Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
The Anatomic and Functional Outcomes of Ozurdex-Aided Vitrectomy in Proliferative Diabetic Retinopathy
Authors Wang M, Luan R, Liu B, Gong Y, Zhao J, Chen X, Yang Q, Liu J, Liu J , Shao Y , Li X
Received 21 November 2023
Accepted for publication 21 February 2024
Published 8 March 2024 Volume 2024:17 Pages 1199—1213
DOI https://doi.org/10.2147/DMSO.S445607
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Manqiao Wang,* Rong Luan,* Boshi Liu, Yi Gong, Jinzhi Zhao, Xiteng Chen, Qianhui Yang, Jingjie Liu, Juping Liu, Yan Shao, Xiaorong Li
Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Shao; Xiaorong Li, Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin, People’s Republic of China, Tel +86 17622651170; +86 18622818042, Email [email protected]; [email protected]
Purpose: To investigate the 3-months outcomes of patients who underwent intraoperative intravitreal injection of Ozurdex for proliferative diabetic retinopathy (PDR).
Methods: This is a prospective randomized controlled clinical trial (ChiCTR2100043399). Seventy-one patients with PDR who had indications for surgery without intravitreal injection history within 3 months preoperatively were enrolled. Patients were randomly divided into three groups based on the medicine injected intraoperatively: Ozurdex, Conbercept, and Control group. The primary outcome is the best-corrected visual acuity (BCVA) within 3 months postoperatively. The secondary outcomes include the intraocular pressure (IOP), mean sensitivity, central retinal thickness and vessels perfusion.
Results: The BCVA and the mean sensitivity improved in the three groups (F = 130.8, P < 0.0001; F = 34.18, P < 0.0001), but there was no statistical difference among the three groups (F = 0.858, P = 0.552; F = 0.964, P = 0.452). The IOP was no significant differences among the three groups within 3 months postoperatively (F = 0.881, P = 0.533). Compared with the other two groups, central retinal thickness (CRT) and outer retinal layer (ORL) thickness decreased significantly in patients of the Ozurdex group (F = 3.037, P = 0.008; F = 2.626, P = 0.018), especially in the diabetic macular edema (DME) patients (F = 2.761, P = 0.0164; F = 2.572, P = 0.0240). In macular region, superficial vascular plexus (SVP), intermediate capillary plexus (ICP) and deep capillary plexus (DCP) perfusion were not shown statistical difference at 3 months postoperatively in the all three groups compared with 1 day postoperatively (P > 0.05).
Conclusion: Compared with the other two groups, anatomical outcomes was improved significantly in Ozurdex group for DR patients. Ozurdex may help to improve the visual acuity and visual sensitivity, and there is no significant difference in the change of IOP and microvascular improvement.
Clinical Trial Registration: This trial is registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn, registration number ChiCTR2100043399).
Keywords: proliferative diabetic retinopathy, ozurdex, pars plana vitrectomy, optical coherence tomography angiography, microperimetry
Diabetic retinopathy (DR) is a common and specific complication of diabetes. According to statistics, about 34.6% of diabetic patients worldwide suffer from retinopathy, of which 30% are vision threatening.1 At present, it is believed that the early changes of DR are mainly caused by high glucose and microvascular changes, inflammatory factors, and retinal neurodegeneration.2 Clinically, DR can be divided into proliferative and non-proliferative diabetic retinopathy according to whether there is retinal hemorrhage and neovascularization in patients’ fundus. Proliferative diabetic retinopathy (PDR) mainly showed optic disc or other parts neovascularization, preretinal hemorrhage, or vitreous hemorrhage.3 Vitrectomy should be considered for PDR patients with vitreous hemorrhage that interfere with vision.1 For the pathological changes of DR, the use of anti-VEGF drugs or anti-inflammatory drugs after vitrectomy may reduce postoperative bleeding, relieve diabetic macular edema (DME), and help postoperative recovery.2,4,5 Meanwhile, the study found that vitreous inflammatory cytokines and chemokines could not be reduced effectively by Conbercept, but may lead to early postoperative macular edema (ME) in patients with PDR.6 Ozurdex (0.7mg, Dexamethasone intravitreal implant, Allergan Pharmaceuticals Ireland), as a dexamethasone sustained-release agent, has become a second-line drug for the treatment of DME. The maximum benefit occurs 2–3 months after injection, and the therapeutic effect can be maintained for up to 6 months in the human eye.4 Many clinical trials in DME showed that Ozurdex can effectively improve visual acuity, but there is no difference compared with anti-VEGF drugs, and can significantly reduce retinal thickness, but there may be a risk of high intraocular pressure.7–9 However, few research has been focus on intraoperative intravitreal injection of Ozurdex for vitrectomy in PDR patients. Therefore, PDR patients who needed pars plana vitrectomy (PPV) were selected and given Ozurdex injection intraoperatively. The control groups were selected as the injection group of anti-VEGF drug Conbercept and the non-injection group to observe the postoperative recovery of retinal anatomical structure and visual function of PDR patients under the effects of different drugs.
