Back to Journals » Journal of Inflammation Research » Volume 15
The Alterations of Serum IgG Fucosylation as a Potential Additional New Diagnostic Marker in Advanced Endometriosis
Authors Sołkiewicz K , Krotkiewski H , Jędryka M , Czekański A , Kratz EM
Received 5 October 2021
Accepted for publication 3 December 2021
Published 13 January 2022 Volume 2022:15 Pages 251—266
DOI https://doi.org/10.2147/JIR.S341906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Katarzyna Sołkiewicz, 1 Hubert Krotkiewski, 2 Marcin Jędryka, 3, 4 Andrzej Czekański, 3, 4 Ewa Maria Kratz 1
1Department of Laboratory Diagnostics, Division of Laboratory Diagnostics, Faculty of Pharmacy, Wroclaw Medical University, Wroclaw, 50-556, Poland; 2Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, 53-114, Poland; 3Department of Oncology, Gynecological Oncology Clinic, Faculty of Medicine, Wroclaw Medical University, Wroclaw, 53-413, Poland; 4Department of Oncological Gynecology, Wroclaw Comprehensive Cancer Center, Wroclaw, 53-413, Poland
Correspondence: Ewa Maria Kratz
Department of Laboratory Diagnostics, Division of Laboratory Diagnostics, Faculty of Pharmacy, Wroclaw Medical University, Borowska Street 211A, Wroclaw, 50-556, Poland
Tel +48 71 784 01 52
Fax +48 71 784 01 54
Email [email protected]
Background: Endometriosis is an inflammatory disease leading to the growth of endometrial-like tissue outside of the uterus, which affects approximately 10% of young women of reproductive potential. The diagnosis of this disease is difficult, often invasive and time-consuming, therefore non-invasive diagnostic methods are strongly desirable in endometriosis detection. The aim of our project was to investigate whether any associations exist between the expression of serum IgG fucosylation and advanced stages of endometriosis. We were also interested in whether native serum IgG (s-IgG) fucosylation analysis, without prior IgG isolation, could provide a panel of parameters helpful in non-invasive diagnostics of advanced endometriosis.
Methods: IgG fucosylation was examined using a lectin-ELISA test with fucose-specific lectins: AAL and LCA, specific for core fucose α 1,6-linked, as well as LTA and UEA which recognize α 1,3- and α 1,2-linked fucose, respectively.
Results: ROC curve and cluster analysis showed s-IgG reactivities with the panel of fucose-specific lectins AAL, LCA and LTA.
Conclusion: s-IgG reactivity with the panel of fucose-specific lectins AAL, LCA and LTA can be taken into account as a useful diagnostic and clinical tool to differentiate women with advanced endometriosis. Moreover, it has been shown that the analysis of native IgG fucosylation directly in serum, without prior time-consuming, expensive IgG isolation, is sufficient to distinguish advanced stages of endometriosis from a control group of healthy women.
Keywords: serum IgG, IgG fucosylation, lectin-ELISA, endometriosis
Introduction
Endometriosis, a painful, chronic inflammatory disease, is characterized by the growth of endometrial-like tissue outside of the uterus. It affects about 10% of young women of reproductive age. The symptoms of endometriosis are often nonspecific, and thus the clinical diagnosis of the disease is very difficult.1 Laparoscopic visualization is currently the only way to definitively detect endometriosis lesions, and remains the gold standard for its diagnosis.2–4 Endometriosis is divided into four stages based on anatomic location and disease severity: minimal, mild, moderate and severe - in accordance with the revised American Fertility Society (rAFS) classification,5 which has been updated by the American Society for Reproductive Medicine (ASRM).6 The etiology of endometriosis is not fully understood, and many factors may be involved in the development and progression of the disease.7,8 The immunological aspects of endometriosis pathophysiology are thought to be related to the innate immune response, but the exact mechanisms remain unknown.9–11 Numerous studies support the fact that women with endometriosis have altered immune parameters, with which may be associated decreased immuno-surveillance, reduced T-lymphocyte-mediated cytotoxicity to autologous endometrial cells, and impaired recognition of ectopic endometrial cells secondary to defective NK-cell (natural killer) activity.12–14 Glycosylation is a common process of the post-translational modification of proteins, including antibodies, and produces a variety of glycan structures that encode a variety of information. Even slight modification of glycans by the addition or removal of a single sugar can dramatically alter a protein or antibody’s structure and function, as well as interactions with the specific receptors that modulate biological responses. Immunoglobulin G (IgG) is involved in the recognition, neutralization and elimination of toxic antigens or pathogens, and is represented in the highest percentage from all immunoglobulins in blood plasma.15 IgG molecule consists of two heavy and two light chains that together form two portions of antigen-binding Fab fragments and one portion of crystallizable constant fragment, Fc. Two N-glycans are linked to the heavy chains at Asn297 in the CH2 domain of the protein backbone (Fc part). These Fc glycans are in part located in a cavity between the two heavy chains and influence the conformation of the protein.16,17 IgG molecules are glycoproteins, and oligosaccharides attached to the IgG Fc region are important for antibody functionality such as binding to cellular Fc receptors and complement activation. The core moiety consists of a bi-antennary heptameric structure of mannose and N-acetylglucosamine (GlcNAc), further decorated with terminal and branching residues including galactose, sialic acid, fucose, and GlcNAc. Variation in this composition influences antibody affinity to FcγR and thus antibody effector activity.18–20 Although considerable progress has been made in understanding the structural and functional consequences of the presence or absence of various sugar moieties in IgG Fc carbohydrates, the role of their glycosylation is not yet fully understood and defined. In addition to the oligosaccharide core, more than 95% of the bi-antennary complex type structure of the final IgG glycan carries a N-acetylglucosamine on both arms,21,22 and 85% are fucosylated.23 In mammals, the core fucosylation (α1,6-fucosylation) is the attachment of fucose to the innermost GlcNAc at the reducing terminus of the N-glycans by α1,6-fucosyltransferase, FUT8. Fucosylation is one of the major glycan modifications and regulates a huge variety of physiological processes, including immune response, signal transduction, and cell adhesion. The absence of core fucose in IgG1 glycans has been shown to result in increased binding affinity to FcγRIIIa and FcγRIIIb, due to glycan-glycan interactions between Asn162 found only in FcγRIII and Asn297.20,24,25 Fucosylation also plays a key role in the commitment, differentiation, and flexibility of immune cells, and also in immune processes and diseases.26 Fucosylated glycans participate in a variety of physiological and/or pathological processes including tissue development, cell adhesion, fertilization, angiogenesis and malignancy,27 as well as in tumor metastasis.28 The discovery how important role fucosylation plays in immune cell development and functional regulation significantly broadened the scope of fucosylation investigations.26 The alterations in glycans fucosylation has been observed in variety of inflammatory conditions, among which there is rheumatoid arthritis (RA),29–33 chronic pancreatitis,34 Crohn’s disease35 and sclerosing cholangitis.36 Changes in IgG fucosylation have been reported eg in neonatal alloimmune thrombocytopenia, showing significantly reduced levels of fucosylated IgG, which suggests the specific regulation of IgG fucosylation and its potential role in autoimmunity.37 In recent years, significant progress has been made in understanding the mechanisms that regulate the activity of IgG antibodies in vivo. However, to the best of our knowledge, nothing is known about the role of serum IgG fucosylation in endometriosis etiology and disease progression, or the utility of this parameter for disease diagnostics. Therefore, the aim of our present study was to investigate whether any associations exist between the expression of serum IgG fucosylation and advanced stages of endometriosis. We were also interested in whether native serum IgG fucosylation analysis, without prior IgG isolation, may reach a panel of parameters which would be helpful in the non-invasive diagnostics of advanced endometriosis.
