of visceral adiposity and is considered as a diagnostic criterion for poor cardiovascular health.29,30 Supplementation of CRE conferred a significant reduction of up to 9.53% in the waist circumference. These results suggest CRE could be an effective option for reducing body weight and fat in overweight individuals.
Individuals with CRE supplementation experienced a significant reduction in total cholesterol, triglycerides, serum LDL, HDL, and VLDL, which indicated the impact of the extract on dyslipidemia. Concomitantly, a significant improvement in the serum HDL levels was observed with the treatment. As reported by Fischer et al, hyperlipidemia, associated with obesity, is a known risk factor for CVD as well as atherosclerosis.31 The reduced circulating lipid levels reflect an improved fat metabolism and reduced fat deposition in the body, thus implying possible cardiovascular benefits for overweight subjects. The other clinical and hematological parameters remained within their normal levels without any significant changes, suggesting the safety of the extract.
In support of the clinical data, 3T3-L1 fibroblasts exposed to CRE showed a significant reduction in lipid accumulation, as well as reduced expression of PPARγ and aP2 genes. Peroxisome proliferator-activated receptor γ is one of the master regulators of adipocyte differentiation, lipogenesis, and survival.32 Adipocyte protein 2 (aP2), or the fatty acid-binding protein (FABP4), is an adipocyte-specific protein, highly expressed in mature adipocytes.33 It plays a vital role in intracellular fatty acid transport and metabolism and helps in maintaining glucose and lipid homeostasis.34 In obese animals, the deletion of aP2 is known to reduce insulin resistance. Thus, a reduction in critical obesity-related transcripts suggests that CRE reduces adipogenesis by interfering with adipocyte differentiation and fatty acid metabolism.
Similarly, in the in vivo study, weight gain in mice was significantly reduced by 30–46% with Cyperus extract supplementation in HFD-fed mice. The serum total cholesterol and triglycerides were reduced in the CRE-treated animals along with weight reduction. In HFD fed mice, the lipids, including HDL, were higher than control. HDL has been reported to increase in diet-induced obese mice.35 Unlike humans, rodents carry the majority of cholesterol in HDL rather than LDL,36 which could explain the difference in HDL in human and animal studies.
The hematological parameters and organ weight remained in the normal range, suggesting that the extract induced no adverse effects and was safe even at the highest dose of 200 mg/kg in rats. Since there was no significant difference in food consumption among the animals upon the treatment, we could rule out satiety as a contributory factor for weight reduction. Leptin, a hormone related to maintaining energy balance, plays a crucial role in controlling obesity.37 As reported by Clément et al, the amount of leptin circulation is proportional to the amount of fat in an individual. The primary physiological role of leptin is to restrain food intake by binding it to its receptor in the brain and inducing energy expenditure.38 Obesity promotes multiple cellular processes that attenuate leptin signaling, leading to leptin resistance and accumulation of leptin in serum.39 CRE showed a significant dose-dependent reduction in the serum leptin levels, suggesting that the anti-obesity activity may be mediated by relieving leptin resistance and inducing energy expenditure. Stress is another factor known to be involved in obesity. It can cause physiological and neuroendocrinological changes associated with increased food intake and adipogenesis.40 Corticosterone (cortisol in humans), a glucocorticoid (GC) hormone, has been reported to redistribute white adipose tissue to the abdominal region, increasing appetite with a preference for energy-dense food.41 Supplementation with CRE reduced the HFD-induced serum corticosterone levels.
Interestingly, the glucocorticoids released during stress can stimulate adipocyte leptin gene expression.42 Macedo et al have reported an association between HFD induced TG levels, stress, and leptin levels.43 Serum triglyceride levels reduce the transport of leptin to the brain and cause leptin resistance in obesity.44 We observed a reduction in TG, leptin, and the stress hormone corticosterone in treated animals, suggesting that the CRE may act on this axis to reduce stress and serum TG, further reducing leptin resistance and inducing energy expenditure.
In conclusion, overweight individuals supplemented with CRE reported a statistically significant decrease in their clinical symptoms like weight, BMI, and waist circumference and improved serum lipid profiles by the end of the study duration. The extract effectively prevented weight gain induced by a high-fat diet in mice and showed a significant reduction in adipogenesis in vitro. Although the detailed mechanism of action needs to be further explored, anti-hyperlipidemic activity and reduction in stress could be the mechanism of weight loss. Combating leptin resistance could be another possible mechanism of action of the extract. This is the first clinical trial reporting the anti-adipogenic effect of a standardized C. rotundus extract containing Stilbenoid dimers to the best of our knowledge. The clinical study was conducted in a single location with few subjects, which is the limitation of the study. Estimation of biomarkers like leptin and adiponectin in the clinical study would have confirmed the preclinical study on the mechanism of action of CRE. Being the first study in humans, these parameters were not measured, which is also a limitation of the study. Future more extensive cohort studies will help understand the benefits of this extract in other comorbidities associated with obesity.
