Back to Journals » Infection and Drug Resistance » Volume 12
The Anti-Mycobacterial Activity Of Ag, ZnO, And Ag- ZnO Nanoparticles Against MDR- And XDR-Mycobacterium tuberculosis
Authors Heidary M, Zaker Bostanabad S, Amini SM, Jafari A, Ghalami Nobar M, Ghodousi A, Kamalzadeh M, Darban-Sarokhalil D
Received 30 June 2019
Accepted for publication 2 October 2019
Published 4 November 2019 Volume 2019:12 Pages 3425—3435
DOI https://doi.org/10.2147/IDR.S221408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Mohsen Heidary,1,2 Saeed Zaker Bostanabad,3,4 Seyed Mohammad Amini,5 Alireza Jafari,6 Mostafa Ghalami Nobar,4,7 Arash Ghodousi,8 Morteza Kamalzadeh,9 Davood Darban-Sarokhalil1
1Department of Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran; 2Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran; 3Microbiology Department, Islamic Azad University-Parand Branch, Tehran, Iran; 4Mycobacteriology Department, Massoud Laboratory, Tehran, Iran; 5Radiation Biology Research Center, Iran University of Medical Sciences (IUMS), Tehran, Iran; 6Inflammatory Lung Diseases Research Center, Department of Internal Medicine, Razi Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran; 7Reference Health Laboratory of Iran (RHL), Ministry of Health and Medical Education, Tehran, Iran; 8Emerging Bacterial Pathogens Unit, Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, Milano, Italy; 9Quality Control, Department, Razi Vaccine and Research Institute, Agricultural Research Education and Extension Organization, Karaj, Iran
Correspondence: Davood Darban-Sarokhalil
Department of Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Email [email protected]
Background: Nowadays, tuberculosis (TB) is one of the top ten leading causes of mortality worldwide. The emergence of multidrug-resistant (MDR) – and extensively drug-resistant (XDR) – Mycobacterium tuberculosis (M. tuberculosis) is identified as one of the most challenging threats to TB control. Thus, new and safe nano-drugs are urgently required for the elimination of TB. The aim of this study was to investigate the anti-bacterial effects of Ag, ZnO, and Ag-ZnO nanoparticles (NPs) on MDR- and XDR-M. tuberculosis.
Materials and methods: In this study, Ag, ZnO, and Ag-ZnO NPs were synthesized by the chemical reduction and chemical deposition methods. NPs were characterized using ultraviolet–visible spectroscopy, dynamic light scattering, and transmission electron microscopy. Then, various dilutions of NPs were prepared and their minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) were determined against M. tuberculosis strains using the broth microdilution and agar microdilution methods. Finally, MTT test and cell culture assay were performed.
Results: The effects of concentrations of 1–128 μg/mL Ag NPs, ZnO NPs, 2Ag: 8ZnO, 8Ag:2ZnO, 3Ag: 7ZnO, 7Ag:3ZnO, and 5Ag:5ZnO on M. tuberculosis strains were investigated. MIC results showed the inhibitory effect of 1 μg/mL of all NPs against XDR-M. tuberculosis. In addition, the concentrations of 4 μg/mL Ag, 8 μg/mL 5Ag:5ZnO, 8 μg/mL 7Ag:3ZnO, 32 μg/mL 3Ag:7ZnO, 16 μg/mL 8Ag:2ZnO, and 64 μg/mL 2Ag:8ZnO inhibited MDR-M. tuberculosis growth. However, MBC results indicated the inability of Ag, ZnO and Ag-ZnO NPs, either in combination or alone, to kill MDR- or XDR-M. tuberculosis.
Conclusion: To the best of our knowledge, this is the first study to evaluate the effects of Ag and ZnO NPs against MDR and XDR strains of M. tuberculosis. According to the results, Ag and ZnO NPs showed bacteriostatic effects against drug-resistant strains of M. tuberculosis. Therefore, these NPs may be considered as promising anti-mycobacterial nano-drugs. However, further studies are required to affirm the bactericidal effects of these NPs against TB.
Keywords: Mycobacterium tuberculosis, silver, zinc oxide, nanoparticle, MDR-TB, XDR-TB
Introduction
Tuberculosis (TB) is one of the top ten leading causes of death accounting for a high mortality rate compared to other infectious diseases worldwide. According to the latest reports of WHO, about 10.4 million new cases including 6.2 million men, 3.2 million women, and 1 million children are infected with TB worldwide.1,2 Recently, the emergence of multidrug-resistant (MDR)- and extensively drug-resistant (XDR)-Mycobacterium tuberculosis has complicated TB control,3,4 necessitating advanced research and novel tools for the treatment and eradication of TB during the End-TB era.5 As novel potential platforms, nanoscale structures can destroy bacterial structures thereby inhibiting their growth. Employing these NPs has several advantages, including the provision of noninvasive cell access, production of ROS, and increased localized activities of ionizing radiation.6 Silver (Ag) and zinc oxide (ZnO) nanoparticles (NPs) are two nanotechnology products with numerous applications in various fields of health care and medical sciences in recent years.7–9 Ag and ZnO NPs possess a wide range of antibacterial properties and can rarely lead to the development of bacterial resistance.10 The unique physicochemical properties of Ag NPs, including small-scale size, ability to interact with biomolecules, and possessing a uniform morphology have reinforced their antimicrobial activity.11 Moreover, the antibacterial effects of ZnO NPs against eczema, slight excoriation disorders, wound, and hemorrhoids have long been known.12 To the best of our knowledge, few studies have investigated the effects of Ag and ZnO NPs against MDR and XDR strains of M. tuberculosis. Accordingly, the aim of this study was to evaluate the antibacterial activity of Ag, ZnO, and Ag-ZnO NPs against H37Rv, MDR, and XDR strains of M. tuberculosis using minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests.