Materials and Methods
Study Design and Population
This was a prospective randomized controlled clinical trial (ChiCTR2100043399). We collected patients with PDR who were hospitalized in the ophthalmology department of Tianjin Medical University Eye Hospital from February 2021 to January 2023. The inclusion criteria were as follows: (1) Age: ≥18 years old; (2) Diagnosis of PDR (according to the International Classification of Diabetic retinopathy10), but without tractional retinal detachment (TRD); (3) Patients with surgical indications, including unabsorbed vitreous hemorrhage, diabetic macular edema associated with posterior hyaloidal traction, and anterior segment neovascularization with media opacities;11 (4) Patients can undergo a comprehensive ophthalmic examination, such as the best-corrected visual acuity (BCVA) assessment with the Early Treatment Diabetic Retinopathy Study (ETDRS) charts at 5m, fundus examination, visual function examination and optical coherence tomography angiography (OCTA) examination after vitrectomy. The exclusion criteria were as follows: (1) history of previously high ocular pressure or diagnosed glaucoma; (2) other retinal diseases including but not limited: wet age-related macular degeneration (wAMD), retinal vein occlusion (RVO), and other neovascular retinopathy; (3) dense vitreous hemorrhage after surgery; (4) injection of anti-VEGF drugs or dexamethasone within 3 months preoperatively; (5) an intraocular filling of silicone oil or inert gas; (6) history of any intraocular surgery and intraocular laser therapy within 3 months preoperatively; (7) severe heart, lung, liver or kidney function impairment or advanced cancer.
PDR patients were randomly divided into three groups by the randomization table with a 1:1:1 ratio: Ozurdex group, Conbercept group and control group. The preliminary experiment was included 5 patients every group to calculate the sample size. According to the preliminary experiment, it is assumed that the BCVA 3-month postoperatively would be approximately 0.3 (Log MAR) in Ozurdex and Conbercept group, and that would be 0.5 (Log MAR) in control group. A sample size of 16 per group achieves 80% power at a two-sided significance level (alpha) of 0.05. Thus, allowing for 20% of participants loss to 3-month follow-up, a total of 58 participants are required for this study. We observed the recovery of retinal anatomical structure and visual function at 1 day, 1 week, 1 month, and 3 months postoperatively. BCVA (Log MAR) and intraocular pressure (IOP) were recorded.
Operation Methods
All patients received levofloxacin eye drops 4 times a day for 3 days preoperatively. All procedures were performed by the same experienced ophthalmologist. Under retrobulbar anesthesia, PPV was performed combined with cataract surgery and panretinal photocoagulation (PRP). The patients with proliferative membrane were treated with membrane peeling, and the inner limiting membrane (ILM) peeling was not performed during the operation. Vitrectomy system (Alcon Laboratories, Inc., Forth Worth, TX, USA) and 27G equipment were used to complete the surgery. The cutting rate was 7500 times /min in linear mode. Intraocular perfusion pressure was maintained at 25 ~ 30 mmHg (1 mmHg = 0.133 kPa). The 27G cannula and conjunctival incision were not sutured. Postoperatively, the intraocular pressure was measured with the fingers, and the eyes were bandaged with tobramycin and dexamethasone eye ointment.
Optical Coherence Tomography Angiography Measurements Scans Evaluation
All participants were imaged with Swept-Source OCTA (SS-OCTA) with a scanning speed of 200,000 A scan per second (VG200; SVision Imaging, Ltd., Luoyang, China). 12 × 12-mm scans were performed in the center of the macula for each participant, which consisted of 1024 horizontal A lines at 1024 vertical locations with two repeated scans in each fixed location.
