Back to Journals » Infection and Drug Resistance » Volume 16
The 100 Most-Cited Articles in COVID-19 Vaccine Hesitancy Based on Web of Science: A Bibliometric Analysis
Authors Liu B, You J, Huang L, Chen M, Shen Y, Xiong L, Zheng S, Huang M
Received 13 February 2023
Accepted for publication 22 April 2023
Published 2 May 2023 Volume 2023:16 Pages 2625—2646
DOI https://doi.org/10.2147/IDR.S408377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Suresh Antony
Bo Liu,1,2 Junjie You,1 Lingyi Huang,1 Mengling Chen,1 Yushan Shen,1 Longyu Xiong,1 Silin Zheng,3 Min Huang2
1School of Nursing, Southwest Medical University, Luzhou, Sichuan Province, 646000, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan Province, 646000, People’s Republic of China; 3Nursing Department, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan Province, 646000, People’s Republic of China
Correspondence: Silin Zheng, Nursing Department, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan Province, 646000, People’s Republic of China, Tel +86 13002866667, Email [email protected] Min Huang, Department of Respiratory and Critical Care Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan Province, 646000, People’s Republic of China, Tel +86 18982482624, Email [email protected]
Purpose: To perform a bibliometric analysis of the 100 most-cited articles (T100 articles) on COVID-19 vaccine hesitancy to characterize current trends.
Methods: The data of the bibliometric analysis were retrieved from the Web of Science Core Collection (WoSCC) database on January 29, 2023, and the results were sorted in descending order by citations. Two researchers independently extracted the characteristics of the top 100 cited articles, including title, author, citations, publication year, institution, country, author keywords, Journal Cited Rank, and impact factor. Excel and VOSviewer were used to analyze the data.
Results: The T100 articles ranged from 79 to 1125 citations, with a mean of 208.75. The T100 articles were contributed by 29 countries worldwide, of which the USA ranked first with 28 articles and 5417 citations. The T100 articles were published in 61 journals; the top three citations were VACCINES, NATURE MEDICINE, and EUROPEAN JOURNAL OF EPIDEMIOLOGY, and the number of citations was 2690, 1712, and 1644, respectively. Professor Sallam, M(n=4) from Jordan, is the author who participated in the most published articles. Catholic University of the Sacred Heart (n=8) had the most T100 articles.
Conclusion: It is the first bibliometric analysis of the T100 articles in the field of COVID-19 vaccine hesitancy. We carefully analyzed and described the characteristics of these T100 articles, which provide ideas for further strengthening COVID-19 vaccination and fighting against the epidemic in the future.
Keywords: COVID-19, vaccine hesitancy, bibliometric analysis, citation
Introduction
This year marks the fourth year of the Coronavirus Disease 2019 (COVID-19) epidemic, which has had a massive impact on the public health, production, and economies of countries around the world. Vaccination is known to be the most cost-efficient way to prevent infectious diseases and prevent the spread of epidemics.1,2 However, vaccine hesitancy is a growing and significant barrier to vaccination. Vaccine hesitancy is defined as a delay in acceptance or refusal of vaccines,3,4 despite the availability of vaccine services. Some studies indicate that vaccine hesitancy is influenced by several factors, including but not limited to confidence, complacency, and convenience.5–7 In addition, the COVID-19 vaccine is a novel product, and there may be greater hesitancy with a new vaccine than with other vaccines that are well known. COVID-19 vaccine hesitancy is prevalent worldwide, although the rates vary across countries/regions. A cross-sectional survey of community‐based research in Turkey showed that 45.3% of participants hesitated to receive the COVID‐19 vaccine.8 The Freeman et al9 study showed that COVID-19 vaccine hesitancy is up to 28.3% in the UK. In addition, many studies have shown that social media significantly impacts vaccine hesitancy. Misinformation, scandal, or negative emotions can reduce people’s confidence in vaccines, leading to high vaccine hesitancy rates.10–14 Choudhary et al15 report that vaccine hesitancy is reportedly a widespread challenge in India, particularly in rural areas, due to misinformation and mistrust. On August 31st, 2022, the US Food and Drug Administration (FDA) granted emergency authorization to use Pfizer and Moderna’s bivalent COVID-19 vaccines. The experts strongly recommend receiving a booster dose with a bivalent COVID-19 vaccine if you are eligible for one to ensure you are protected against the current circulating variants of the virus.16 However, Pfizer was reported by the media recently to have produced a COVID-19 variant virus, which has generated much discussion. Whether this is true remains to be seen, but it will undoubtedly impact the COVID-19 vaccination.
