Back to Journals » Cancer Management and Research » Volume 13
TGIF1 Knockdown Inhibits the Proliferation and Invasion of Gastric Cancer via AKT Signaling Pathway
Authors Zhang J , Zhang F, Fan J, Feng B
Received 17 March 2020
Accepted for publication 19 December 2020
Published 18 March 2021 Volume 2021:13 Pages 2603—2612
DOI https://doi.org/10.2147/CMAR.S254348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ahmet Emre Eşkazan
Jing Zhang,1 Feiyan Zhang,2 Jiye Fan,3,4 Bin Feng5
1Pharmacy Department, Hebei Chemical and Pharmaceutical College, Shijiazhuang, Hebei 050026, People’s Republic of China; 2Department of Outpatient Operating Room, Heze Municipal Hospital, Heze City, Shandong Province, 274000, People’s Republic of China; 3Department of Pharmaceutical Engineering, Hebei Chemical and Pharmaceutical College, Shijiazhuang, Hebei 050026, People’s Republic of China; 4College of Life Science, Hebei Normal University, Shijiazhuang, Hebei 050024, People’s Republic of China; 5Department of Gastrointestinal Surgery, Heze Municipal Hospital, Heze City, 274000 Shandong Province, People’s Republic of China
Correspondence: Bin Feng
Department of Gastrointestinal Surgery, Heze Municipal Hospital, No. 2888, Caozhou Road, Mudan District, Heze City 274000, Shandong Province, People’s Republic of China
Email [email protected]
Introduction: Gastric cancer is a kind of cancer with high mortality. TGIF1, as a transcription inhibitor, can inhibit the transcription of specific genes. The purpose of this study was to investigate the role of TGIF1 in gastric cancer by knocking down TGIF1.
Methods: The expression of TGIF1 was detected by qPCR and Western blotting; CCK8 assay, colony formation assay, transwell, and wound-healing assay were used to evaluate the proliferation, migration, and invasion of gastric cancer cells; cell apoptosis was analyzed by flow cytometry and Hoechst-PI double staining; cell cycle was detected by flow cytometry. Gelatinase experiment was performed to detect the expression level of MMP-2; apoptosis related proteins and AKT singling pathway were assessed by Western blotting.
Results: Knockdown of TGIF1 inhibited the proliferation, migration, and invasion of gastric cancer cells and promoted apoptosis. TGIF1 knockdown down-regulated the expression levels of MMP-2, Bcl2, CyclinD1, and p-Akt, and up-regulated the expression levels of Bax and Caspase3. These data suggested that knockdown of TGIF1 inhibited the development of gastric cancer via AKT signaling pathway.
Conclusion: TGIF1 knockdown inhibited the proliferation, migration, and invasion and promoted apoptosis of gastric cancer cells via the AKT signaling pathway, suggesting that TGIF1 is considered a potential inhibitor in gastric cancer.
Keywords: TGIF1, gastric cancer, AKT pathway, apoptosis, proliferation
Introduction
Gastric cancer ranks fifth in the global incidence of malignant tumors and third in the mortality of malignant tumors.1 At present, the treatment of gastric cancer mainly comprises surgery assisted radiotherapy and chemotherapy, but the treatment effect is not ideal. Moreover, due to the high recurrence and metastasis rate of gastric cancer, about 40% – 70% of patients have tumor recurrence,2 and the 5-year survival rate is less than 30%.3
The occurrence and development of gastric cancer is a complex process, involving the inactivation of many tumor suppressor genes or the activation of cancer genes. It is of great significance to study the pathogenesis and mechanism of gastric cancer, for its diagnosis and treatment.4
TG-interacting factor 1 (TGIF1) is a highly conserved transcription factor in different types of vertebrates, which plays an important role in regulating fetal head and face, brain development, and differentiation of various cells.5–8 TGIF1 is expressed in hematopoietic stem cells and participates in the regulation of many physiological processes. There has been a lot of research on its mechanism. Early studies have shown that TGIF1 protein is a transcriptional inhibitor, which can inhibit the transcription of specific genes.9,10 The abnormal functioning of TGIF1 is related to many diseases. Several studies have proven that TGIF1 is closely related to the human genetic disease HPE which occurs in the fetal development stage.11,12 On the other hand, the change of expression level and functional activity of TGIF1 is related to the occurrence and development of various cancers such as urothelial cancer,7,13 liver cancer,14 and lung cancer.15 In addition, the expression level of TGIF1 is also significantly increased in lung cancer cells, indicating that TGIF1 may be related to the occurrence or development of lung cancer.15 However, the effect and mechanism of TGIF1 on gastric cancer still remain unclear. In this study, the expression of TGIF1 gene was silenced by RNA interference to explore its effect and mechanism on the proliferation and apoptosis of gastric cancer cells, in order to provide a theoretical basis for the diagnosis and treatment of the disease.