OCT/OCTA images selected with good scan quality (more than 7/10 signal strength as defined by the manufacturer) and without severe motion artefacts were included. Retinal layers were segmented using a validated semiautomated segmentation algorithm and manually calibrated by two experienced ophthalmologists for accuracy. Record the central retinal thickness (CRT), macular ganglion-cell layer complex (GCC) thickness, retinal nerve fiber layer (RNFL) thickness, ganglion cell-inner plexus layer (GCL) thickness, inner nuclear layer (INL) thickness, the outer retinal layer (ORL) thickness and pigment-epithelial detachment layer thickness with macular region diameter of 1mm at OCT. Record perfusion of superficial vascular plexus (SVP), intermediate capillary plexus (ICP), deep capillary plexus (DCP) and choriocapillaris with macular region diameter of 1mm at OCTA.
MP-3 Microperimetry Measurement
The macular function was measured using fundus-monitored microperimetry (MP-3, Nidek, Gamagori, Japan). Microscopic field measurements were performed using macular integrity assessment. A 4-2-staircase strategy with Goldmann III-sized stimuli (white, 200 ms) on the background luminance of 31.4 ASB was used with 45 stimulus locations within 12°. The eccentric circle was drawn at 12° from the fovea. The maximum luminance of the MP-3 is 10,000 ASB, which results in the stimulus dynamic range between 0 and 34 dB. The results of mean sensitivity in the macular area were analyzed.
Statistical Analysis
Fisher’s exact test was used to compare baseline differences in gender and eye laterality (OD/OS) among the three groups. Age, duration of diabetes, glycated hemoglobin (HbA1c), Serum total cholesterol (TC), triglyceride (TG), carbamide (URE), and creatinine (CRE) were subjected to normal distribution. One-way ANOVA test was performed for data with normal distribution, and Kruskal–Wallis test was applied if not available among the three groups. Tukey’s multiple comparison test and paired comparison were used as post hoc tests following one-way ANOVA test and the Kruskal–Wallis test. Two-way repeated measures ANOVA for all groups was applied. Tukey’s multiple comparison test were used in the comparisons among groups. The ratio of postoperative DME was also used fisher’s exact test. A p value < 0.05 was considered statistically significant.
Results
Patient Characteristics
Totally 71 eyes of 71 patients were enrolled in this study from February 2021 to January 2023. 2 patients underwent reoperation due to retinal detachment or vitreous hemorrhage, and 60 patients completed the 3 months follow-up period. The baseline characteristics of 71 subjects who took part in this study are shown in Table 1 and there were no significant differences among the three groups.
 |
Table 1 Demographic Features and Clinical Characteristics of the Patients |
Efficacy
The BCVA was improved significantly after treatment in Ozurdex group and Conbercept group within 3 months, while that was decreased at 3 months in the control group after improving within 1 month (Ft = 130.8, Pt < 0.0001; Fg = 1.402, Pg = 0.253; F = 0.858, P = 0.552). The IOP was no significant differences among the three groups within 3 months postoperatively (F = 0.881, P = 0.533). The mean sensitivity was improved significantly in Ozurdex group within 3 months, while that in Conbercept group and control group only increased within 1 month (Ft = 34.18, Pt < 0.0001; Fg = 1.263, Pg = 0.290; F = 0.964, P = 0.452). The CRT of patients in Ozurdex group continued to decrease and the average CRT was <300 um at 3 months postoperatively, while the CRT of patients in the Conbercept group and the control group began to increase again from 1 month and the average CRT was >300 um at 3 months postoperatively (F = 3.037, P = 0.008). ORL thickness decreased in all three groups at 3 months postoperatively compared to 1 day postoperatively. The ORL thickness decreased in both Ozurdex group and Conbercept group at 1 month, with the Ozurdex group showing the most significant decrease and eventually the average ORL thickness reaching <200 um, while Conbercept group showed an increase in ORL thickness after 1 month. In the control group, the ORL thickness showed a trend of increasing and then decreasing (F = 2.626, P = 0.018). Choriocapillaris perfusion (CCP) fluctuated over 3 months in all three groups (F = 3.249, P = 0.005). (Table 2, Figure 1: “F”-statistic, “P”-significance. “t”-time. “g”-group. “Ft” and “Pt” represented differences in measurements changes over time within the same group. “Fg” and “Pg” represented differences in measurements changes between different groups, at the same time. “F” and “P” represented differences in measurements changes over time between different groups.)