Several theories or tools have been used to analyze factors contributing to vaccine hesitancy. Such as the Health Belief Model (HBM),17 Protective Motivation Theory (PMT),18 Theory of Planned Behavior (TPB),19 3Cs model, MoVac-COVID19S,20–22 DrVac-COVID19S,23,24 VAX scale,25 VHS scale26 and Vaccine Conspiracy Beliefs Scale(VCBS),27 etc. These tools and theories provide a comprehensive understanding of the underlying factors behind vaccine hesitancy, enabling public health officials to develop tailored interventions to address vaccine hesitancy and promote vaccine uptake. On the other hand, some countries/regions also call for multiple vaccinations to ensure that the body’s immune system has a continuous defensive role against COVID-19. For example, China implemented 10 new epidemic prevention measures last December, which have significantly impacted both domestically and internationally.Thus, a fourth dose of the COVID-19 vaccine has been called for in China.
Citation analysis is a critical component of bibliometric analysis, which is an important method for assessing the impact of research articles.28 In the context of our rapidly evolving and complex social landscape, coupled with the significant variability in COVID-19 vaccine coverage across countries/regions, it is imperative to identify and review critical articles in the field. It will allow us to improve our understanding of the field and assess the potential impact of such articles on future research directions.
Methods
The study was a retrospective bibliometric analysis, and there was no need for institutional review board approval.
Data Extraction
The Web of Science Core Collection (https://www.webofscience.com/wos/) was searched on January 29, 2023, for all COVID-19 vaccine hesitancy-related articles, and the results were sorted in descending order according to their citations. The search strategy is as follows: (TS=(COVID-19) OR TS=(Corona Virus Disease 2019) OR TS=(SARS-CoV-2) OR TS=(2019-nCoV)) AND (TS=(Vaccine hesitancy)). Inclusion criteria: The literature topic is related to COVID-19 vaccine hesitancy. Exclusion criteria: animal literature, book chapters, book reviews, conferences. Two researchers carefully and independently used the same search strategy to search and review the abstracts or full texts of the retrieved articles in several rounds, excluding articles unrelated to COVID-19 vaccine hesitancy. In case of ambiguity, a third researcher would judge again to resolve the disagreement. After screening out the T100 articles, a pre-established data collection form was used to collect the following information: Title, author, journal, author keywords, publication year, institution, country (subject to the first author), Journal Citation Reports (JCR Q1–Q4), the impact factor (IF), citation number, article type, the average number of citations.
Statistical Analysis
Microsoft EXCEL 2019 calculated descriptive statistical analysis, including title, years, journal, country (subject to the first author), total citation, average citation, and IF. In addition, using Microsoft Excel to analyze the T100 articles’ characteristics, we classified North Ireland, Wales, and England into the UK. The T100 articles categories and document types were analyzed by the “Analyze Results” function module in Web of Science.VOSviewer1.6.18 was used for Visual analysis, including the co-countries/regions network, co-institutions network, and the T100 articles source. Nodes represented specific elements such as country, author, or institution. The size of the node indicated the quantity or frequency of publication. The larger the node, the more often the element was present. A line between nodes meant that these appeared together in an article in the T100. The thicker the line, the more often they appeared together.
Results
Characteristics of the T100 Articles
A total of 3167 COVID-19 vaccine hesitancy-related articles fit the Web of Science search strategy, and the T100 articles were sorted in descending based on the number of their citations (Table 1). The T100 articles ranged from 79 to 1125 citations; the total number of citations reached 20875, with a mean of 208.75. The publication years were concentrated in 2021 (n=71). Only one article was cited more than 1000 times, written by Lazarus, JV, and published in NATURE MEDICINE in 2021, describing potential COVID-19 vaccine acceptance and influence factors.
 |
Table 1 Top 100 Articles Cited Article in COVID-19 Vaccine Hesitancy |
A total of 29 countries participated in the T100 articles, of which 8 countries had ≥3 T100 articles (Table 2). The USA ranked first with 28 T100 articles and 5417 citations. The UK ranked second with 12 T100 articles and 3229 citations, followed by France (1691 citations), China (1250 citations), and Italy (1243 citations), tied for third with 7 T100 articles. For information on all countries of T100 articles can be found in Table 2.