Materials And Methods
Identification Of Mycobacterial Strains
The MDR, XDR, and H37Rv (ATCC 27294) strains of M. tuberculosis were obtained from Massoud laboratory, Tehran, Iran. For confirmation, Ziehl Neelsen staining was carried out. In addition, following decontamination by the Petroff’s method, samples were inoculated into Lowenstein–Jensen (LJ) media (Merck, Germany) and incubated at 37°C for 56 days. Next, bacterial suspension was prepared and adjusted to the turbidity of 1 McFarland standard (3×108 CFU/mL).13
DNA Extraction
Mycobacterial DNAs were extracted using phenol-chloroform isoamyl alcohol DNA extraction method according to the study of Torkaman et al.14 Simply, 1.5 mL of a loopful of bacterial culture was incubated at 80°C for 20 mins. Then, it was re-suspended in a freshly prepared Tris-EDTA buffer containing lysozyme. Next, proteinase K was added, and the mixture was heated at 56°C for 30 mins. Finally, 80 μL of neutralizing buffer was added to the mixture and an equal volume of chloroform-isoamyl alcohol (24:1) was added to the tube. Mycobacterial DNAs were precipitated with isopropanol, washed with 70% ethanol, and re-dissolved in TE buffer.
Polymerase Chain Reaction Assay
Mycobacterial strains were identified as M. tuberculosis complex using PCR method via an Eppendorf thermocycler (Eppendorf, Germany). The extracted DNA was amplified using the following primers: IS6110-F (5′-CCTGCGAGCGTAGGCGTCGG-3′) and IS6110-R (5′-CTCGTCCAGCGCCGCTTCGG-3′).15 DNA template was amplified in 35 cycles consisting of denaturation at 95°C for 30 s, annealing at 65°C for 30 s, and extension at 72°C for 20 s.16
Drug Susceptibility Testing
The drug susceptibility testing used for the identification of MDR and XDR strains of M. tuberculosis was based on the agar proportion method. In this assay, the capability of strains to grow on LJ medium containing a critical concentration (the lowest concentration that inhibits wild strains) of a single drug was investigated. MDR strain of M. tuberculosis was defined as M. tuberculosis strain resistant to at least isoniazid and rifampin (first-line anti-TB drugs). XDR strain of M. tuberculosis was defined as the MDR strain resistant to at least one fluoroquinolone and a second-line injectable drug (amikacin, capreomycin, or kanamycin).17
Synthesis Of Ag NPs
Ag NPs were synthesized through a chemical reduction method with some modifications. Briefly, 0.5 mL silver nitrate (0.02 mM) (Merck, Germany) and 20 mL sodium citrate (0.08 M) (Merck, Germany) were mixed and then, 0.5 mL fresh ice-cooled sodium borohydride (0.02 M) (Merck, Germany) was injected to the mixture under vigorous stirring for 15 mins.18
Synthesis Of ZnO NPs
ZnO NPs were prepared based on a chemical precipitation method. Briefly, 1.2 g sodium hydroxide (Merck, Germany) was dissolved in 25 mL 100% methanol (Dr. Mojallali, Iran) and 1.097 g zinc acetate (Merck, Germany) was dissolved in 25 mL 100% methanol (Dr. Mojallali, Iran) under mild stirring for 60 mins. Then, zinc acetate was added dropwise to the sodium hydroxide solution. The final mixture was vigorously stirred for 3 hrs and the obtained ZnO NPs were centrifuged, washed, and dried for 48 hrs. The washing/drying process was repeated three times.19
Preparation Of Ag-ZnO NPs
Various ratios/dilutions of Ag-ZnO NPs were prepared following the mixture of Ag and ZnO NPs. These ratios/dilutions included 2Ag: 8ZnO, 8Ag:2ZnO, 3Ag: 7ZnO, 7Ag:3ZnO, and 5Ag:5ZnO.