 |
Table 2 Observation Index Statistics of Patients in Ozurdex Group (n = 22), Conbercept Group (n = 25), and Control Group (n = 24) |
Postoperative observation of PDR patients with DME showed that BCVA (Log MAR) of the patients improved from 1.517 ± 0.595 to 0.319 ± 0.132 within 3 months after injection of Ozurdex. BCVA increased from 1.558 ± 0.475 to 0.510 ± 0.401 at 1 month and followed by a slight decrease to 0.534±0.540 at 3 months after injection of Conbercept, but there was no significant difference in visual acuity among 3 groups (Ft = 68.81, Pt < 0.0001; Fg = 0.112, Pg = 0.894; F = 0.471, P = 0.875). The IOP was also no significant differences among the three groups within 3 months postoperatively (F = 0.5533, P = 0.8142). The mean sensitivity improved over 3 months in both Ozurdex group and Conbercept patients, while control patients showed a decrease at 7 days followed by a gradual recovery (Ft = 28.45, Pt < 0.0001; Fg = 0.427, Pg = 0.656; F = 1.210, P = 0.310). The CRT decreased in both Ozurdex group and Conbercept group over 3 months. The final average CRT in Ozurdex group was <300um, while the average CRT in Conbercept group was still >300um at 3 months. The CRT in control group continued to increase over 1 month, but decreased to a similar thickness to Conbercept group by 3 months (F = 2.761, P = 0.0164). The ORL thickness decreased in both Ozurdex group and Conbercept group over 3 months, with Ozurdex group showing the most significant decrease in ORL thickness and eventually the average ORL thickness reaching <200um, while the control group continued to increase in thickness for 1 month and then decreased to a similar thickness as Ozurdex group at 3 months (F = 2.572, P = 0.0240). Compared among the three groups, no significant differences in the number of patients with DME persistent 1-month and 3-month postoperatively (P1m=0.182; P3m=0.167) (Table 3, Figure 2).
 |
Table 3 Statistical Observation Indicators of DME Patients Injected with Ozurdex (n = 12), Conbercept (n = 14) or Without Drugs (n = 11) |
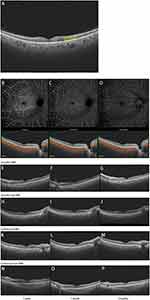
Postoperative observation of PDR patients without DME showed that BCVA of the patients improved within 3 months after injection of Ozurdex and Conbercept. But BCVA of the patients without drug injections improved gradually within 1 week and decreased gradually to 3 months. However, there was no significant difference in BCVA among the 3 groups (Ft = 61.44, Pt < 0.0001; Fg = 0.780, Pg = 0.4671; F = 1.899, P = 0.0673). The IOP was also no significant differences among the three groups within 3 months postoperatively (F = 1.210, P = 0.2999). The mean sensitivities of patients injected with Ozurdex increased gradually within 3 months, while the mean sensitivity of patients who did not inject drugs or injected with Conbercept increased within 1 month and then decreased significantly (Ft = 9.510, Pt = 0.0004; Fg = 1.643, Pg = 0.210; F = 1.368, P = 0.2405). The thickness of GCC layer was slightly thicker in Conbercept group at 1 day postoperatively and then decreased to the thickness similar to that of Ozurdex from 1 week postoperatively. The GCC thickness in the control group remained relatively stable but slightly higher than the other two groups (F = 3.765, P = 0.0026). RNFL thickness in Conbercept group was slightly thicker at 1 day postoperatively and then decreased from 7 days postoperatively to a thickness similar to that of Ozurdex and control groups until 3 months postoperatively (F = 2.732, P = 0.0191). The CCP in the three groups fluctuated over 3 months. CCP was 0.513 ± 0.035, 0.529±0.098, and 0.483 ± 0.134 in Ozurdex, Conbercept and control groups at 3 months postoperatively, respectively (F = 3.668, P = 0.0032). Compared among the three groups, 2 patients in Conbercept group and 5 patients in control group developed DME 1 month postoperatively (P = 0.075). And another one patient was developed DME in control group 3 month postoperatively (P = 0.031). (Table 4, Figure 3) The representative cases are shown in Figure 4.