Materials and Methods
Blood sera were collected from women with advanced endometriosis (stage III and IV) (E; n = 40, median age 34 years, interquartile range (IQR) 30.5–40.5) and from a group of women without endometriosis (NE: non-endometriosis; n = 36, median age 39 years, IQR 33.5–42.0), at the Department of Oncological Gynecology, Wroclaw Comprehensive Cancer Center, Poland. E and NE groups underwent surgical interventions, mainly laparoscopic, and after histological verification were classified to the proper group. One day after the surgery, once the diagnosis was established, the serum for the study was obtained. Women with endometriosis were classified by the extent and severity of disease according to the revised American Fertility Society (rAFS) classification. Blood sera from healthy women (C: control group, n = 19, median age 39 years IQR 35.0–48.0), were collected at the Department of Laboratory Diagnostics, Wroclaw Medical University. The non-endometriosis group, with severe dysplasia – CIN 3 (cervical intraepithelial neoplasia grade 3) or leiomyomas, was histologically confirmed with benign ovarian cysts. The control group consisted of healthy, premenopausal and non-pregnant women, without any symptoms or history related to endometriosis. All serum samples used in this study were collected from women on any day of the menstrual cycle. The study was conducted in agreement with the Helsinki–II-declaration and the protocol was approved by the Bioethics Human Research Committee of the Wroclaw Medical University (Permission No. KB-293/2016 and KB-719/2018). Before starting the study, all participants gave written and informed consent.
The IgG fucosylation profile and degree was determined using a modified lectin-ELISA test. The method was based on the reactivity of IgG sugar moieties with four fucose-specific biotinylated lectins (Vector Laboratories Inc., Burlingame, CA, USA): 1) Aleuria aurantia lectin (AAL, recognizing fucose α1,6-linked to the N-acetylglucosamine core of N-glycans and with lower affinity fucoses α1,2-, α1,3- and α1,4-linked of the outer arms,38 2) Lens culinaris agglutinin (LCA, recognizing sequences containing fucosylated tri-mannose N-glycan core sites), 3) Lotus tetragonolobus agglutinin (LTA, specifically reacting with fucose α1,3-linked to GlcNAc, characteristic for Lewisx oligosaccharide structures, however, it can also slightly react with fucose typical for Lewisa and Lewisy structures. The expression of terminal sialic acid α2,3-linked in glycoprotein glycan structures disturb the recognition by LTA of fucose α1,3-linked.39 The fourth lectin was Ulex europaeus agglutinin (UEA), specific to antennary fucoses α1,2-linked to Gal and α1,3-linked to GlcNAc (Lewisy sugar structures), but the presence of UEA-reactive fucose α1,2-linked prevents the formation of sialyl-Lewisx glycan structures.40
Lectin-ELISA
The lectin-ELISA test was performed according to the procedure previously described,41 with slight modification. In short: the microtiter plates were incubated with 0.01 mg/mL protein G solution (Abcam, United States) in 10mM TBS pH 7.4 (2h, 37°C, and next at 4°C overnight). Next, the plates were coated with native/isolated IgG diluted with 10 mM TBS-T0.1% (pH 7.4) in the amount of 500 ng IgG in 50 µL solution per well, and incubated (3h, 37°C). Additionally, in this step, for examination of LCA and AAL reactivities, the plates bound with IgG were reduced with dithiothreitol (DTT) (70 min, 37°C). The plates were incubated for 90 min at 37°C with biotinylated lectins diluted with 10mM TBS-T0.1% as follows: AAL-1:2000, LCA-1:2000, LTA-1:100, UEA-1:250. In the next step the plates were incubated with phosphatase-labelled ExtrAvidin for 1h at room temperature, and then the phosphatase reaction was developed at 37°C with a substrate, p-nitrophenyl-phosphate. The reaction was stopped with 100 µL of 1mM NaOH per well and the absorbance was measured at 405 nm with a reference filter λ=630 nm, using Mindray-96A microplate reader (Shenzhen Mindray Bio-Medical Electronics Co, China). After each incubation step the plates were extensively washed with 10 mM TBS-T0.1%, pH 7.4. All samples were analyzed in duplicate. Blank sample background absorbance was measured for samples containing all reagents, except biological sample which was replaced by 10 mM TBS-T0.1%, pH 7.4. The relative reactivities of samples’ with lectins were expressed in absorbance units (AU). IgG levels, necessary for the calculation of IgG amount to lectin-ELISA, in whole sera (s-IgG), had been previously measured using the turbidimetric method,42 and those in IgG isolates (i–IgG) were estimated by the BCA method (described below).