Materials and Methods
Agents
Primary antibodies, including anti-TGIF1 (Cat # ab52955), Bcl-2 (Cat # ab32124), Active-Caspase3 (Cat # ab32042), Bax (Cat # ab182733), AKT (Cat # ab8805), p-AKT (Cat # ab38449), Cyclin D1 (Cat # ab134175), and anti-tubulin (Cat # ab210797) were purchased from Abcam (Cambridge, UK). HRP sheep anti-rabbit/mouse secondary antibodies were obtained from PTG Company.
Cell Culture and Transfection
Human gastric cancer cell lines AGS and MKN45 were obtained from the cell bank of Shanghai Chinese Academy of Sciences and cultured in DMEM medium (HyClone, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin in a 37°C humidified incubator containing 5% CO 2. The culture medium was changed 24 hours later, and then changed every 2–3 days according to the growth state of cells. The cells were transfected with TGIF1-specific siRNA (si-TGIF1) according to the instructions of Lipofectamine 2000 transfection kit (Invitrogen; Thermo Fisher Scientific, Inc.) at logarithmic stage. Non-specific siRNA was used as the control. The interfere sequences used were the following:
TGIF1-siNC: 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ
TGIF1-siRNA1: 5ʹ-GGCUGUAUGAGCACCGUUATT-3ʹ
TGIF1-siRNA2: 5ʹ-GCGUUGCUGUCCCAGCAAATT-3ʹ
TGIF1-siRNA3: 5ʹ-AGUGGAUGUUGCACUCAAATT-3ʹ
TGIF1-siRNA4: 5ʹ-CAGUGGAUUUCAGCUUCUATT-3ʹ
Fluorescence Quantitative PCR
The total RNA of cells was extracted by RNA extraction kit. After reverse transcription, some of them were used for downstream experiments, and the rest were frozen in a refrigerator at - 80°C. SYBR method was used to detect the mRNA level of each sample and the data were analyzed by 2 −ΔCT method. The primers (GENEWIZ, Inc) were as follows:
TGIF1-qPCR-up: 5ʹ-TGAGCACCGTTACAATGCCT-3ʹ
TGIF1-qPCR-down: 5ʹ-GAAGTCCTGGTTGAGGTCCG-3ʹ
human-β-actin-up: 5ʹ-CCCGAGCCGTGTTTCCT-3ʹ
human-β-actin-down: 5ʹ-GTCCCAGTTGGTGACGATGC-3ʹ
Cell Proliferation and Growth Ability Detection
Cell Counting Kit-8 (CCK8) assay was performed to detect cell proliferation. When the fusion rate reached 90%, cells were washed with PBS, digested with trypsin, and the cell suspension was prepared and counted. About 1000 cells were seeded into each well of a 96-well-plate. The cell viability was detected every 24 hours. Before detection, 10 μL CCK8 reagent was added to each hole and incubated in a 37°C incubator for 1.5 hours. The OD value was detected with 450 nm excitation light by enzyme labeling instrument and the proliferation curve was drawn.
Flat plate clone formation test was used to evaluate the ability of cell cloning. The cells of each group in logarithmic growth period were digested with trypsin, and 500 cells were inoculated into a 35 mm cell culture dish for 2–3 weeks. When there were visible clones in the dish, the culture was terminated and fixed with 4% POM for 20 minutes, stained with crystal violet for 30 minutes, then counted under a microscope. Finally, the clone formation rate was calculated.
Clone formation rate = (number of clones/number of inoculated cells) × 100%.