 |
Table 4 Statistical Observation Indicators of Non-DME Patients Injected with Ozurdex (n = 10), Conbercept (n = 11) or Without Drugs (n = 13) |
Safety
No serious adverse events such as neovascular glaucoma, endophthalmitis, or other diseases related to medical operations were found. During follow-up, no serious systemic adverse events related to drugs such as myocardial infarction and cerebral infarction were found. No postoperative intraocular hypertension in Ozurdex group. However, the retinal detachment was found 1 month postoperatively in one patient in Ozurdex group and one in Conbercept group. Two patients in Conbercept group and two in Control group had a recurrence of vitreous hemorrhage from 1 to 3 months after postoperatively. Three patients in Control group received intravitreal injection of Conbercept due to macular edema during follow-up from 1 to 3 months postoperatively.
Discussion
In this study, we found that injections of the drug helped in the improvement of visual acuity, visual sensitivity, and the restoration of the anatomical structure of the retinal layers, regardless of whether the patients developed DME or not. In patients who developed DME, we found that injections of Ozurdex did prolong the stabilization of the inner retinal layer structure in patients than injections of Conbercept, thus allowing for longer-term stabilization of vision. In patients without DME, injections of Ozurdex prolonged the time to visual sensitivity improvement compared to injections of Conbercept and no injections of the drug. We also found that both Ozurdex and Conbercept were helpful in the anatomical recovery of the central retinal layer, GCC layer, and ORL. However, we did not find any statistical differences for SVP, ICP and DCP of the three groups in this study. In terms of safety, only the Ozurdex group had no recurrence of vitreous hemorrhage during the follow-up period, suggesting that Ozurdex may have a positive effect on reducing the probability of postoperative vitreous hemorrhage recurrence.
Both Ozurdex and Conbercept can effectively treat DME, and both can significantly reduce the CRT of patients.12,13 In terms of pathophysiology, corticosteroids can inhibit the production of pro-inflammatory mediators, reduce VEGF synthesis indirectly, and decrease the loss of endothelial tension-binding proteins.14 Besides reducing VEGF level, Conbercept treatment also significantly reduced some inflammatory factors, such as intercellular cell adhesion molecule-1 (ICAM-1), macrophage inflammatory protein-1 (MIP-1), IL-1β, IL-6 and TNF-α protein levels in aqueous fluid of PDR patients.15 Ozurdex releases small amounts of corticosteroids in a time-dependent manner. Pharmacokinetic data from a nine-month study of Ozurdex showed that the dexamethasone implant was effective immediately after implantation, in high concentrations in the vitreous and retina for up to 60 days, and could be maintained for up to 6 months after administration, gradually losing its efficacy.4 As a result, Ozurdex stays in the eye longer and has a longer duration of action than Conbercept, so there is a greater decrease in CRT for patients with DME who are injected with Ozurdex. The retinal GCC layer mainly includes the nerve fiber layer (NFL) and GCL. It has been found that NFL14.06±1.52 μm and GCL36.48 ± 8.39 μm in the normal macular fovea. If there is a significant increase in GCL, it may indicate diffuse swelling of cells in this layer, an early sign of DME progression, even in well-controlled diabetes.16 Therefore, the postoperative decrease of GCC layer in the Ozurdex group and the Conbercept group without DME in this study may be due to the subsidence of the diffuse swelling of ganglion cells and inner plexus cells that occurred preoperatively by the action of the drug. It17,18 was shown in many studies that thinning of the RNFL layer occurs in patients with DM or early DR without significant microvascular alterations, suggesting neurodegeneration. However, the RNFL layer thickness was consistently thicker than normal in all three groups of patients with non-DME in our study. It may contribute to the microvascular and structural changes in the RNFL, such as intracellular and extracellular edema and hemorrhage, that occur with disease progression, leading to an increase in RNFL thickness at the time of OCTA assessment. ORL includes the outer plexiform layer (OPL), the outer nuclear layer (ONL), the outer external limiting membrane (ELM), the photoreceptors’ segments layer (PRL) and the pigment epithelium (RPE). Based on the OCT presentation, DME is divided into 3 main types: namely cystoid macular edema (CME), plasmacytoid retinal detachment (SRD) and sponge-like retinal swelling.19 Among them, the cystoid spaces of the CME is mainly located in the inner nuclear layer (INL) and the outer plexiform layer (OPL).20 SRD occurs in the extracellular fluid pool between the photoreceptor outer segment (PROS) and the RPE.20 Spongy retinal swelling can be showed up in the fovea of the OPL.20 A number of studies have found the intraocular levels of potent proangiogenic cytokine (VEGF) and proinflammatory cytokine are both increased in DME.19,21 Thus, after drug injection, patients with DME showed a significant decrease in CRT and ORL thickness, while the group without drug injection showed a trend toward persistent worsening of edema, a result also consistent with the findings of others that both Conbercept and Ozurdex can treat DME. However, the DME did not subside after injection sometimes, which may be due to the effect of multiple types of pathogenic factors or the mismatch between pathogenic factors and injection drugs.22 It found that PPV combined with ILM peeling was effective in reducing the retinal thickness and helping vision recovery in patients with DME, but this effect tended to decrease over time, so a significant number of patients required additional treatment.23,24 Coincidentally, our study also found that the PDR patients with DME, who were without drug injection, could show a significant decrease in CRT and ORL thickness at 3 months with the patient’s postoperative recuperation.