 |
Table 2 Countries of Origin of the T100 |
The T100 articles were published in 61 different journals. The VACCINES published the most T100 articles and had 2690 citations, followed by PLOS ONE, with 7 T100 articles and 918 citations. VACCINE ranked third, with 6 T100 articles and 1285 citations. The journal of Quartile in category was mainly distributed in Q1–Q3, including 36 journals in Q1, 16 in Q2, and 5 in Q3. In addition, 3 journals were not in the 2021 edition of JCR. The impact factors of the journals ranged from 2.1322 −96.2167, and the number of citations ranged from 79–2690. The ANNALS OF INTERNAL MEDICINE has the most average citations per article, with 626 citations (Table 3).
 |
Table 3 Journals Publishing the Top 100 |
A total of 283 institutions worldwide participated in the research of the T100 articles, of which 14 institutions participated in more than 3 articles, as seen in Table 4. The Catholic University of the Sacred Heart from Italy participated in 8 T100 articles, ranking first and having 1181 citations. The University of Jordan and the University of Oxford ranked second, with 7 T100 articles. The University of Jordan is the most cited institution, with 2392 citations. It is worth noting that the institutions in which more than 3 T100 articles were involved, of which 7 (50%) were from the UK.
 |
Table 4 Institutions Contributing to the 100 Most Cited Articles (Number of Publication≥3) |
Overall, 672 researchers worldwide participated in the study of the T100 articles, and who participated in more than 3 T100 articles can be seen in Table 5. Sallam, M from the University of Jordan and Freeman, D from the University of Oxford, with the most T100 articles as the first or corresponding author (Articles that the first author is both corresponding authors). Besides, Sallam, M with the most number of citations. Notably, the 14 researchers ranked 5–18 in Table 5 all contributed to 3 T100 similar articles, so they had the same number of citations. Of the T100 articles, 82 were articles, 11 were reviews, 4 were Editorial Material, and 2 were Letters. These articles belong to 30 categories of Web of Science, of which the top 3 are Immunology (n = 28), Medicine Research Experimental (n = 22), and Public Environmental Occupational Health (n = 22) (Table 6).
 |
Table 5 The Most Productive Authors in T100 Articles |
 |
Table 6 Web of Science Categories in the T100 Articles |
Furthermore, we analyzed the T100 articles published per continent. Ten countries in Europe published 35 T100 articles with 8616 citations. There were no publications from South America or Antarctica. It is worth noting that even though only two North American countries published T100 articles, they published 35 articles with 6225 citations (Table 7).
 |
Table 7 The T100 Articles in per Contient |
Co-Countries/Regions Network Visualization
All institutions met the criteria when the minimum number of articles published by the countries/regions was set to 1. The co-countries/regions network is revealed in Figure 1. The co-countries/regions network, including 53 institutions, was divided into 7 clusters represented by different colors. The largest cluster of red and green consists of 15 institutions. In addition, the circles of the USA were significantly larger than others, and total link lines and strength were also significantly more than others, indicating that the USA participated in the most studies in these 100 articles.
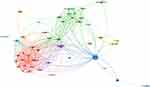 |
Figure 1 Co-countries/regions network visualization. |
Co-Institutions Network Visualization
Using Vosviewer to analyze the co-institutions network of the T100 articles. The co- institutions network can be seen in Figure 2. It involved 124 institutions and was divided into 7 clusters represented by different colors. 24 institutions were included in red clusters, which were the largest. However, from Figure 2, the cooperation strength between the institutions with a large number of publications seems weak.
 |
Figure 2 Co-institutions network visualization. |
Citation Source Density Visualization
Previously, we used Microsoft Excel to analyze the published journals of the T100 articles in Table 3. When we used Vosviewer to analyze, the generated density visualization more intuitively reflected the participation of each journal in this field. Each dot has a color and represents a journal. By default, colors range from blue to green to yellow to red, indicating increasing numbers of T100 articles (Figure 3).
 |
Figure 3 Citation source density visualization. |
Co-Citation Network of Sources Visualization
A co-citation relationship exists between two journals when they are cited simultaneously in 1 or more of the same publications. The co-citation network of the source is revealed in Figure 4. A total of 1546 journals had co-citation relationships. When setting the minimum number of citations of a source to 10, 54 journals met the criteria. From Figure 4, it is known that VACCINE has the highest co-citation frequency (8465 times). In addition, VACCINE-Basel, Human Vaccine & Immunotherapeutics, and Plos One have more than 3000 times co-citations. In general, the above four journals could significantly influence the field.
 |
Figure 4 Co-citation network of sources visualization. |
Discussion
In this study, we evaluated the current status of T100 articles on COVID-19 vaccine hesitancy by analyzing the field’s institutions, authors, countries, and journals. It can help to understand the current information regarding COVID-19 vaccine hesitancy quickly. It can also provide ideas for future research in this area.