IgG Isolation
Immunoglobulin G was isolated from blood serum using affinity chromatography on a Protein A/Protein G Sepharose column, according to the method described earlier by Ey et al43 and in our previous study.41
Statistical Analysis
Statistical analysis was performed using the statistical software STATISTICA 13.3PL (StatSoft, Poland). Obtained results were presented as means and standard deviations (SD), and distributions of the values within the analyzed groups were presented as box-whisker plots with median and interquartile (25th-75th percentile) ranges. The values of examined parameters did not fit normal distribution according to a Shapiro–Wilk W-test, and thus to determine differences among the studied groups the nonparametric Mann–Whitney U-test was used. The correlations were estimated according to the Spearman rank test with a 95% confidence interval. A two-tailed p-value of less than 0.05 was considered as significant. The diagnostic significance of the examined parameters was analyzed using receiver operating characteristic (ROC) curves. Additionally, cluster analysis was performed for fucose expression in s-IgG and i–IgG, only for those reactivities with lectins for which the area under the curve (AUC) values, determined in ROC analysis, were simultaneously moderate or high, and differentiated the E group from the control group of healthy women. In this analysis, the results are presented as a dendrogram, beginning from one cluster in which all subjects (patients and healthy women) are gathered. The subjects were clustered, those which were similar in terms of values of all the analyzed traits were grouped together and different ones formed a separate cluster. In brief, a greater distance of separation reflects greater differences in subject characteristics. Using an Euclidean metric on the original data points were analyzed and calculated the similarities between samples with no reference to the clinical status of the samples. The order of statistical analyses performed in the present study was based on our previous experience.41,42
Results
The relative reactivities of serum IgG glycans with fucose-specific biotinylated lectins are presented in Table 1 as mean absorbance values and standard deviations (SD) for each analyzed group. The distributions of IgG glycans relative reactivities with lectins used, measured for the control, E and NE groups, are shown in Figure 1.
 |
Table 1 Relative Reactivities of Serum Native IgG and Isolated Serum IgG Glycans with Fucose-Specific Lectins |
The expression of AAL-reactive core fucose in s-IgG was significantly lower in E and NE groups (0.029 ± 0.010 AU, median 0.027 AU, p < 0.000001 and 0.028 ± 0.014 AU, median 0.026 AU, p < 0.000001, respectively) than for the control group (0.056 ± 0.015 AU, median 0.054 AU). The relative reactivities of i–IgG with AAL were significantly higher for the control group (0.140 ± 0.043 AU, median 0.126 AU) than in E and NE groups (0.055 ± 0.033 AU, median 0.045 AU, p < 0.000001 and 0.093 ± 0.022 AU, median 0.090 AU, p = 0.0000026, respectively). The relative reactivities of s-IgG with LCA were significantly higher for the E group (0.587 ± 0.124 AU, median 0.563 AU) than those measured for the NE (0.461 ± 0.109 AU, median 0.452 AU, p = 0.000020) and control (0.467 ± 0.093 AU, median 0.467 AU, p = 0.000677) groups (Table 1, Figure 1).
The relative reactivities of s-IgG and i–IgG glycans with LTA specific to fucose α1,3-linked were significantly lower in the control group (0.213 ± 0.293 AU, median 0.114 AU and 0.185 ± 0.155 AU, median 0.143 AU, respectively) than in E (0.421 ± 0.149 AU, median 0.397 AU, p = 0.000008 and 0.603 ± 0.347 AU, median 0.503 AU, p < 0.000001, respectively) and NE (0.352 ± 0.152 AU, median 0.327 AU, p = 0.00052 and 0.540 ± 0.280 AU, median 0.462 AU, p < 0.000001, respectively) groups (Table 1, Figure 1).
The relative reactivity of s-IgG with UEA reactive with fucose α1,2-linked was lower in the E and NE groups (0.048 ± 0.032 AU, median 0.042 AU and 0.055 ± 0.041 AU, median 0.045 AU, respectively) than for the control group (0.067 ± 0.041 AU, median 0.054), however, these differences were insignificant (p > 0.05). Relative reactivities of i–IgG with UEA were significantly higher for the control group (0.193 ± 0.097 AU, median 0.172 AU) than for the E (0.012 ± 0.015 AU, median 0.008 AU, p < 0.000001) and NE (0.016 ± 0.010 AU, median 0.013 AU, p < 0.000001) groups (Table 1, Figure 1).
The Spearman rank test was used to analyze the correlations between relative reactivities of lectins tested with IgG glycans (s-IgG and i–IgG), and the results of the analysis are shown in Table 2 and Figure 2. A high positive correlation between the relative reactivities of AAL and LCA (r = 0.566, p < 0.000001) with glycans of i–IgG was observed. Average and weak negative correlations were also observed between the relative reactivities of s-IgG and i–IgG glycans with AAL and LTA (r = - 0.350, p = 0.0005; r = - 0.292, p = 0.004, respectively). High positive correlations exist between AAL and UEA relative reactivities with i–IgG glycans (r = 0.610, p < 0.000001). For s-IgG relative reactivities with AAL and UEA, the correlation was average and weaker than for i–IgG (r = 0.437, p = 0.000009). Average negative correlation between relative reactivities with LCA and LTA (r = - 0.352, p = 0.0004) of i–IgG glycans was observed. Positive average correlation between LCA and UEA relative reactivities (r = 0.475, p = 0.000001) with i–IgG glycans was observed. A weak negative correlation was observed between i–IgG glycans relative reactivities with LTA and UEA (r = - 0.270, p = 0.008) (Table 1).
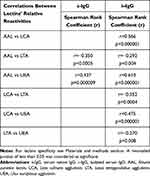 |
Table 2 The Correlations Between Relative Reactivities of IgG Glycans with Fucose-Specific Lectins |
The comparison of s-IgG and i–IgG relative reactivities with lectins used shows the presence of a high positive correlation in LTA relative reactivities examined for s-IgG and i–IgG (r = 0.583; p < 0.000001). Average positive correlations in AAL, as well as in UEA relative reactivities with IgG glycans were observed between s-IgG and i–IgG (r = 0.405; p = 0.000045 and r = 0.465; p = 0.000002, respectively). The positive correlation between relative reactivities of s-IgG and i–IgG glycans with LCA was weak (r = 0.257; p = 0.011) (Figure 2).