Characterization Of Ag-ZnO NPs
Particle size and size distribution of the synthetized Ag and ZnO NPs were determined by dynamic light scattering (DLS) (Scatteroscope-I, Korea). The samples were diluted to be suitable for DLS characterization and the experiment was carried out at room temperature (24°C).20
The ultraviolet–visible (UV–Vis) spectrum absorption of Ag and ZnO NPs were recorded by UV–Vis spectrometer (Analytik Jena, Germany) with an optical resolution of 0.01nm full width at half maximum. The spectrum response was taken within a range of 200–800 nm at regular time intervals in a 4×1×1 cm path quartz cuvette.20
In addition, the exact concentration of silver and zinc ions were determined using inductively coupled plasma mass spectrometry (ICP-MS, Varian VISTA PRO, USA).
Finally, the morphology, average particle size, size distribution, and crystallinity of the particles were characterized by transmission electron microscopy at an operating voltage of 100 kV (TEM, Zeiss-EM10C, Germany). TEM samples were prepared by homogenizing a suspension of ZnO/ethanol or Ag/deionized water with 1 mM concentration in an ultrasonic vibrator and dropping a few droplets of the mixed suspension on the holy carbon-coated grid copper mesh 300 followed by the solvent evaporation at room temperature.21
Broth Microdilution Method
A specific broth microdilution test for M. tuberculosis called the Microplate Alamar Blue Assay (MABA) was performed to determine the MIC of various concentrations of Ag NPs, ZnO NPs, and various ratios/dilutions of Ag-ZnO NPs.22 In this method, the wells of sterile 96-well plates (Dr. Mojallali, Iran) in rows B to G of columns 3 to 11 received 100 µL Middle-brook 7H9 broth (BD diagnostics, USA). To minimize the evaporation of Middle-brook 7H9 broth during incubation, 200 µL of sterile deionized water was added to all outer-perimeter wells. Then, 100 µL of the concentrations of 1 µg/mL, 2 µg/mL, 4 µg/mL, 8 µg/mL, 16 µg/mL, 32 µg/mL, 64 µg/mL, and 128 µg/mL of Ag and ZnO NPs, as well as ratios/dilutions of 2Ag: 8ZnO, 8Ag:2ZnO, 3Ag: 7ZnO, 7Ag:3ZnO, and 5Ag:5ZnO mixed NPs were added to the wells in rows B to G of columns 2 and 3. The wells in rows B to G of column 2 were considered as negative control (bacteria-free). Serial dilution was prepared from column 3 to column 10. Next, 100 µL of the suspension of MDR, XDR, and H37Rv strains of M. tuberculosis with a turbidity equal to 1 McFarland standard (3×108 CFU/mL) were added to the wells in rows B to G of columns 3 to 11. The wells in rows B to G of column 11 were considered as positive control (drug-free). The plates were sealed with Parafilm and incubated at 37°C for 5 days. Then, 50 µL of Alamar Blue reagent (BD diagnostics, USA) was added to all wells in the microplate and followed for up to 2 days. A blue color in the well was interpreted as no growth of M. tuberculosis, and a pink color was scored as bacterial growth. The MIC was considered as the lowest concentration of the NPs which prevented a color change from blue to pink. Each two rows of 96-well plates carried one M. tuberculosis strain. To elucidate, the wells of rows B and C were treated with M. tuberculosis H37Rv, the wells of rows D and E were treated with the MDR strain, and the wells of rows F and G were treated with the XDR strain.
Agar Microdilution Method
A routine agar microdilution test was performed for M. tuberculosis strains treated with Ag and ZnO NPs as well as various ratios/dilutions of Ag-ZnO NPs to determine their MBC.21 This test was performed for the wells that prevented a color change from blue to pink (MIC positive tests). The contents of these wells, including a mixture of the M. tuberculosis strains, Middle-brook 7H9 broth, and the NPs were cultured on LJ medium and incubated at 37°C for 56 days. Next, McCartney bottles were removed from the incubator and the colony-forming units (CFU) of M. tuberculosis strains were counted.
MTT Test
Cell viability following exposure to Ag and ZnO NPs was evaluated using the MTT test.23 THP-1 cells were seeded in 96-well plate at 1×104 cell/well in RPMI 1640 medium. Serial dilutions of Ag and ZnO NPs were prepared. Then, cells were treated with Ag and ZnO NPs in a 5% CO2 incubator at 37°C for 24 hrs. Since the THP-1 cells were non-adherent, microplates were centrifuged at 1200 rpm for 5 mins to allow cells to attach to the bottom of the plate. Supernatant was carefully removed and replaced with 100 µL of ready-to-use RPMI 1640. Next, 10 µL of the MTT stock solution was added to each well and the microplates were incubated at 37°C for 4 hrs. Then, 50 μL of DMSO was added to each well and completely mixed. After incubation at 37°C for 10 mins, the plate was read at 570 nm by a microplate reader.