With the largest number of T100 articles, the USA is the dominant country in terms of contributions to the development of COVID-19 vaccine hesitancy, and this may have to do with the fact that the USA is a more developed country and has more resources or financial support at its disposal.29 However, it is noteworthy that although there was only one article in Spain, the number of citations was 1225. The VACCINE journal published the most T100 articles and the highest number of citations during this multi-year period. In addition, we collected the Quartile in category and impact factor of the journals, most of which were in Q1, and the total impact factor of all the journals is as high as 841.6061. However, there were fewer publications in journals with exceptionally high impact factors, such as Nature and Nature Medicine. Several factors can influence the decision of which journal to submit manuscripts to, including acceptance rate, impact factor, speed of manuscript processing, and the overall reputation of the journal.30 Authors in the field of COVID-19 vaccine hesitancy tend to publish in corresponding journals rather than general medicine journals. The Catholic University of the Sacred Heart in Italy was the largest contributor to the T100 articles, but most institutions involved in the T100 articles were from the UK.
Our study shows that Europe and North America lead in the world’s COVID-19 vaccine hesitancy field, with the maximum number of publications and global impacts. It appears that their research receives significant attention and recognition on a global scale. Research platforms with high-quality and advanced technology are critical in promoting research development. Additionally, Europe and North America invest more in research, facilitating its smooth progression. As shown in Figure 1, Europe and North America cooperate closely, enabling them to exchange experiences and resources and expand their research capabilities. The collaborative network density among institutions with many publications is low, suggesting a lack of close collaboration and communication, as seen in Figure 2. Therefore, important to promote more vaccine hesitancy research through cross-national/regional collaborations in the future.
As mentioned above, the USA had the most T100 articles, but vaccine acceptance rates varied from a low of 12% to a high of 91.4%.31 One study32 showed that about 20% of Americans have explicitly refused COVID-19 vaccines, not including those who have delayed vaccinations. For those Americans who are already parents, their attitudes influence their decision to vaccinate their children. A study by Ruiz et al33 showed that accepting the COVID-19 vaccine in children was strongly associated with parents’ intentions to receive it for themselves, and up to 1/3 of parents reported pediatric vaccine hesitancy.
Vaccine hesitancy has become one of the most important factors affecting public health.34 The vaccine were potential side effects, safety, how well it works, and not trusting the government, which they were worried about.35 Therefore, measures must be taken to protect public health and quality of life. From the government level, it is necessary to strengthen top-level planning, formulate reasonable vaccination policies, increase the convenience of vaccination, monitor online information, and punish misinformation. Medical institutions and voluntary public health groups should disseminate knowledge about COVID-19 vaccines to the public to increase their understanding and willingness to be vaccinated. A cross-sectional survey36 conducted in India showed that the willingness to be vaccinated increased by 29% among participants who received professional education from medical and health workers. At the same time, health workers’ attitudes toward vaccination can impact the public, so health workers should set a good example. Individuals need to learn to distinguish between truth and misinformation, and it is also important to seek professional help or information from official reports.
This study also has some limitations. First, we selected only the Web of Science core collection database as the source of retrieval data and did not consider other databases such as PubMed, Scopus, CNKI, etc. Second, we are and will remain in the era of information explosion, and the information is updated much faster than our cognition, so we cannot guarantee whether some new high-quality articles are excluded. Finally, the software’s algorithm is also an important factor affecting the results. We cannot do what software engineers should do but only passively accept the use of the software.
Conclusion
To our knowledge, this study is the first reported attempt to analyze the top 100 most cited articles in the field of COVID-19 vaccine hesitancy. Through the bibliometric analysis, our study sheds light on various aspects of the field, including the most influential journal, country, and author. This information may be useful to researchers, policymakers, and practitioners interested in understanding the field’s current state and identifying potential areas for future research. In addition, it is also possible to use our study’s findings to address vaccine hesitancy, a critical public health issue. Infectious disease control and prevention efforts can be undermined by vaccine hesitancy. Researchers and policymakers can identify effective strategies to address vaccine hesitancy by identifying the most influential articles and authors in the field, such as developing targeted communication campaigns, addressing misinformation and conspiracy theories, and improving vaccine access.