ROC Curve Analysis
ROC curve analysis of relative reactivities of s-IgG glycans with fucose-specific lectins in advanced endometriosis and control groups identified parameters with a sensitivity and specificity, respectively: for AAL 0.950 and 0.737 (AUC 0.926 – high clinical value); for LCA 0.825 and 0.579 (AUC 0.775 – moderate clinical value); for LTA 0.925 and 0.842 (AUC 0.862 – moderate clinical value); for UEA 0.600 and 0.632 (AUC 0.631 – limited clinical value). For i–IgG the obtained results were the following: for AAL 0.875 and 1.0 (AUC 0.953 – high clinical value); for LCA 0.950 and 0.947 (AUC 0.978 – high clinical value); for LTA 0.825 and 0.947 (AUC 0.923 – high clinical value); for UEA 0.975 and 1.0 (AUC 0.997 – high clinical value). For the determination of cut-off points (Figure 3 and Table 3) the Youden index method was used. The clinical value of a laboratory test, based on AUC value, can be defined as: 0–0.5 – zero, 0.5–0.7 – limited, 0.7–0.9 – moderate and > 0.9 high.44
 |
Table 3 Receiver Operating Characteristic Curve Analysis of Serum Native IgG and Isolated Serum IgG Relative Reactivities with Fucose-Specific Lectins |
Cluster Analysis
The relative reactivities with AAL, LCA and LTA were selected from the panel of parameters investigated among the expression of s-IgG and i–IgG glycans used for differentiation the group of patients with endometriosis from the group of healthy women. They were taking into account the values of relative reactivities with those lectins which simultaneously meet the following criteria: they differentiate the endometriosis women from the control group of healthy women, as well as having moderate and high clinical value according to the results of ROC analysis (AUC ≥ 0.775). The analysis was performed for 59 samples, for which all three selected parameters were determined, and was conducted separately for s-IgG and i–IgG. In the case of s-IgG, the first cluster could be separated as a group of 1 control sample (Figure 4 and Table 4) at 87% distance. The next cluster, separated at 50.7% distance (Cluster 2), consists of 18 samples from which 16 samples were from women with endometriosis (40% from the whole endometriosis group). The next cluster (Cluster 3 at 46.7% distance) was separated as a homogenous group of 16 endometriosis samples (40% of the whole endometriosis group). The last cluster (Cluster 4) consists of 8 endometriosis samples and 16 samples from the control group (84% of the whole control group). For i–IgG (Figure 5 and Table 5) the first cluster was separated at distance 97.5%. This group was homogenous and consists of 9 endometriosis samples (22.5% of the whole group). The next cluster (No 2) was separated at 75% distance, in which all samples from the control group were gathered (100%). Cluster 2 and 3 (homogenous, composed of only endometriosis samples) were distinguished at distance 52%.
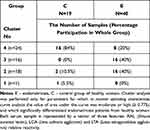 |
Table 4 Results of Cluster Analysis for Values of Serum Native IgG Relative Reactivities with AAL, LCA and LTA |
 |
Table 5 Results of Cluster Analysis for Values of Isolated Serum IgG Relative Reactivities with AAL, LCA and LTA |
Discussion
While the IgG fucosylation study is not a new field in glycoimmunology, to the best of our knowledge this is the first investigation to analyse the human serum IgG fucosylation profile and degree in endometriosis using lectin-ELISA. This method, contrary to other methods used for analysis of the quantitative and qualitative composition of glycoprotein glycans, enables us to assess the interactions that occur in a living organism between monosaccharide residues of glycoprotein glycans and the corresponding ligands, which are particularly interesting and valuable in our opinion. We compared the obtained results with those for isolated serum IgG, aiming to check whether native serum IgG fucosylation analysis, without prior IgG isolation, is enough and satisfactory for the differentiation of advanced endometriosis from the control group of healthy women.
We demonstrated that for native and isolated serum IgG, the expression of AAL-reactive core fucose was significantly lower in patients with non-endometriosis and advanced endometriosis when compared to the healthy women, and AAL-reactivity significantly varied between E and NE groups only for isolated IgG. In the case of the second lectin specific for core fucose, LCA, the obtained relative reactivity values were significantly different for s-IgG and i–IgG between the endometriosis group and the group of healthy women, and only for s-IgG did the expression of LCA-reactive fucose significantly differ between NE and E groups, which is particularly important considering the need to differentiate endometriosis from other gynaecological inflammatory diseases. On the other hand, only for i–IgG do the relative reactivities with LCA differentiate NE from the control group. The observed differences in AAL and LCA relative reactivities between analyzed groups of s-IgG and i–IgG may be caused by unnecessary identical specificity of both lectins, because AAL, except α1,6-linked fucose, may also detect antennary fucose α1,3-linked as a part of Lewisx oligosaccharide structures, which could influence the obtained results. Moreover, taking into account the above information, the most plausible explanation for the observed differences in s-IgG and i–IgG reactivities with AAL and LCA may be the slightly different sugar residue availability for the lectin. Due to the fact that methods of determination based on the simplest procedures, ie without the need to carry out complicated and time-consuming isolation procedures of the glycoprotein from the biological material, are the most valuable in terms of their possible application in the diagnostics of endometriosis, and other diseases, the comparisons of reactivity with fucose-specific lectins carried out for native IgG and IgG isolated from the sera are, in our opinion, of particular importance. The observed strong positive correlations between AAL and LCA reactivity (r = 0.566, p < 0.000001) for i–IgG only seem to confirm the hypothesis about differences in sugar moieties availability for lectins between s-IgG and i–IgG.
Despite the fact that endometriosis is a benign disease, accompanied by the development of inflammation,45,46 its infiltration of peripheral organs and invasion of distant organs resembles the metastasis of malignant disease.47–49 Fucosylation of the IgG Fc region is regulated to drive appropriate pro-inflammatory effector cell functions, and changes leading to the modification of IgG Fc glycans are key regulators of antibody activity in vivo. While Fc sialylation plays an active role in anti-inflammatory signaling and in the maturation of adaptive immune responses, fucosylation of the Fc is regulated to drive appropriate pro-inflammatory effector cell functions. Reduced core fucosylation is typical for inflammatory conditions and abnormal glycosylation patterns of glycoproteins are associated with variety of diseases, such as inflammation or cancer, as shown in many previous studies.50–52 Endometriosis induces a variable amount of inflammatory reactions in the pelvic environment, depending on the stage and morphologic appearance of disease.53,54 The inflammatory reaction associated with endometriosis has been demonstrated both in vitro and in vivo by the infiltration of immune cells and the presence of a number of primary and secondary inflammatory mediators in tissues and body fluids.55,56 The molecular mechanism by which fucosylation affects ADCC has long remained unknown. Studies of Okazaki et al57 showed that the afucosylated antibody binds to the FcγRIIIa receptor with greater affinity than the fucosylated version (31–21 fold). Increased affinity is achieved by engaging in additional non-covalent (enthalpy driven) interactions. Ferrara et al20 and Mizushima et al25 demonstrated that the fucose moiety clashes with saccharide units of the N-glycan of the receptor FcγRIIIa, thus imposing steric hindrance and thereby explaining the unfavorable interaction in the fucosylated state. This explanation is consistent with previous data showing that the N-glycan of FcγRIIIa is compatible with the afucosylated N-glycan of IgG-Fc.58,59 In the present research we showed that the s-IgG and i–IgG glycans, except for AAL- and LCA-reactivity detecting core fucose, may also react with LTA and UEA, which may suggest the presence of α1,3-linked, and α1,2-linked antennary fucose, characteristic for Lewisx and Lewisy oligosaccharide structures, respectively. The relative reactivities of s-IgG and i–IgG glycans with LTA were significantly reduced in the group of healthy women in comparison to the endometriosis and non-endometriosis groups. The antennary fucoses, as part of Lewisx/sialyl-Lewisx and Lewisy oligosaccharide structures, are involved in eg recognition between cells, embryo development or disease processes.60,61 The expression of UEA-reactive glycans of s-IgG and i–IgG was very low and the significant differences between endometriosis and NE group versus control group, and between E versus NE group were observed for i–IgG only. However, as documented in many studies investigating IgG fucosylation, IgG is mainly core-fucosylated.20,26,62 In our previous studies on IgG glycosylation we reported the presence of LTA- and UEA-reactive fucose in synovial fluid IgG glycans,63 and for glycans of human seminal plasma IgG.64 Flögel et al65 and Gornic et al66 in their study documented that serum IgG may be also decorated by UEA-reactive fucose. Zauner et al67 reported that in IgG, fucose can be attached not only to the first GlcNAc residues in the core glycan part (core fucose) but also to the terminal Gal residues of the glycan antennas. Therefore, it was not a big surprise for us that serum IgG glycans may react also with UEA and LTA, which are specific to antennary fucose. The observed weak and strong positive correlation for s-IgG and i–IgG oligosaccharide structures, respectively, between AAL and UEA relative reactivities may suggest the simultaneous increased expression of glycans, not necessarily the same, which are decorated by core fucose as well as by UEA-reactive fucose α1,2-linked.