Cell Culture
The THP-1 cell line (human monocytic leukemia cell line) was provided by Razi Vaccine and Serum Research Institute, Tehran, Iran. The THP-1 cells were transferred to culture media containing 9 mL RPMI 1640 medium (Gibco, USA) with 10% FBS (Gibco, USA) and 100 units/mL of penicillin/streptomycin (Sigma-Aldrich, USA). Next, cells were cultured in 96-well microplates and incubated at 37°C in a humidified incubator with 5% CO2 atmosphere to assess the cell viability. About 24 hrs before treatment of THP-1 with M. tuberculosis strains, phorbol myristate acetate(SIGMA, USA) was added to wells in order to activate phagocytosis of bacteria. Then, 10 µL of bacterial suspension with turbidity equal to 1 McFarland standard (3×108 CFU/mL) was added to each well. After incubation at 37°C for 3 hrs, the infected macrophages were treated with various ratios/dilutions of Ag and ZnO NPs and incubated at 37°C for 24 hrs. Finally, the macrophages were lysed with 0.05% SDS and cultured in LJ medium.18
Statistical Analysis
In the current study, statistical analyses were performed using Microsoft office excel software (Version 2017). A p-value less than 0.05 was considered statistically significant (p<0.05). Data were expressed as mean±SD. Diagrams were drawn with Origin 2015 (OriginLab Co., USA) software. TEM micrographs were analyzed using Digital Micrograph® and Origin 2015.
Results
Mycobacterial Strains
Following Ziehl Neelsen staining, acid-fast bacilli were observed in all 100 fields of the light microscope with 1000x magnification. Sputum specimens were culture-positive for M. tuberculosis complex and molecular amplification testing confirmed the genetic relatedness of strains to M. tuberculosis. According to the results of drug susceptibility testing (agar proportion method), these M. tuberculosis strains were determined as MDR and XDR.
Characterization Of NPs
Based on the DLS assay, Ag and ZnO NPs were mono-dispersed and homogeneous. The UV–Vis spectrum absorption of Ag and ZnO NPs in the range of 300−500 nm exhibited an absorption peak at 420 and 350 nm, respectively (Figures 1 and 2). Furthermore, the average zeta potential values of Ag and ZnO NPs were reported −1.5 and −9.3, respectively, which is indicative of their small size and high stability.
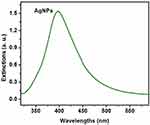 |
Figure 1 UV-visible spectrum absorption of Ag nanoparticles. |
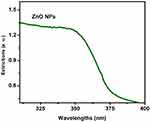 |
Figure 2 UV-visible spectrum absorption of ZnO nanoparticles. |
The TEM micrographs showing the morphology, size, and size distribution of the Ag and ZnO NPs are presented in Figures 3 and 4. As expected, the majority of Ag and ZnO NPs were spherical with respective average particle sizes of 5.4±2.6 nm and 9.3±3.9 nm (Figures 5 and 6).
 |
Figure 3 TEM image of Ag nanoparticles on a scale of 50 nm. |
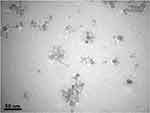 |
Figure 4 TEM image of ZnO nanoparticles on a scale of 50 nm. |
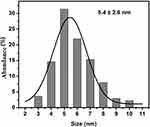 |
Figure 5 Size distribution of Ag nanoparticles. |
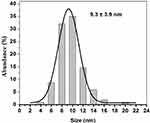 |
Figure 6 Size distribution of ZnO nanoparticles. |
The results of ICP-MS were used for determining the initial concentrations of Ag and ZnO NPs. Based on our results, the concentration of silver and zinc ions was 512 µg/mL.
Broth Microdilution Method
The MICs of Ag and ZnO NPs as well as various ratios/dilutions of Ag-ZnO NPs were assessed against MDR, XDR, and H37Rv strains of M. tuberculosis by the MABA test.
According to the results, 1 µg/mL of Ag NPs, ZnO NPs, 2Ag:8ZnO NPs, 8Ag:2ZnO NPs, 3Ag: 7ZnO NPs, 7Ag:3ZnO NPs, and 5Ag:5ZnO NPs was the lowest concentration inhibiting the growth of the XDR strain of M. tuberculosis (Figures 7–9).
 |
Figure 7 The MIC results of concentrations of 1 µg/mL to 128 µg/mL of 5Ag:5ZnO on H37Rv, MDR, and XDR strains of M. tuberculosis. |
 |
Figure 8 The MIC results of 1−128 µg/mL for 2Ag:8ZnO on the H37Rv, MDR, and XDR strains of M. tuberculosis. |
 |
Figure 9 The MIC results of concentrations of 1–128 µg/mL for 8Ag:2ZnO on the H37Rv, MDR, and XDR strains of M. tuberculosis. |
The concentrations of 4 µg/mL Ag NPs, 8 µg/mL 5Ag:5ZnO NPs, 8 µg/mL 7Ag:3ZnO NPs, 32 µg/mL 3Ag:7ZnO NPs, 16 µg/mL 8Ag:2ZnO NPs, and 64 µg/mL 2Ag:8ZnO NPs were the lowest concentrations inhibiting the growth of the MDR strain of M. tuberculosis (Figures 7–9).
Furthermore, 1 µg/mL Ag NPs, 1 µg/mL 5Ag:5ZnO NPs, 16 µg/mL 7Ag:3ZnO NPs, 32 µg/mL of 3Ag:7ZnO NPs, 16 µg/mL 8Ag:2ZnO NPs, and 32 µg/mL 2Ag:8ZnO NPs were the lowest concentrations inhibiting the growth of M. tuberculosis H37Rv (Figures 7–9).