Conclusion
From the present results, it can be concluded that herbal composition from the rhizomes of C. rotundus comprising Piceatannol, Scirpusin A, and Scirpusin B could be potent and safe health adjuvant for managing hypercholesterolemia and obesity in humans. Thus, Cyperus rotundus extract is safe for human consumption and is also effective in managing weight and hypercholesterolemia in humans.
Data Sharing Statement
The authors confirm that all the data pertaining to the clinical trial are included in the main manuscript and the supplementary section.
Acknowledgments
The animal study was conducted in collaboration with LIVEON BIOLABS Pvt Ltd, Bangalore, and the clinical study was conducted in Government Ayurvedic Medical College (GAMC), Mysore by Syncretic Clinical Research Services (SMO) and ClinWorld Private Limited (CRO). The authors thank Dr. Rajendra V (Principle investigator, GAMC, Mysore), Dr. Srinivasa M (Investigator for handling adverse events, Srinivasa Health clinic, Mysore), and Mr. Raveendranath Gaddam (Statistician) for their contribution to the clinical study. The authors gratefully acknowledge all the participants, doctors, and clinical trial monitors for their contribution.
Disclosure
All the authors are affiliated with Sami Labs Limited or Sabinsa Corporation. Data described in the current study partly forms the basis for the patent applications. Dr Muhammed Majeed has a patent Composition comprising scirpusin A and scirpusin B and anti-adipogenesis/anti-obesity potential thereof issued to Sami Labs; Dr Kalyanam Nagabhushanam has a patent US9782450 issued to Sami Labs Limited, a patent US10172903 issued to Sami Labs Limited, a patent US9387193 issued to Sami Labs Limited; Dr Beena Bhat has a patent US9387193 issued to Sami Labs; Dr Anjali Pandey reports a patent United States Of America US9387193; Dr Sarang Bani reports a patent United States of America US9387193. The authors report no other conflicts of interest in this work.
References
1. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi:10.1001/jama.289.1.76
2. World Health Organization. Obesity and overweight; 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
3. Garg SK, Maurer H, Reed K, Selagamsetty R. Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab. 2014;16(2):97–110. doi:10.1111/dom.12124
4. Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104(4):531–543. doi:10.1016/S0092-8674(01)00240-9
5. Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15(9):639–660. doi:10.1038/nrd.2016.75
6. Gooda Sahib N, Saari N, Ismail A, Khatib A, Mahomoodally F, Abdul Hamid A. Plants’ metabolites as potential antiobesity agents. Sci World J. 2012;2012:436039. doi:10.1100/2012/436039
7. Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5(3):93.
8. Chou Y, Ho C, Pan M. Stilbenes: chemistry and molecular mechanisms of anti-obesity. Curr Pharmacol Rep. 2018;4:202–209. doi:10.1007/s40495-018-0134-5
9. Almagro L, Belchí-Navarro S, Sabater-Jara AB, et al. Bioproduction of trans-resveratrol from Grapevine cell cultures. In: Ramawat KG, Mérillon J-M, editors. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013:1683–1713.
10. Kershaw J, Kim K-H. The therapeutic potential of piceatannol, a natural stilbene, in metabolic diseases: a review. J Med Food. 2017;20(5):427–438. doi:10.1089/jmf.2017.3916
11. Hong EH, Heo EY, Song JH, et al. Trans-scirpusin A showed antitumor effects via autophagy activation and apoptosis induction of colorectal cancer cells. Oncotarget. 2017;8(25):41401–41411. doi:10.18632/oncotarget.17388
12. Sano S, Sugiyama K, Ito T, Katano Y, Ishihata A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J Agric Food Chem. 2011;59(11):6209–6213. doi:10.1021/jf104959t
13. Nakajima K, Taguchi H, Endo T, Yosioka I. The constituents of Scirpus fluviatilis (TORR.) A. GRAY. I.: the structures of two new hydroxystilbene dimers, Scirpusin A and B. Chem Pharm Bull. 1978;26(10):3050–3057. doi:10.1248/cpb.26.3050
14. Majeed M, Kalyanam N, Sarang B, et al.; Inventors; Sami Labs Limited (Bangalore, IN), assignee. Composition comprising scirpusin A and scirpusin B and anti-adipogenesis/anti-obesity potential thereof; 2016.