Based on the results of our previous study concerning sialylation of serum IgG in endometriosis41 we also observed the presence of strong positive correlations between the specific reactivity of s-IgG and i–IgG with AAL, LCA and UEA, and their reactivity with sialo-specific lectin MAA (data not shown). The above observation is in accordance with the fact that highly sialylated IgG with a high content of fucose has a protective effect, because increased expression of fucose in IgG glycans contributes to a decrease in antibody-dependent cytotoxicity,68–71 increases the stability of the Fc fragment of IgG62 and, together with an increase in sialic acid expression, enhances the anti-inflammatory properties of IgG.50–52 Comparing the lectins’ relative reactivities in each of the analyzed groups for s-IgG and i–IgG glycans, they more or less differ from each other, which may be due to differences between native s-IgG and i–IgG isolated from serum in the availability of sugar residues for lectins.41 It should be taken into account that N-glycans are present not only in the IgG Fc region. Estimates of the percentage of Fab-glycosylated IgG in healthy individuals range from ∼15% to 25%, depending on the method used for glycans detection and analysis. As for the Fc region, the amounts and types of Fab glycans can vary under certain physiological and pathological conditions.72 The observed higher level of LCA-reactive core fucose expression in i–IgG, on the contrary to s-IgG, in the group of healthy women in comparison to the E group, may be due to the fact that IgG N-glycans may be expressed and detected in Fc and Fab IgG regions as well, and the process of IgG isolation may have a positive impact on the core fucose availability for lectins, especially those which compose glycans of Fc region. This seems to be confirmed by the positive correlations observed between the relative reactivities of s-IgG and i–IgG oligosaccharides with fucose-specific lectins which, however significant, were weak, and strong only for LTA reactivity.
In our previous study42 we reported that the values of total serum IgG concentration were significantly reduced in women with advanced stages of endometriosis in comparison to the healthy controls. We hypothesized that this phenomena may be associated with the follicular phase of the patients’ menstrual cycle, as well as may be caused by the treatment of women with glucocorticoids used eg for allergies. The therapy used may eliminate the inflammatory reaction accompanying endometriosis. Unfortunately, we have no information about allergic diseases diagnosed or menstrual cycle phases in the studied groups of women.42 As in the present study we observed that some serum samples showed extremely high IgG relative reactivity with the lectins used, we wanted to check the possible cause for these results, and we decided to analyse the concentrations of commonly known and routinely determined parameters of inflammation CA 125 and hsCRP, analyzed and discussed in our previous study.42 We noticed that from nine samples with extremely high reactivity of IgG glycans with fucose-specific lectins, only three have CA 125 levels slightly exceeding physiological level (data not shown), and only one has a slightly increased hsCRP concentration. This additionally confirms the complexity of the processes that accompany the development of inflammation, the interrelationships of which are not fully understood, and their course may be influenced by many factors that are not always recognized. Kokot et al42 also observed significant negative correlations between serum total IgG concentrations and the levels of CA 125 and hs-CRP. In the present study, we observed the presence of significant negative correlations between total serum IgG levels and IgG relative reactivity with LTA, specific to α1,3-linked fucose of Lewisx oligosaccharide structures, and LCA detecting core fucose (data not shown), which indicates that decreased total serum IgG levels are accompanied by an increased degree of IgG fucosylation. On the other hand, significant positive correlations between CA 125 levels and relative reactivities of IgG glycans with LTA and LCA (data not shown) may additionally confirm that degree of IgG fucosylation is closely related to the development of inflammation.