Cell Invasion and Migration Ability Detection
Transwell assay was used to assess the invasion and migration ability of AGS and MKN45 cells. For invasion assay, 100 μL prepared Matrigel (1:6 diluted by serum-free medium) was added to the upper chamber of transwell chamber (Millipore, Billerica, MA, USA), placed in a carbon dioxide incubator for 4–6 hours at 37°C to form the glue, then 500 μL of serum-free culture solution was added and it was left for half an hour to hydrate the basement membrane. 100 μL cell suspension (1 × 105 cells) was added to the upper chamber and 500 μL complete culture medium was added to the lower chamber. After being left overnight, the cells were moved from the upper chamber to lower chamber, and then fixed with 4% paraformaldehyde for 30 minutes after PBS cleaning, stained with 0.1% crystal violet for 20 minutes, observed and counted under the microscope. The procedure of migration experiment is similar to that of invasion experiment. Except the transwell chamber did not need to be treated with glue, and the cell number was 5000.
The migration ability was also detected by wound-healing assay. When the cells were 95% fused, a 10 μL pipette tip was used to make an artificial wound, and they were cultured with serum-free medium in a 5% CO2 incubator at 37°C. The micrographs were taken at 0, 24, and 48 hours respectively and the migration area of the cells was analyzed to evaluate the migration ability of AGS and MKN45 cells.
Gelatinase Experiment
After 24 hours of transfection, the cells were washed with serum free medium and cultured in serum free medium. After 24 hours, the supernatant was collected and centrifuged to remove the cells. The supernatant of the diluted buffer solution without mercapto ethanol was added to each hole of the vertical electrophoresis bath and the samples were separated by SDS-page (0.5 mg/mL gelatin). Image scanner (Amersham) and Image Quant TL v2003 software were used to analyze the activity of MMPs.
Cell Apoptosis Detection
Cell apoptosis rate was analyzed with flow cytometry. After 24 hours of transfection, the cells were resuspended with 1 × buffer and the cell density was adjusted to 1–5 × 106 cells/mL. 100 µL cell suspension and 5 μL annexin V/FITC were added to 5 mL flow tube, then incubated at room temperature in the dark for 5 min, followed by adding 10 µL 20 µg/mL of double staining PI. Cell samples were analyzed using flow cytometric analysis and Flow Jo software.
Hoechst-PI fluorescence double staining experiment was performed to detect cell apoptosis. After 24 hours of transfection, Hoechst 33342 and PI staining solution were added to the culture solution. The fluorescence was observed by different excitation of fluorescence microscope. Hoechst staining was observed by blue light excited by ultraviolet light, and PI staining was observed by red light excited by green light.
Western Blot
After 24 hours of transfection, the protein was extracted with RIPA lysate. 20 µL protein samples were added into each pore of the vertical electrophoresis tank and separated by SDS-PAGE. Then, the protein was transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with 5% skim milk for 1 hour, the PVDF membrane was probed with primary antibodies (1:1,000) at 4°C overnight and secondary (1:5,000) antibodies at room temperature for 1 hour. Then, immunoblotting was performed with ECL.
Cell Cycle Analysis
Flow cytometry was used to analyze cell cycle. After 24 hours of transfection, 70% ethanol was added to fix the cells at 4°C overnight. 500 μL PI was added and maintained in the dark for 30 min. Flowjo software was used to analyze the flow results.
Statistical Analyses
SPSS 18.0 software was used for statistical analysis. The gray values of Western blot positive bands were measured by Image J analysis software, and the internal reference bands were used as protein quantitative reference. Compared with t-test, P < 0.05 was statistically significant.
Results
Knockdown of TGIF1 Inhibits the Growth Capacity of Gastric Cancer Cells
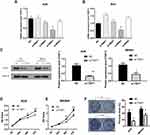
To study the role of TGIF1 in gastric cancer, AGS and MKN45 cell lines were selected. The expression level of TGIF1 was detected by qPCR. The results were as follows (Figure 1A), and si-RNA3 was selected as the interference plasmid to knock down TGIF1. In addition, the interference effect of other siRNAs was not significant, which may be due to the unreasonable sequence design. The increase in TGIF1 level after transfection of siRNA1 and siRNA4 in MKN45 cells may be due to the change of other genes’ expression. Then, AGS and MKN45 cells were transfected with si-RNA3 to construct TGIF1 knockdown cell lines (si-TGIF1) with negative interference sequence as negative control group (NC). RNA and protein of NC and si-TGIF1 groups were collected. The results of qPCR showed that the mRNA level of TGIF1 in si-TGIF1 group was significantly lower than that in the NC group (Figure 1B). The protein level was evaluated with Western blotting. As shown in Figure 1C, the expression level of TGIF1 in si-TGIF1 group was decreased significantly compared with that in the NC group (P<0.05).