Agar Microdilution Method
Agar microdilution method was performed to evaluate the MBCs of Ag and ZnO NPs as well as ratios/dilutions of 2Ag: 8ZnO, 8Ag:2ZnO, 3Ag: 7ZnO, 7Ag:3ZnO, and 5Ag:5ZnO mixed NPs against MDR, XDR, and H37Rv strains of M. tuberculosis. After treatment, M. tuberculosis strains were cultured on LJ medium and incubated at 37°C for 56 days. Then, the CFUs of M. tuberculosis strains were reported according to the protocols of World Health Organization (Table 1). The Ag and ZnO NPs as well as ratios/dilutions of Ag-ZnO NPs showed concentration-dependent activity against M. tuberculosis strains. In fact, the CFUs of strains were decreased with increased concentrations of the NPs (Table 2). However, the concentrations of 1 µg/mL, 2 µg/mL, 4 µg/mL, 8 µg/mL, 16 µg/mL, 32 µg/mL, 64 µg/mL, and 128 µg/mL of Ag, ZnO, and Ag-ZnO NPs were unable to kill M. tuberculosis strains. Therefore, none of these concentrations had bactericidal effects on M. tuberculosis strains.
 |
Table 1 Grading Scale For The Culture Of Mycobacterium tuberculosis On The LJ Media |
 |
Table 2 The MBC Results Of Ag, ZnO, And Ag-ZnO Nanoparticles On H37Rv, MDR, And XDR Strains Of M. tuberculosis |
MTT Test
MTT test was applied to determine the survival of THP-1 cells treated with the concentrations of 1 µg/mL, 2 µg/mL, 4 µg/mL, 8 µg/mL, 16 µg/mL, 32 µg/mL, 64 and 128 µg/mL of Ag NPs, ZnO NPs, 2Ag: 8ZnO, 8Ag:2ZnO, 3Ag: 7ZnO, 7Ag:3ZnO, and 5Ag:5ZnO. The results of MTT assay showed the high toxicity of 128 µg/mL Ag NPs against the THP-1 cell line, leading to the destruction of half of the cells (Figure 10). Apparently, the higher toxicity of Ag NPs on THP-1 cells compared to that of ZnO NPs is due to using sodium borohydride in Ag NPs synthesis. In addition, the number of viable THP-1 cells increased with decreased concentrations of Ag and ZnO NPs as well as ratios/dilutions of Ag-ZnO NPs (Figure 10).
 |
Figure 10 The percentage viability of THP-1 cells in exposure of Ag, ZnO, and Ag-ZnO nanoparticles. |
Cell Culture
Cell culture was performed to determine the bactericidal effects of Ag, ZnO, and Ag-ZnO NPs against MDR, XDR, and H37Rv strains of M. tuberculosis phagocytized by the THP-1 cell line. The results showed poor antibacterial activities of the Ag, ZnO, and Ag-ZnO NPs. As expected, these results confirmed the results of the agar microdilution method (Table 2). The CFUs of M. tuberculosis strains decreased with increased concentrations of Ag, ZnO, and Ag-ZnO NPs. However, the Ag, ZnO, and Ag-ZnO NPs were unable to eliminate the M. tuberculosis strains in the THP-1 cells and the strains treated with NPs showed growth in the LJ medium.
Discussion
Nowadays, TB is one of the most common infectious diseases in developing countries and has remained as one of the main causes of morbidity and mortality worldwide.24 Immediately after the introduction of antibiotic therapy for TB treatment, the emergence of drug-resistant M. tuberculosis was reported during 1940–1950. Soon after, it was found that patients suffering from MDR- and XDR-TB were less likely to be cured.25,26
As potential novel therapeutic options, Ag and ZnO NPs can destroy drug-resistant bacteria. Although the exact mechanism of Ag NPs on bacteria is still unknown, two antibacterial mechanisms are confirmed including contact killing and ion-mediated killing. In addition, the proposed antibacterial mechanism of ZnO NPs is the ROS producing capacity, which destroys the bacterial membrane and decreases DNA, proteins, and survival.27,28
In order to develop an effective therapeutic approach for TB infection, small-sized Ag and ZnO NPs were synthesized. Ag NPs were prepared through a chemical approach by which Ag ions were reduced with sodium borohydride in the presence of citrate ions. This approach has been completely discussed in our previous reports.7,11 On the other hand, ZnO NPs were precipitated in methanol solution using zinc acetate and sodium hydroxide19 and were synthesized in a non-aqueous medium. Although previous studies have shown the prolonged stability of ZnO NPs,11,19 fresh NPs were used here for each assay. The hydrodynamic diameters of both Ag and ZnO NPs obtained by the DLS instrument were larger than those obtained by TEM micrographs in virtue of different analysis systems. TEM is a quantitative instrument, while DLS is an intensity-based one. Therefore, TEM gives the exact sizes of NPs in the dry state (core size); whereas DLS shows the hydrodynamic diameter in the solvated state (core size+adsorbed atoms).29,30 In addition, the larger hydrodynamic diameters of small metal NPs compared to those obtained by TEM micrographs have been attributed to the flocculation of small particles.31