To check the utility of the results obtained for the differentiation of s-IgG and i–IgG fucosylation in a way that reflects their clinical characteristics typical for advanced endometriosis, versus a group of healthy women, we analyzed the values of core fucose expression (AAL and LCA reactivity) and the expression of LTA- and UEA-reactive fucose, using ROC curve analysis. For s-IgG and i–IgG, the cut-off point for AAL reactivity was 0.044 AU (AUC 0.926) with sensitivity 95% and specificity 73.5%, and 0.082 AU (AUC 0.953) with sensitivity 87.5% and specificity 100%, respectively, which reflects that this parameter has a high clinical value. For LCA, the proposed cut-off point was 0.47 AU (AUC 0.775) with sensitivity 82.5% and specificity 57.9%, and 0.734 AU (AUC 0.978) with sensitivity 95% and specificity 94.7% for s-IgG and i–IgG, respectively, which proves that this parameter has moderate and high clinical value, respectively. The ROC curve analysis of LTA relative reactivity shows that for s-IgG the cut-off point was 0.226 AU (AUC 0.862, which shows a moderate clinical value) with sensitivity 92.5% and specificity 84.2%, but for i–IgG this parameter has a high clinical value and the proposed cut-off point was 0.273 AU with AUC 0.923, sensitivity 82.5% and specificity 94.7%. Although the results of the ROC analysis showed that for i-IgG, but not for s-IgG, UEA relative reactivity also has high clinical value (AUC 0.997, cut-off point 0.045 AU, sensitivity 97.5%, specificity 100%) and may be taken into account as a specific marker for endometriosis, we selected only AAL, LCA and LTA reactivity for the cluster analysis, taking into consideration only these parameters which simultaneously differentiate patients with advanced endometriosis from healthy subjects and have at least moderate clinical value, documented by ROC curve analysis. It should also be mentioned that ROC curve analysis was also performed to check the utility of s-IgG and i-IgG fucosylation determination in a way that reflects their clinical characteristics, typical for the group of women without endometriosis (NE group) but with other gynaecological diseases, versus the advanced endometriosis group and the group of healthy women, however, the results obtained showed no clinical value (data not shown). Another reason for this selection was the aim of our study, which was to check whether any changes in the profile and degree of s-IgG fucosylation could become additional diagnostic markers enabling the differentiation of women with advanced endometriosis from the control group of healthy women. Cluster analysis additionally confirmed the utility of the values of the AAL, LCA and LTA relative reactivity with IgG glycans for differentiation of advanced endometriosis patients from the group of healthy subjects. For s-IgG the fourth cluster differed with regard to the clinical characteristics of women, gathering 84% of samples from the whole group of healthy women (16 from 19) and 8 samples from the endometriosis group (20% from the whole E group). From three clusters formed for i-IgG, cluster 2 gathered 100% of samples from the group of healthy women and only 5% of samples from women with endometriosis .
The aim of our study was to check whether the analysis of immunoglobulin G fucosylation profile and degree may be usable as markers differentiating endometriosis from the physiological state, and the results obtained in the present study seem to meet this criterion. Especially important are the findings which inform us that while for IgG isolates the differences between the E and control group were more spectacular, the IgG fucosylation analysis directly in serum, without prior IgG isolation, may also be diagnostically useful in advanced endometriosis. The lectin-ELISA method we used for this purpose, which mimics the interaction between glycan and lectin that occurs in vivo, including the availability of sugar moieties, additionally enabled us to deepen our knowledge about the molecular mechanisms of these reactions. However, we must remember that most of the lectins used do not have an absolute specificity and therefore can bind to similar carbohydrate structures with various affinities. Under unfavorable conditions, or due to the unavailability of the most preferred attachment site, lectins may bind to other structures for which they have a lower affinity.73 Endometriosis has a profound impact on women’s lives, including associated pain and infertility, which decrease the quality of their lives. For many women, the road to the diagnosis of endometriosis is long and full of obstacles, therefore any additional biomarker which can help with faster and more accurate non-invasive diagnostics of endometriosis is especially valuable, and searching for them should be a priority. An additional finding resulting from our research is the re-confirmation of one from our previous studies, that in order to be able to draw correct conclusions about the diagnostic usefulness of the parameters determined in the sera of patients with endometriosis, the control group should be composed of completely healthy women, and not non-endometriosis patients suffering from other gynaecological diseases.41,42 Of key importance here is the inflammation accompanying both endometriosis and other female disorders, which affects the expression of many biochemical parameters, including changes in IgG glycosylation, often regardless of the disease it is associated with. The current research additionally confirms our previous observations on this topic.41,42 Moreover, the lack of a representative group of patients with early-stage endometriosis makes it impossible the verification of analyzed in this study parameters and further evaluation whether they may be useful biomarkers the early stages development of endometriosis, which is a limitation of our study, but also a direction of future investigations.
Conclusion
Endometriosis has a profound effect on women’s lives, and in many cases the path to diagnosis of endometriosis is long and full of obstacles. The relatively fast, non-invasive diagnosis of disease should be a priority, unfortunately the diagnostic process of endometriosis is difficult, expensive and often invasive, therefore the search for new patient-friendly diagnostic methods and parameters is necessary. Based on current knowledge, disease development is associated with inflammatory processes, especially in the advanced stages of endometriosis, markers of which can also be detected in peripheral blood serum. In light of the above information and the results obtained in the present study, the analysis of the profile and degree of serum IgG fucosylation may be very promising in the diagnostics of advanced endometriosis, and may be useful as an additional parameter for medical interviews and tests. The proposed panel of parameters which are the expression in IgG glycans of AAL- and LCA-reactive core fucose and α1,3-linked fucose recognized by LTA, could be taken into account as a useful clinical marker to diagnose women with advanced endometriosis, however, its clinical utility in everyday practice for disease diagnostics needs to be evaluated in further studies. It would also be interesting to check whether the determinations of the parameters we selected could be helpful in the diagnosis of the early stages of endometriosis, enabling the detection of the disease at an early stage of its development, which would be particularly valuable from the patients’ point of view.
Institutional Review Board Statement
The study procedures followed in the study were conducted in agreement with the Helsinki-II-declaration and the protocol was approved by the Bioethics Human Research Committee of the Wroclaw Medical University (Permission No. KB-293/2016 and KB-719/2018). Written informed consent was obtained from recruited patients.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This research was financed through a subsidy by the Polish Ministry of Health, realized under the topic, according to the records in the Simple system with the number SUB.D270.21.096.
Disclosure
Authors declare no conflict of interests.
References
1. Agarwal SK, Chapron C, Giudice LC, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. 2019;220(4):354. doi:10.1016/j.ajog.2018.12.039
2. Johnson NP, Hummelsho L. Consensus on current management of endometriosis. Hum Reprod. 2013;28:1552–1568. doi:10.1093/humrep/det050
3. Dunselman G, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400–412. doi:10.1093/humrep/det457
4. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935. doi:10.1016/j.fertnstert.2014.02.012
5. Andrews WC, Buttram VC, Behrman SJ. Revised American fertility society classification of endometriosis: 1985. Fertil Steril. 1985;44:7–8.