15. Peerzada AM, Ali HH, Naeem M, Latif M, Bukhari AH, Tanveer A. Cyperus rotundus L.: traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol. 2015;174:540–560. doi:10.1016/j.jep.2015.08.012
16. Jung SH, Kim SJ, Jun BG, et al. Alpha-cyperone, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced COX-2 expression and PGE2 production through the negative regulation of NFkappaB signalling in RAW 264.7 cells. J Ethnopharmacol. 2013;147(1):208–214. doi:10.1016/j.jep.2013.02.034
17. Kamala A, Middha SK, Karigar CS. Plants in traditional medicine with special reference to Cyperus rotundus L: a review. 3 Biotech. 2018;8(7):309. doi:10.1007/s13205-018-1328-6
18. Trivedi VP, Mann AS. Vegetable drugs regulating fat metabolism in caraka (Lekhaniya Dravyas). Q J Crude Drug Res. 1972;12(4):1988–1999. doi:10.3109/13880207209083244
19. Lemaure B, Touche A, Zbinden I, et al. Administration of cyperus rotundus tubers extract prevents weight gain in obese Zucker rats. Phytother Res. 2007;21(8):724–730. doi:10.1002/ptr.2147
20. Mohamed GA. Iridoids and other constituents from Cyperus rotundus L. rhizomes. Bull Fac Pharm Cairo Univ. 2015;53(1):5–9. doi:10.1016/j.bfopcu.2015.01.001
21. Lin S-Q, Zhou Z-L, Zhang H-L, Yin W-Q. Phenolic glycosides from the rhizomes of Cyperus rotundus and their antidepressant activity. J Korean Soc Appl Biol Chem. 2015;58(5):685–691. doi:10.1007/s13765-015-0092-0
22. Hu Q-P, Cao X-M, Hao D-L, Zhang -L-L. Chemical composition, antioxidant, DNA damage protective, cytotoxic and antibacterial activities of cyperus rotundus rhizomes essential oil against foodborne pathogens. Sci Rep. 2017;7(1):45231. doi:10.1038/srep45231
23. Morrison S, McGee SL. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte. 2015;4(4):295–302. doi:10.1080/21623945.2015.1040612
24. Setoguchi Y, Oritani Y, Ito R, et al. Absorption and metabolism of piceatannol in rats. J Agric Food Chem. 2014;62(12):2541–2548. doi:10.1021/jf404694y
25. Kwon JY, Seo SG, Heo YS, et al. Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J Biol Chem. 2012;287(14):11566–11578. doi:10.1074/jbc.M111.259721
26. Tung YC, Lin YH, Chen HJ, et al. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules. 2016;21(11):1419. doi:10.3390/molecules21111419
27. Brown JD, Buscemi J, Milsom V, Malcolm R, O’Neil PM. Effects on cardiovascular risk factors of weight losses limited to 5–10. Transl Behav Med. 2016;6(3):339–346. doi:10.1007/s13142-015-0353-9
28. Ranasinghe C, Gamage P, Katulanda P, Andraweera N, Thilakarathne S, Tharanga P. Relationship between body mass index (BMI) and body fat percentage, estimated by bioelectrical impedance, in a group of Sri Lankan adults: a cross sectional study. BMC Public Health. 2013;13(1):797. doi:10.1186/1471-2458-13-797
29. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi:10.1016/S0140-6736(05)67402-8
30. Lam BC, Koh GC, Chen C, Wong MT, Fallows SJ. Comparison of Body Mass Index (BMI), Body Adiposity Index (BAI), Waist Circumference (WC), Waist-To-Hip Ratio (WHR) and Waist-To-Height Ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS One. 2015;10(4):e0122985. doi:10.1371/journal.pone.0122985
31. Fischer S, Schatz U, Julius U. Practical recommendations for the management of hyperlipidemia. Atheroscler Suppl. 2015;18:194–198. doi:10.1016/j.atherosclerosissup.2015.02.029
32. Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25(6):293–302. doi:10.1016/j.tem.2014.04.001
33. Spiegelman BM, Frank M, Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983;258(16):10083–10089. doi:10.1016/S0021-9258(17)44608-4
34. Elmasri H, Karaaslan C, Teper Y, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23(11):3865–3873. doi:10.1096/fj.09-134882
35. Yin W, Carballo-Jane E, McLaren DG, et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res. 2012;53(1):51–65. doi:10.1194/jlr.M019927
36. Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr. 2005;135(11):2499–2502. doi:10.1093/jn/135.11.2499
37. Ahima RS. Revisiting leptin’s role in obesity and weight loss. J Clin Invest. 2008;118(7):2380–2383. doi:10.1172/JCI36284
38. Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi:10.1038/32911
39. Myers MG
40. Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obesity Reviews. 2001;2(2):73–86. doi:10.1046/j.1467-789x.2001.00027.x
41. Fardet L, Feve B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74(15):1731–1745. doi:10.1007/s40265-014-0282-9
42. Masuzaki H, Ogawa Y, Hosoda K, et al. Glucocorticoid regulation of leptin synthesis and secretion in humans: elevated plasma leptin levels in Cushing’s syndrome. J Clin Endocrinol Metab. 1997;82(8):2542–2547. doi:10.1210/jcem.82.8.4128
43. Macedo IC, Medeiros LF, Oliveira C, et al. Cafeteria diet-induced obesity plus chronic stress alter serum leptin levels. Peptides. 2012;38(1):189–196. doi:10.1016/j.peptides.2012.08.007
44. Banks WA, Coon AB, Robinson SM, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53(5):1253–1260. doi:10.2337/diabetes.53.5.1253
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.