It has been shown that the normal subfoveal choroidal thickness (SFCT) is 272~448 μm.25 Compared with normal subjects, the choroid thickness will be reduced by the presence of DME, but at the same time, the choroid can be thickened in the initial stage of DR and become thinner with the progression of DR.26,27 Studies have shown that the overexpression of some cytokines is associated with the progression of DR, including monocyte chemokine protein-1 (MCP-1), platelet-derived growth factor, VEGF, insulin-like growth factor 1, pigment epithelium-derived factor, and CXC mode-chemokine ligand, can lead to choroid thickening.28–30 As an anti-inflammatory agent, Ozurdex can effectively reduce the inflammatory response and inflammatory factors,4 while Conbercept can neutralize VEGF to reduce the overexpressed cytokines,13 they both could restore the thickened choroid to a normal level. Some studies have found that CCP is significantly reduced in NPDR and PDR patients compared with normal people, but there is no significant difference between the two groups.31 In this study, the CCP of all three groups of patients experienced fluctuations both under the overall comparison and under the non-DME comparison over three months, but ultimately the difference in CCP between the Ozurdex group and the Conbercept group was not significant, and it was slightly higher than in the control group. This could indicate that when irreversible damage to retinal microvascular changes has occurred in patients with PDR, drug injection does not completely improve CCP, but it delays further deterioration of microvascular changes compared with uninjected drugs.
Studies have proved that compared with normal people, the vessel density of SCP and DCP in the early stage of DR will decrease, reflecting the vascular injury in the early stage of DR, and with the progress of DR, a large range of non-perfusion areas may eventually appear in the retina.32 At the same time, compared with SCP, DCP and DVP can better reflect the vascular changes of DR, and the injury time is longer, which makes some non-perfusion areas irreversible.33–35 There were no statistical differences for SVP, ICP and DCP of the three groups in this study, probably because patients who have developed PDR requiring surgery have irreversible damage to the fundus microvascular circulation and injectable drugs do not improve retinal microcirculation in the short term.
In conclusion, we have demonstrated that drug injection can produce a more stable improvement in visual acuity and visual sensitivity while playing a crucial role in the restoration of retinal anatomy. In DME patients, injection of Ozurdex is more effective than Conbercept in improving the structure of the macula, restoring the shape of the fovea, and prolonging the structural and functional stability. However, there was no significant difference in the improvement of macular vessels density and perfusion with or without drug injection.
This study has limitations. The global COVID-19 pandemic has made our follow-up work more difficult, so some cases were lost to follow-up in the 3 months, which also affected the data results to a certain extent. The follow-up period of 3 months in this clinical trial is also relatively short, and extending the follow-up period or add more time points to obtain more definite conclusions will be the focus in our next clinical trial.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was reviewed and approved by the Ethics Committee of Tianjin Medical University Eye Hospital (2020KY-33) and adhered to the tenets of the Declaration of Helsinki. Before enrolled into the study, each patient signed a written informed consent. This trial is registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn, registration number ChiCTR2100043399). Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Consent for Publication
The work described has not been published before (except in the form of an abstract or as part of a published lecture, review, or thesis). It is not under consideration for publication elsewhere. Its publication has been approved by all co-authors, if any. Its publication has been approved (tacitly or explicitly) by the responsible authorities at the institution where the work is carried out.