6. Canis M, Donnez JG, Guzick DS, et al. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi:10.1016/S0015-0282(97)81391-X
7. Kyama CM, Debrock S, Mwenda JM, D’Hooghe TM. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol. 2003;2(1):123. doi:10.1186/1477-7827-1-123
8. Hyun Ahn S, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int. 2015;2015:795976. doi:10.1155/2015/795976
9. Vlahos NF, Kalampokas T, Fotiou S. Endometriosis and ovarian cancer: a review. Gynecol Endocrinol. 2010;26(3):213–219. doi:10.1080/09513590903184050
10. Czyzyk A, Podfigurna A, Szeliga A, Meczekalski B. Update on endometriosis pathogenesis. Minerva Ginecol. 2017;69(5):447–464. doi:10.23736/S0026-4784.17.04048-5
11. Ruderman R, Pavone ME. Ovarian cancer in endometriosis: an update on the clinical and molecular aspects. Minerva Ginecol. 2017;69(3):286–294. doi:10.23736/S0026-4784.17.04042-4
12. Oosterlynck DJ, Meuleman C, Waer M, Vandeputte M, Koninckx PR. The natural killer activity of peritoneal fluid lymphocytes is decreased in women with endometriosis. Fertil Steril. 1992;58(2):290–295. doi:10.1016/s0015-0282(16)55224-8
13. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. doi:10.1016/s0015-0282(00)01630-7
14. Patel BG, Lenk EE, Lebovic DI, Shu Y, Yu J, Taylor RN. Pathogenesis of endometriosis: interaction between endocrine and inflammatory pathways. Best Pract Res Clin Obstet Gynaecol. 2018;50:50–60. doi:10.1016/j.bpobgyn.2018.01.006
15. Routier FH, Hounsell EF, Rudd PM, et al. Quantitation of the oligosaccharides of human serum IgG from patients with rheumatoid arthritis: a critical evaluation of different methods. J Immunol Methods. 1998;213:113–130. doi:10.1016/s0022-1759(98)00032-5
16. Borrok MJ, Jung ST, Kang TH, Monzingo AF, Georgiou G. Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem Biol. 2012;7:1596–1602. doi:10.1021/cb300130k
17. Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979–989. doi:10.1016/s0022-2836(02)01250-0
18. Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi:10.1074/jbc.M202069200
19. Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406(6793):267–273. doi:10.1038/35018508
20. Ferrara C, Grau S, Jager C, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi:10.1073/pnas.1108455108
21. Parekh RB, Dwek RA, Sutton BJ, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:52–457. doi:10.1038/316452a0
22. Ramakrishna C, Newo AN, Shen YW, Cantin E. Passively administered pooled human immunoglobulins exert IL-10 dependent anti-inflammatory effects that protect against fatal HSV encephalitis. PLoS Pathog. 2011;7:e1002071. doi:10.1371/journal.ppat.1002071
23. Mizuochi T, Taniguchi T, Shimizu A, Kobata A. Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J Immunol. 1982;129:2016–2020.
24. Shibata-Koyama M, Iida S, Okazaki A, et al. The N-linked oligosaccharide at Fc gamma RIIIa Asn-45: an inhibitory element for high Fc gammaRIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology. 2009;19:126–134. doi:10.1093/glycob/cwn110
25. Mizushima T, Yagi H, Takemoto E, et al. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells. 2011;16(11):1071–1080. doi:10.1111/j.1365-2443.2011.01552.x
26. Li J, Hsu HC, Mountz JD, Allen JG. Unmasking fucosylation: from cell adhesion to immune system regulation and diseases. Cell Chem Biol. 2018;25(5):499–512. doi:10.1016/j.chembiol.2018.02.005
27. Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143:725–729. doi:10.1093/jb/mvn011
28. Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16:158–184. doi:10.1093/glycob/cwl040
29. Brinkman-van der Linden EC, de Haan PF, Havenaar EC, van Dijk W. Inflammation-induced expression of sialyl LewisX is not restricted to alpha1-acid glycoprotein but also occurs to a lesser extent on alpha1-antichymotrypsin and haptoglobin. Glycoconjugate J. 1998;15:177–182. doi:10.1023/a:1006972307166
30. Goodarzi MT, Axford JS, Varanasi SS, et al. Sialyl Lewis(x) expression on IgG in rheumatoid arthritis and other arthritic conditions: a preliminary study. Glycoconj J. 1998;15:1149–1154. doi:10.1023/a:1006920007227
31. Li J, Hsu HC, Ding Y, et al. Inhibition of fucosylation reshapes inflammatory macrophages and suppresses type II collagen-induced arthritis. Arthritis Rheumatol. 2014;66:2368–2379. doi:10.1002/art.38711
32. Ryden I, Pahlsson P, Lundblad A, Skogh T. Fucosylation of α1-acid glycoprotein (orosomucoid) compared with traditional biochemical markers of inflammation in recent onset rheumatoid arthritis. Clin Chim Acta. 2002;317:221–229. doi:10.1016/s0009-8981(01)00803-8
33. Thompson S, Kelly CA, Griffiths ID, Turner GA. Abnormally-fucosylated serum haptoglobins in patients with inflammatory joint disease. Clin Chim Acta. 1989;184:251–258. doi:10.1016/0009-8981(89)90058-2
34. Sarrats A, Saldova R, Pla E, et al. Glycosylation of liver acute phase proteins in pancreatic cancer and chronic pancreatitis. Proteomics Clin Appl. 2010;4:432–448. doi:10.1002/prca.200900150
35. Miyoshi J, Yajima T, Okamoto S, et al. Ectopic expression of blood type antigens in inflamed mucosa with higher incidence of FUT2 secretor status in colonic Crohn’s disease. J Gastroenterol. 2011;46:1056–1063. doi:10.1007/s00535-011-0425-7
36. Maroni L, van de Graaf SFJ, Hohenester SD, et al. Fucosyltransferase 2: a genetic risk factor for primary sclerosing cholangitis and Crohn’s disease–a comprehensive review. Clin Rev Allergy Immunol. 2015;48:182–191. doi:10.1007/s12016-014-8423-1
37. Kapur R, Kustiawan I, Vestrheim A, et al. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood. 2014;123:471–480. doi:10.1182/blood-2013-09-527978
38. Yamashita K, Kochibe N, Ohkura T, Ueda I, Kobata A. Fractionation of L-fucose-containing oligosaccharides on immobilized Aleuria aurantia lectin. J Biol Chem. 1985;260:4688–4693. doi:10.1016/S0021-9258(18)89125-6
39. Yan L, Wilkins PP, Alvarez-Manilla G, Do SI, Smith DF, Cummings RD. Immobilized Lotus tetragonolobus agglutinin binds oligosaccharides containing the Le(x) determinant. Glycoconj J. 1997;14:45–55. doi:10.1023/a:1018508914551