In 2016, studies have suggested the ability of Ag-ZnO NPs to inhibit the growth of the standard laboratory strain of M. tuberculosis H37Rv.32 However, there is no sufficient data regarding the antibacterial effects of Ag, ZnO, and Ag-ZnO NPs against the drug-resistant clinical strains of M. tuberculosis. In the study performed by Jafari et al, the effects of Ag, ZnO, and Ag-ZnO NPs were evaluated against M. tuberculosis H37Rv phagocytized by THP-1 cell lines. They showed the ability of Ag-ZnO NPs to kill M. tuberculosis H37Rv in the THP-1 cells in the concentration of 8192 µg/mL. However, other concentrations of Ag-ZnO NPs did not show any bactericidal activity against M. tuberculosis H37Rv.21
Researchers have demonstrated a broad spectrum of antimicrobial activity of Ag NPs against approximately 700 pathogens. Therefore, Ag NPs are supposedly one of the most promising alternatives to anti-TB agents.33 In the present study, different concentrations of Ag NPs demonstrated concentration-dependent efficacy on all three studied M. tuberculosis strains. The MIC of Ag NPs was 1 µg/mL against XDR and H37Rv strains of M. tuberculosis, while the MDR strain of M. tuberculosis was inhibited within the MIC of 4 µg/mL. Banu et al evaluated the antimicrobial effect of Ag NPs against the MDR and XDR clinical isolates of M. tuberculosis by the MABA method. According to their results, the concentration range of 6.25 to 12.5 µg/mL of Ag NPs could inhibit the growth of MDR and XDR isolates of M. tuberculosis.34
Antibacterial effects of ZnO NPs have always received global notice.35 Our study showed concentration-dependent effects of ZnO NPs against M. tuberculosis strains. Both H37Rv and XDR strains of M. tuberculosis were inhibited at concentrations greater than or equal to 1 µg/mL of ZnO NPs. Moreover, the concentration of 4 µg/mL was determined as the MIC of ZnO NPs against the MDR strain of M. tuberculosis.
The synergistic activities of Ag and ZnO NPs have been proven against various bacteria.21 The results of the current study showed the greater effect of the combination of Ag and ZnO NPs against the M. tuberculosis strains compared to that of each NP alone. The highest synergistic effect was observed when equal volumes of Ag and ZnO NPs were applied simultaneously.
In this study, the MIC of Ag-ZnO NPs against the drug-resistant strains of M. tuberculosis was less than that against sensitive strains. Thus, Ag-ZnO NPs were more effective against resistant strains. In conclusion, antibiotic resistance does not lead to the reduced antibacterial activity of Ag-ZnO NPs against M. tuberculosis. This may probably be due to differences in the mechanism of actions adopted by Ag-ZnO NPs and antibiotics.
MBC results demonstrated that the concentrations less than 128 µg/mL of Ag, ZnO, and Ag-ZnO NPs were not able to kill M. tuberculosis strains. However, it should be noted that the CFUs of M. tuberculosis strains decreased with increased concentrations of Ag and ZnO NPs as well as increased dilution ratio of Ag-ZnO NPs. As expected, cell culture method confirmed the results of the MBC assay and M. tuberculosis strains in the THP-1 cells treated with NPs were grown in the LJ medium. Siddiqi et al reported the complete destruction of Mycobacterium smegmatis and Mycobacterium bovis following exposure to 1000 μg/mL ZnO NPs.36 However, since concentrations more than 100 μg/mL of ZnO NPs were highly toxic to THP-1 cells, we did not use concentrations greater than 128 µg/mL for M. tuberculosis killing. This also applies to Ag and Ag-ZnO NPs.
The results of MTT assay showed that the number of viable THP-1 cells increased with decreased concentrations of the NPs. Therefore, the Ag, ZnO, and Ag-ZnO NPs had a concentration-dependent toxicity against the THP-1 cell line. The results also indicated the lower toxic activity of ZnO NPs in the THP-1 cell line compared to Ag NPs. This may be due to the use of sodium borohydride in Ag NPs synthesis. In the study performed by Lanone et al, toxicity of ZnO NPs on macrophage cell lines was greater than Ag NPs. They synthesized the Ag NPs without using sodium borohydride.37
Although we made the Ag and ZnO NPs by chemical methods, the MIC of various ratios/dilutions of Ag-ZnO NPs was not significantly toxic. By producing biocompatible Ag and ZnO NPs from natural and plant materials, their toxicity could be reduced. Therefore, it is recommended that researchers synthesize biocompatible plant-derived secondary metabolites coating on Ag and ZnO NPs surfaces to decrease the detrimental effects on human health.38
Based on our results, Ag and ZnO NPs showed bacteriostatic activities against M. tuberculosis strains. Since sometimes these bacteria may go to latent phase and survive, further studies should be done on the bactericidal effects of these NPs against TB.39 Therefore, we suggest that the researchers work on the synergism between these NPs and the common anti-TB drugs or other effective anti-TB drugs (i.e., bedaquiline, linezolid, and clofazimine).