Overall, the VACCINE was the most influential journal, and the USA was the most productive country. Sallam, M was the most influential author. More importantly, this article may support a quick review of the state of the COVID-19 vaccine hesitancy field and direct future research trends.
Abbreviations
COVID-19, Coronavirus disease 2019; FDA, Food and Drug Administration; IF, impact factor; JCR, Journal Citation Reports; HBM, health belief model; PMT, protective motivation theory; TPB, theory of planned behavior.
Data Statement
All data generated or analyzed during this study are included in this published article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abbas K, Procter SR, van Zandvoort K, et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: a benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob Health. 2020;8(10):e1264–e1272. doi:10.1016/s2214-109x(20)30308-9
2. Wu J, Li Q, Silver Tarimo C, et al. COVID-19 vaccine hesitancy among Chinese population: a large-scale national study. Front Immunol. 2021;12:781161. doi:10.3389/fimmu.2021.781161
3. Marti M, de Cola M, MacDonald NE, Dumolard L, Duclos P. Assessments of global drivers of vaccine hesitancy in 2014-Looking beyond safety concerns. PLoS One. 2017;12(3):e0172310. doi:10.1371/journal.pone.0172310
4. Doherty IA, Pilkington W, Brown L, et al. COVID-19 vaccine hesitancy in underserved communities of North Carolina. PLoS One. 2021;16(11):e0248542. doi:10.1371/journal.pone.0248542
5. Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. PUBLIC HEALTH. 2021;194:245–251. doi:10.1016/j.puhe.2021.02.025
6. Nazlı ŞB, Yığman F, Sevindik M, Deniz Özturan D. Psychological factors affecting COVID-19 vaccine hesitancy. Ir J Med Sci. 2022;191(1):71–80. doi:10.1007/s11845-021-02640-0
7. Acar-Burkay S, Cristian DC. Cognitive underpinnings of COVID-19 vaccine hesitancy. Soc Sci Med. 2022;301:114911. doi:10.1016/j.socscimed.2022.114911
8. Ikiışık H, Akif Sezerol M, Taşçı Y, Maral I. COVID-19 vaccine hesitancy: a community-based research in Turkey. Int J Clin Pract. 2021;75(8):e14336. doi:10.1111/ijcp.14336
9. Freeman D, Loe BS, Chadwick A, et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol Med. 2022;52(14):3127–3141. doi:10.1017/s0033291720005188
10. Hou Z, Tong Y, Du F, et al. Assessing COVID-19 vaccine hesitancy, confidence, and public engagement: a global social listening study. J Med Internet Res. 2021;23(6):e27632. doi:10.2196/27632
11. Garett R, Young SD. Online misinformation and vaccine hesitancy. Transl Behav Med. 2021;11(12):2194–2199. doi:10.1093/tbm/ibab128
12. Lyu JC, Han EL, Luli GK. COVID-19 vaccine-related discussion on twitter: topic modeling and sentiment analysis. J Med Internet Res. 2021;23(6):e24435. doi:10.2196/24435
13. Lockyer B, Islam S, Rahman A, et al. Understanding COVID-19 misinformation and vaccine hesitancy in context: findings from a qualitative study involving citizens in Bradford, UK. Health Expect. 2021;24(4):1158–1167. doi:10.1111/hex.13240
14. Hernandez RG, Hagen L, Walker K, O’Leary H, Lengacher C. The COVID-19 vaccine social media infodemic: healthcare providers’ missed dose in addressing misinformation and vaccine hesitancy. Hum Vaccin Immunother. 2021;17(9):2962–2964. doi:10.1080/21645515.2021.1912551
15. Choudhary OP, Choudhary P, Singh I. India’s COVID-19 vaccination drive: key challenges and resolutions. Lancet Infect Dis. 2021;21(11):1483–1484. doi:10.1016/s1473-3099(21)00567-3
16. FDA. Coronavirus (COVID-19) Update: FDA authorizes moderna, Pfizer-BioNTech Bivalent COVID-19 vaccines for use as a booster dose; 2022. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use.