Acknowledgment
Manqiao Wang and Rong Luan are co-first authors for this study. Yan Shao and Xiaorong Li are co-correspondence authors for this study. We are very grateful to Dr. Xinyuan Huang, Dr. Mingming Ma, Dr. Shuo Sun, and Dr. Xuehao Cui for their help during the data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
National Natural Science Foundation Project (82360209), Science and technology project of Tibet Autonomous Region (XZ202301YD0029C), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-037A).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern®. Ophthalmology. 2020;127(1):P66–P145. doi:10.1016/j.ophtha.2019.09.025
2. Wang W, Lo ACY. Diabetic Retinopathy: pathophysiology and Treatments. Int J Mol Sci. 2018;19(6):1.
3. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi:10.1016/S0140-6736(09)62124-3
4. Karti O, Saatci AO. Place of intravitreal dexamethasone implant in the treatment armamentarium of diabetic macular edema. World J Diabetes. 2021;12(8):1220–1232. doi:10.4239/wjd.v12.i8.1220
5. Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2015;2015(8):Cd008214. doi:10.1002/14651858.CD008214.pub3
6. Sun H, Zou W, Zhang Z, et al. Vitreous inflammatory cytokines and chemokines, not altered after preoperative adjunctive conbercept injection, but associated with early postoperative macular edema in patients with proliferative diabetic retinopathy. Front Physiol. 2022;13:846003. doi:10.3389/fphys.2022.846003
7. Boyer DS, Yoon YH, Belfort R
8. Patil NS, Mihalache A, Hatamnejad A, et al. Intravitreal steroids compared with anti-VEGF treatment for diabetic macular edema: a meta-analysis. Ophthalmol Retina. 2023;7(4):289–299. doi:10.1016/j.oret.2022.10.008
9. Rosenblatt A, Udaondo P, Cunha-Vaz J, et al. A collaborative retrospective study on the efficacy and safety of intravitreal dexamethasone implant (ozurdex) in patients with diabetic macular edema: the European DME Registry Study. Ophthalmology. 2020;127(3):377–393. doi:10.1016/j.ophtha.2019.10.005
10. Wilkinson CP, Ferris FL
11. Cruz-Iñigo YJ, Acabá LA, Berrocal MH. Surgical management of retinal diseases: proliferative diabetic retinopathy and traction retinal detachment. Dev Ophthalmol. 2014;54:196–203.
12. Parravano M, Allegrini D, Carnevali A, et al. Effectiveness of a hydrophilic curcumin-based formulation in coadjuvating the therapeutic effect of intravitreal dexamethasone in subjects with diabetic macular edema. Front Pharmacol. 2021;12:726104. doi:10.3389/fphar.2021.726104
13. Zhou P, Zheng S, Wang E, et al. Conbercept for treatment of neovascular age-related macular degeneration and visual impairment due to diabetic macular edema or pathologic myopia choroidal neovascularization: a systematic review and meta-analysis. Front Pharmacol. 2021;12:696201. doi:10.3389/fphar.2021.696201
14. Gonzalez-Cortes JH, Martinez-Pacheco VA, Gonzalez-Cantu JE, et al. Current treatments and innovations in diabetic retinopathy and diabetic macular edema. Pharmaceutics. 2022;15(1):122. doi:10.3390/pharmaceutics15010122
15. Xia J-P, Liu S-Q, Wang S. Intravitreal conbercept improves outcome of proliferative diabetic retinopathy through inhibiting inflammation and oxidative stress. Life Sci. 2021;265:118795. doi:10.1016/j.lfs.2020.118795
16. Gerendas BS, Hatz K, Kaider A, et al. Ganglion cell layer thickening in well-controlled patients with type 1 diabetes: an early sign for diabetic retinopathy? Acta Ophthalmol. 2020;98(3):e292–e300. doi:10.1111/aos.14273
17. Gong X, Wang W, Xiong K, et al. Associations between peripapillary retinal nerve fiber layer and choroidal thickness with the development and progression of diabetic retinopathy. Invest Ophthalmol Visual Sci. 2022;63(2):7. doi:10.1167/iovs.63.2.7
18. El-Fayoumi D, Badr Eldine NM, Esmael AF, et al. Retinal nerve fiber layer and ganglion cell complex thicknesses are reduced in children with type 1 diabetes with no evidence of vascular retinopathy. Invest Ophthalmol Visual Sci. 2016;57(13):5355–5360. doi:10.1167/iovs.16-19988