Conclusion
The results of the current study showed that 1 µg/mL of Ag and ZnO NPs can inhibit the growth of the XDR strains of M. tuberculosis. Moreover, 1–64 µg/mL of various dilutions of Ag-ZnO NPs can inhibit the MDR and H37Rv strains of M. tuberculosis. Therefore, Ag and ZnO NPs may be considered as promising options for TB infection therapy and may be used as an alternative to antibiotics. However, more investigations are required, and the simultaneous use of NPs and anti-TB drugs is recommended.
Acknowledgment
This research was supported by grant no: 30-02-96-33128 from Iran University of Medical Sciences.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Nasiri MJ, Imani Fooladi AA, Dabiri H, et al. Primary ethambutol resistance among Iranian pulmonary tuberculosis patients: a systematic review. Ther Adv Infect Dis. 2016;3(5):133–138. doi:10.1177/2049936116661962
2. Mirnejad R, Asadi A, Khoshnood S, et al. Clofazimine: a useful antibiotic for drug-resistant tuberculosis. Biomed Pharmacother. 2018;105:1353–1359. doi:10.1016/j.biopha.2018.06.023
3. Khoshnood S, Heidary M, Haeili M, et al. Novel vaccine candidates against Mycobacterium tuberculosis. Int J Biol Macromol. 2018. doi:10.1016/j.ijbiomac.2018.08.037
4. Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi:10.1016/S0140-6736(10)60410-2
5. Dias HMY, Pai M, Raviglione MC. Ending tuberculosis in India: a political challenge & an opportunity. Indian J Med Res. 2018;147(3):217. doi:10.4103/ijmr.IJMR_1375_16
6. Yaghini E, Pirker KF, Kay CW, Seifalian AM, MacRobert AJ. Quantification of reactive oxygen species generation by photoexcitation of pegylated quantum dots. Small. 2014;10(24):5106–5115. doi:10.1002/smll.201401209
7. Osanloo M, Amini SM, Sedaghat MM, Amani A. Larvicidal activity of chemically synthesized silver nanoparticles against anopheles stephensi. J Pharm Negat Results. 2018;10(1):2.
8. Jafari A, Ghane M, Sarabi M, Siyavoshifar F. Synthesis and antibacterial properties of zinc oxide combined with copper oxide nanocrystals. Orient J Chem. 2011;27(3):811.
9. Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomed. 2007;3(1):95–101. doi:10.1016/j.nano.2006.12.001
10. Fan W, Sun Q, Li Y, Tay FR, Fan B. Synergistic mechanism of Ag+–zn 2+ in anti-bacterial activity against Enterococcus faecalis and its application against dentin infection. J Nanobiotechnology. 2018;16(1):10. doi:10.1186/s12951-018-0336-3
11. Amiri S, Yousefi-Ahmadipour A, Hosseini M-J, et al. Maternal exposure to silver nanoparticles are associated with behavioral abnormalities in adulthood: role of mitochondria and innate immunity in developmental toxicity. Neurotoxicology. 2018;66:66–77. doi:10.1016/j.neuro.2018.03.006
12. Ma L, Zou X, Chen W. A new X-ray activated nanoparticle photosensitizer for cancer treatment. J Biomed Nanotechnol. 2014;10(8):1501–1508. doi:10.1166/jbn.2014.1954
13. Nasiri MJ, Rezaei F, Zamani S, et al. Drug resistance pattern of Mycobacterium tuberculosis isolates from patients of five provinces of Iran. Asian Pac J Trop Med. 2014;7(3):193–196. doi:10.1016/S1995-7645(14)60019-5
14. Torkaman MRA, Nasiri MJ, FaRnia P, Shahhosseiny MH, Mozafari M, Velayati AA. Estimation of recent transmission of Mycobacterium tuberculosis strains among Iranian and Afghan immigrants: a cluster-based study. J Clin Diagn Res. 2014;8(9):DC05. doi:10.7860/JCDR/2014/6788.3956
15. Narayanan S, Parandaman V, Narayanan P, et al. Evaluation of PCR using TRC4 and IS6110 primers in detection of tuberculous meningitis. J Clin Microbiol. 2001;39(5):2006–2008. doi:10.1128/JCM.39.5.2006-2008.2001
16. Farzam B, Fooladi AAI, Izadi M, Hossaini HM, Feizabadi MM. Comparison of cyp141 and IS6110 for detection of Mycobacterium tuberculosis from clinical specimens by PCR. J Infect Public Health. 2015;8(1):32–36. doi:10.1016/j.jiph.2014.08.005
17. Reller LB, Weinstein MP, Woods GL. Susceptibility testing for mycobacteria. Clin Infect Dis. 2000;31(5):1209–1215. doi:10.1086/317441
18. Jafari A, Mosavari N, Movahedzadeh F, et al. Bactericidal impact of Ag, ZnO and mixed AgZnO colloidal nanoparticles on H37Rv Mycobacterium tuberculosis phagocytized by THP-1 cell lines. Microb Pathog. 2017;110:335–344. doi:10.1016/j.micpath.2017.07.010
19. Ghaemi B, Mashinchian O, Mousavi T, Karimi R, Kharrazi S, Amani A. Harnessing the cancer radiation therapy by lanthanide-doped zinc oxide based theranostic nanoparticles. ACS Appl Mater Interfaces. 2016;8(5):3123–3134. doi:10.1021/acsami.5b10056
20. Thatoi P, Kerry RG, Gouda S, et al. Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J Photochem Photobiol. 2016;163:311–318. doi:10.1016/j.jphotobiol.2016.07.029
21. Jafari A, Jafari Nodooshan S, Safarkar R, et al. Toxicity effects of AgZnO nanoparticles and rifampicin on Mycobacterium tuberculosis into the macrophage. J Basic Microbiol. 2018;58(1):41–51. doi:10.1002/jobm.201700289