17. Limbu YB, Gautam RK, Pham L. The health belief model applied to COVID-19 vaccine hesitancy: a systematic review. Vaccines. 2022;10(6):973. doi:10.3390/vaccines10060973
18. Eberhardt J, Ling J. Explaining COVID-19 vaccination intention in younger adults using protection motivation theory. Health Psychol. 2022. doi:10.1037/hea0001231
19. Li Z, Ji Y, Sun X. The impact of vaccine hesitation on the intentions to get COVID-19 vaccines: the use of the health belief model and the theory of planned behavior model. Front Public Health. 2022;10:882909. doi:10.3389/fpubh.2022.882909
20. Chen IH, Wu PL, Yen CF, et al. Motors of COVID-19 vaccination acceptance scale (MoVac-COVID19S): evidence of measurement invariance across five countries. Risk Manag Healthc Policy. 2022;15:435–445. doi:10.2147/rmhp.S351794
21. Pramukti I, Strong C, Chen IH, et al. The Motors of COVID-19 vaccination acceptance scale (MoVac-COVID19S): measurement invariant evidence for its nine-item version in Taiwan, Indonesia, and Malaysia. Psychol Res Behav Manag. 2022;15:1617–1625. doi:10.2147/prbm.S363757
22. Chen IH, Ahorsu DK, Ko NY, et al. Adapting the motors of influenza vaccination acceptance scale into the motors of COVID-19 vaccination acceptance scale: psychometric evaluation among mainland Chinese university students. VACCINE. 2021;39(32):4510–4515. doi:10.1016/j.vaccine.2021.06.044
23. Yeh YC, Chen IH, Ahorsu DK, et al. Measurement invariance of the drivers of COVID-19 vaccination acceptance scale: comparison between Taiwanese and mainland Chinese-speaking populations. Vaccines. 2021;9(3). doi:10.3390/vaccines9030297
24. Fan CW, Chen JS, Addo FM, et al. Examining the validity of the drivers of COVID-19 vaccination acceptance scale using Rasch analysis. Expert Rev Vaccines. 2022;21(2):253–260. doi:10.1080/14760584.2022.2011227
25. Kumar R, Alabdulla M, Elhassan NM, Reagu SM. Qatar healthcare workers’ COVID-19 vaccine hesitancy and attitudes: a national cross-sectional survey. Front Public Health. 2021;9:727748. doi:10.3389/fpubh.2021.727748
26. Yeşiltepe A, Aslan S, Bulbuloglu S. Investigation of perceived fear of COVID-19 and vaccine hesitancy in nursing students. Hum Vaccin Immunother. 2021;17(12):5030–5037. doi:10.1080/21645515.2021.2000817
27. Andrade G. Covid-19 vaccine hesitancy, conspiracist beliefs, paranoid ideation and perceived ethnic discrimination in a sample of University students in Venezuela. VACCINE. 2021;39(47):6837–6842. doi:10.1016/j.vaccine.2021.10.037
28. He L, Fang H, Wang X, et al. The 100 most-cited articles in urological surgery: a bibliometric analysis. Int J Surg. 2020;75:74–79. doi:10.1016/j.ijsu.2019.12.030
29. Rodwin MA. Reforming pharmaceutical industry-physician financial relationships: lessons from the United States, France, and Japan. J Law Med Ethics. 2011;39(4):662–670. doi:10.1111/j.1748-720X.2011.00633.x
30. Zhang Y, Quan L, Xiao B, The DL. 100 top-cited studies on vaccine: a bibliometric analysis. Hum Vaccin Immunother. 2019;15(12):3024–3031. doi:10.1080/21645515.2019.1614398
31. Yasmin F, Najeeb H, Moeed A, et al. COVID-19 vaccine hesitancy in the United States: a systematic review. Front Public Health. 2021;9:770985. doi:10.3389/fpubh.2021.770985
32. Thunström L, Ashworth M, Finnoff D, Newbold SC. Hesitancy Toward a COVID-19 Vaccine. EcoHealth. 2021;18(1):44–60. doi:10.1007/s10393-021-01524-0
33. Ruiz JB, Bell RA. Parental COVID-19 Vaccine Hesitancy in the United States. Public Health Rep. 2022;137(6):1162–1169. doi:10.1177/00333549221114346
34. 2019: a year of challenges and change. MEDICC Rev. 2019;21(1):3. doi:10.37757/mr2019.V21.N1.1
35. AP-NORC Center for Public Affairs Research. Safety concerns remain the main driver of vaccine hesitancy; 2021. Available from https://apnorc.org/projects/safety-concerns-remain-main-driver-of-vaccine-hesitancy/.
36. Kotecha I, Vasavada DA, Kumar P, Nerli LM, Tiwari DS, Parmar DV. Knowledge, attitude, and belief of health-care workers toward COVID-19 Vaccine at a tertiary care center in India. Asian J Soc Health Behav. 2022;5:63–67. doi:10.4103/shb.shb_20_21
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