19. Chung YR, Kim YH, Ha SJ, et al. Role of inflammation in classification of diabetic macular edema by optical coherence tomography. J Diabet Res. 2019;2019:8164250. doi:10.1155/2019/8164250
20. Murakami T, Yoshimura N. Structural changes in individual retinal layers in diabetic macular edema. J Diabet Res. 2013;2013:920713. doi:10.1155/2013/920713
21. Noma H, Mimura T, Yasuda K, et al. Role of inflammation in diabetic macular edema. Ophthalmologica. 2014;232(3):127–135. doi:10.1159/000364955
22. Sadhukhan K, Naskar S. Role of combined therapy of intravitreal ranibizumab and dexamethasone in refractory diabetic macular edema: a Retrospective Study. Maedica. 2021;16(4):615–619. doi:10.26574/maedica.2021.16.4.615
23. Gunay BO, Erdogan G. Evaluation of macular changes in the long term after pars plana vitrectomy with internal limiting membrane peeling for diabetic macular edema. Ophthalmologica. 2021;244(3):237–244. doi:10.1159/000514992
24. Rush RB, Rush SW. Pars plana vitrectomy with internal limiting membrane peeling for treatment-naïve diabetic macular edema: a Prospective, Uncontrolled Pilot Study. Clin Ophthalmol. 2021;15:2619–2624. doi:10.2147/OPTH.S320214
25. Zeng J, Li J, Liu R, et al. Choroidal thickness in both eyes of patients with unilateral idiopathic macular hole. Ophthalmology. 2012;119(11):2328–2333. doi:10.1016/j.ophtha.2012.06.008
26. Hassan H, Cheema A, Tahir MA, et al. Comparison of choroidal thickness in eyes of diabetic patients with eyes of healthy individuals using optical coherence tomography in a tertiary care hospital. Pak J Med Sci. 2022;38(1):254–260. doi:10.12669/pjms.38.1.4443
27. Gerendas BS, Waldstein SM, Simader C, et al. Three-dimensional automated choroidal volume assessment on standard spectral-domain optical coherence tomography and correlation with the level of diabetic macular edema. Am J Ophthalmol. 2014;158(5):1039–1048. doi:10.1016/j.ajo.2014.08.001
28. Nomura Y, Takahashi H, Fujino Y, et al. Association between aqueous humor cxc motif chemokine ligand 13 levels and subfoveal choroidal thickness in normal older subjects. Retina. 2016;36(1):192–198. doi:10.1097/IAE.0000000000000668
29. Yokouchi H, Baba T, Misawa S, et al. Correlation between serum level of vascular endothelial growth factor and subfoveal choroidal thickness in patients with POEMS syndrome. Graefe’s Arch Clin Exp Ophthalmol. 2015;253(10):1641–1646. doi:10.1007/s00417-014-2843-8
30. Zhang X, Ma J, Wang Y, et al. Elevated serum IGF-1 level enhances retinal and choroidal thickness in untreated acromegaly patients. Endocrine. 2018;59(3):634–642. doi:10.1007/s12020-017-1511-2
31. Conti FF, Qin VL, Rodrigues EB, et al. Choriocapillaris and retinal vascular plexus density of diabetic eyes using split-spectrum amplitude decorrelation spectral-domain optical coherence tomography angiography. Br j Ophthalmol. 2019;103(4):452–456. doi:10.1136/bjophthalmol-2018-311903
32. Safi H, Nourinia R, Safi S, et al. Retinal vascular response to hyperoxia in patients with diabetes mellitus without diabetic retinopathy. J Ophthalmolo. 2021;2021:9877205. doi:10.1155/2021/9877205
33. Toto L, D’Aloisio R, Chiarelli AM, et al. A custom-made semiautomatic analysis of retinal nonperfusion areas after dexamethasone for diabetic macular edema. Transl Vis Sci Technol. 2020;9(7):13. doi:10.1167/tvst.9.7.13
34. Kashani AH, Chen CL, Gahm JK, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retinal Eye Res. 2017;60:66–100. doi:10.1016/j.preteyeres.2017.07.002
35. Toto L, D’Aloisio R, Di Nicola M, et al. Qualitative and quantitative assessment of vascular changes in diabetic macular edema after dexamethasone implant using optical coherence tomography angiography. Int J Mol Sci. 2017;18(6). doi:10.3390/ijms18061181
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.