22. Franzblau SG, Witzig RS, McLaughlin JC, et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36(2):362–366.
23. Lopes LQS, de Oliveira PSB, de Souza Filho WP, et al. Glycerol monolaurate nanocapsules for biomedical applications: in vitro toxicological studies. Naunyn-Schmiedeberg’s Arch Pharmacol. 2019;392(9):1–10.
24. Nachega JB, Chaisson RE. Tuberculosis drug resistance: a global threat. Clin Infect Dis. 2003;36(Supplement_1):S24–S30. doi:10.1086/344657
25. Zignol M, Dean AS, Falzon D, et al. Twenty years of global surveillance of antituberculosis-drug resistance. N Engl J Med. 2016;375(11):1081–1089. doi:10.1056/NEJMsr1512438
26. Yang S-H, Zhan P, Sun M, Zhang Y-P, Ma N-L. Perfusing chemotherapy by percutaneous lung puncture in the treatment of extensive drug resistant pulmonary tuberculosis. J Thorac Dis. 2012;4(6):624.
27. Yun’an Qing LC, Li R, Liu G, et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomedicine. 2018;13:3311. doi:10.2147/IJN.S177627
28. Tiwari V, Mishra N, Gadani K, Solanki PS, Shah N, Tiwari M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front Microbiol. 2018;9:1218. doi:10.3389/fmicb.2018.01218
29. Amini SM, Kharrazi S, Rezayat SM, Gilani K. Radiofrequency electric field hyperthermia with gold nanostructures: role of particle shape and surface chemistry. Artif Cells Nanomed Biotechnol. 2018;46(7):1452–1462. doi:10.1080/21691401.2017.1373656
30. Zarchi AAK, Amini SM, Salimi A, Kharazi S. Synthesis and characterisation of liposomal doxorubicin with loaded gold nanoparticles. IET Nanobiotechnol. 2018;12(6):846–849. doi:10.1049/iet-nbt.2017.0321
31. Amini SM, Kharrazi S, Jaafari MR. Radio frequency hyperthermia of cancerous cells with gold nanoclusters: an in vitro investigation. Gold Bull. 2017;50(1):43–50. doi:10.1007/s13404-016-0192-6
32. Jafari A, Mosavi T, Mosavari N, et al. Mixed metal oxide nanoparticles inhibit growth of Mycobacterium tuberculosis into THP-1 cells. Int J Mycobacteriol. 2016;5:S181–S183. doi:10.1016/j.ijmyco.2016.09.011
33. Salomoni R, Léo P, Montemor A, Rinaldi B, Rodrigues M. Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol Sci Appl. 2017;10:115. doi:10.2147/NSA.S133415
34. Banu A, Rathod V. Biosynthesis of monodispersed silver nanoparticles and their activity against Mycobacterium tuberculosis. J Nanomed Biotherapeut Discov. 2013;3(110):2. doi:10.4172/2155-983X.1000110
35. Yusof NAA, Zain NM, Pauzi N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against gram-positive and gram-negative bacteria. Int J Biol Macromol. 2019;124:1132–1136. doi:10.1016/j.ijbiomac.2018.11.228
36. Siddiqi KS, Ur Rahman A, Tajuddin HA. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. 2018;13(1):141. doi:10.1186/s11671-018-2532-3
37. Lanone S, Rogerieux F, Geys J, et al. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part Fibre Toxicol. 2009;6(1):14. doi:10.1186/1743-8977-6-14
38. Amini SM. Preparation of antimicrobial metallic nanoparticles with bioactive compounds. Mater Sci Eng. 2019;109809. doi:10.1016/j.msec.2019.109809
39. Nasiri MJ, Haeili M, Ghazi M, et al. New insights in to the intrinsic and acquired drug resistance mechanisms in mycobacteria. Front Microbiol. 2017;8:681. doi:10.3389/fmicb.2017.00681
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