40. Loris R, De Greve H, Dao-Thi MH, Messens J, Imberty A, Wyns L. Structural basis of carbohydrate recognition by lectin II from Ulex europaeus, a protein with a promiscuous carbohydrate binding site. J Mol Biol. 2000;301:987–1002. doi:10.1006/jmbi.2000.4016
41. Sołkiewicz K, Krotkiewski H, Jędryka M, Kratz EM. Variability of serum IgG sialylation and galactosylation degree in women with advanced endometriosis. Sci Rep. 2021;11:5586. doi:10.1038/s41598-021-85200-x
42. Kokot I, Piwowar A, Jędryka M, Sołkiewicz K, Kratz EM. Diagnostic significance of selected serum inflammatory markers in women with advanced. Int J Mol Sci. 2021;22(5):2295. doi:10.3390/ijms22052295
43. Ey PL, Prowse SJ, Jenkin CR. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978;15:429–436. doi:10.1016/0161-5890(78)90070-6
44. Bossuyt X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmun Rev. 2009;8:543–548. doi:10.1016/j.autrev.2009.01.013
45. Eisenberg VH, Zolti M, Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun Rev. 2012;11:806–814. doi:10.1016/j.autrev.2012.01.005
46. Berkes E, Muzinic A, Rigo J, Tinneberg HR, Oehmke F. The analysis of the human plasma N-glycome in endometriosis patients. Eur J Obstet Gynecol Reprod Biol. 2013;171:107–115. doi:10.1016/j.ejogrb.2013.08.008
47. Corzo C, Barrientos Santillan N, Westin SN, Ramirez PT. Updates on Conservative Management of Endometrial Cancer. J Minim Invasive Gynecol. 2018;25(2):308–313. doi:10.1016/j.jmig.2017.07.022
48. Heong V, Ngoi N, Tan DS. Update on immune checkpoint inhibitors in gynecological cancers. J GynecolOncol. 2017;28(2):e20. doi:10.3802/jgo.2017.28.e20
49. Menderes G, Hicks C, Black JD, Schwab CL, Santin AD. Immune checkpoint inhibitors in gynecologic cancers with lessons learned from non-gynecologic cancers. Expert Opin Biol Ther. 2016;16(8):989–1004. doi:10.1080/14712598.2016.1177018
50. Saldova R, Royle L, Radcliffe CM, et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17:1344–1356. doi:10.1093/glycob/cwm100
51. Ohmi Y, Ise W, Harazono A, et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun. 2016;7:11205. doi:10.1038/ncomms11205
52. Komaromy A, Reider B, Jarvas G, Guttman A. Glycoprotein biomarkers and analysis in chronic obstructive pulmonary disease and lung cancer with special focus on serum immunoglobulin G. Clin Chim Acta. 2020;506:204–213. doi:10.1016/j.cca.2020.03.041
53. Halme J, Becker S, Haskill S. Altered maturation and function of peritoneal macrophages: possible role in pathogenesis of endometriosis. Am J Obstet Gynecol. 1987;156:783189. doi:10.1016/0002-9378(87)90333-4
54. Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum. Fertil Steril. 2004;81:652–661. doi:10.1016/j.fertnstert.2003.07.037
55. Khan KN, Masuzaki H, Fujishita A, et al. Regulation of hepatocyte growth factor by basal and stimulated macrophages in women with endometriosis. Hum Reprod. 2005;20:49–60. doi:10.1093/humrep/deh525
56. Khan KN, Kitajima M, Hiraki H, et al. Immunopathogenesis of pelvic endometriosis: role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol. 2008;60:383–404. doi:10.1111/j.1600-0897.2008.00643.x
57. Okazaki A, Shoji-Hosaka E, Nakamura K, et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol. 2004;336:1239–1249. doi:10.1016/j.jmb.2004.01.007
58. Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032. doi:10.1074/jbc.M510171200
59. Shibata-Koyama M, Iida S, Misaka H, et al. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcγRIIIb and MHC class II expression on the phagocytotic neutrophils. Exp Hematol. 2009;37(3):309–321. doi:10.1016/j.exphem.2008.11.006
60. Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41–53. doi:10.1093/glycob/cwg054
61. Chen HL. Lewis glyco-epitopes: structure, biosynthesis, and functions. In: Wu AM, editor. The Molecular Immunology of Complex Carbohydrates-3. New York, Dordrecht, Heidelberg, London: Springer; 2011:53–80.
62. Castilho A, Gruber C, Thader A, et al. Processing of complex N-glycans in IgG Fc-region is affected by core fucosylation. MAbs. 2015;7:863–870. doi:10.1080/19420862.2015.1053683
63. Kratz EM, Borysewicz K, Katnik-Prastowska I. Terminal monosaccharide screening of synovial immunoglobulins G and A for the early detection of rheumatoid arthritis. Rheumatol Int. 2010;30(10):1285–1292. doi:10.1007/s00296-009-1139-5
64. Kratz EM, Ferens-Sieczkowska M, Faundez R, Kątnik-Prastowska I. Changes in fucosylation of human seminal IgG and secretory component of IgA in leukocytospermic patients. Glycoconj J. 2014;31(1):51–60. doi:10.1007/s10719-013-9501-y
65. Flögel M, Lauc G, Gornik I, Macek B. Fucosylation and galactosylation of IgG heavy chains differ between acute and remission phases of juvenile chronic arthritis. Clin Chem Lab Med. 1998;36(2):99–102. doi:10.1515/CCLM.1998.018
66. Gornik I, Maravić G, Dumić J, Flögel M, Lauc G. Fucosylation of IgG heavy chains is increased in rheumatoid arthritis. Clin Biochem. 1999;32(8):605–608. doi:10.1016/s0009-9120(99)00060-0
67. Zauner G, Selman MH, Bondt A, et al. Glycoproteomic analysis of antibodies. Mol Cell Proteomics. 2013;12:856–865. doi:10.1074/mcp.R112.026005
68. Niwa R, Shoji-Hosaka E, Sakurada M, et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64(6):2127–2133. doi:10.1158/0008-5472.can-03-2068
69. Niwa R, Natsume A, Uehara A, et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306(1–2):151–160. doi:10.1016/j.jim.2005.08.009
70. Masuda K, Kubota T, Kaneko E, et al. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol Immunol. 2007;44(12):3122–3131. doi:10.1016/j.molimm.2007.02.005
71. Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112(6):2390–2399. doi:10.1182/blood-2008-03-144600
72. Van de Bovenkamp FS, Hafkenscheid L, Rispens T, Rombouts Y. The emerging importance of IgG Fab glycosylation in immunity. J Immunol. 2016;196:1435–1441. doi:10.4049/jimmunol.1502136
73. Van Damme EJM. Lectins as tools to select for glycosylated proteins. In: Gevaert K, Vandekerckhove J, editors. Gel-Free Proteomics. Springer Protocols; 2011:289–297.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