CCK8 assay was performed to detect cell proliferation. The results showed that TGIF1 knockdown inhibited the proliferation of AGS and MKN45 cells (Figure 1D and E). In addition, the colony forming ability of si-TGIF1 cells was decreased significantly compared with the NC group (P<0.05, Figure 1F). Taken together, knockdown of TGIF1 significantly inhibited the growth capacity of gastric cancer cells in vitro.
TGIF1 Knockdown Inhibits the Invasion and Migration of Gastric Cancer Cells
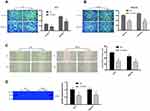
Transwell assay was performed to assess the effect of TGIF1 on the invasion and migration in gastric cancer cells. After transfection of si-RNA, the number of migrating cells and invading cells decreased significantly (Figure 2A and B). In parallel, from the results of wound-healing assay, TGIF1 knockdown inhibited the migration of both AGS and MKN45 cells (Figure 2C). Furthermore, the results of gelatinase spectrum showed that the expression level of MMP-2 protein in si-TGIF1 group was decreased significantly compared with the NC group (Figure 2D). These results suggested that TGIF1 knockdown inhibited the invasion and migration of gastric cancer cells in vitro.
TGIF1 Knockdown Promotes the Apoptosis of Gastric Cancer Cells
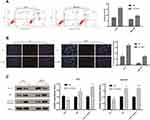
The apoptosis of AGS and MKN45 cells was detected using cell flow-cytometry. After 48 hours of transfection, cells were double stained by Annexin V and PI. As shown in Figure 3A, the apoptosis rates of AGS and MKN45 cells in si-TGIF1 group were increased significantly compared with the NC group. Moreover, the results of Hoechst-PI double staining showed that the apoptotic ratio of si-TGIF1 group was significantly higher than that of NC group both in AGS and MKN45 cells (Figure 3B). To further investigate the mechanism of apoptosis, Western blot analysis was adopted to determine the effect of si-TGIF1 on the expression levels of apoptosis-related proteins. The results indicated that TGIF1 knockdown down-regulated the expression of anti-apoptotic Bcl-2, but up-regulated the expression of pro-apoptotic Bax and Caspase3 (Figure 3C). Taken together, knockdown of TGIF1 promoted the apoptosis of gastric cancer cells by regulating the expression of apoptosis-related proteins.
TGIF1 Knockdown Blocks Cell Cycle of Gastric Cancer Cells
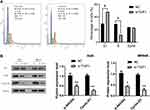
Then, we detected the effect of TGIF1 on cell cycle in AGS cells using flow cytometry. As shown in Figure 4A, the cells in G1 phase of TGIF1 knockdown increased significantly compared with the control cells; moreover, the cells in S phase of TGIF1 knockdown increased significantly compared with the control cells. These results indicated that TGIF1 knockdown blocked the transformation progression of AGS cells from G1 phase to S phase.
TGIF1 Affects the AKT Signaling Pathway in Gastric Cancer Cells
To further investigate the mechanism of TGIF1 in gastric cancer cells, the activity of PI3K/AKT signaling pathway was detected using Western blotting. AKT pathway, as a key pathway of cell proliferation and apoptosis, is involved in tumor progression and metastasis. AKT pathway is often activated in gastric cancer.16 Western blot analysis showed that there was no significant difference in total AKT expression between si-TGIF1 group and NC group, but TGIF1 knockdown reduced the level of the phosphorylated form p-Akt and its downstream protein Cyclin D1 both in AGS and MKN45 cells (Figure 4). Therefore, the activation of AKT signaling pathway was suppressed by TGIF1 knockdown. These results suggested that TGIF1 affects the development of gastric cancer cells by regulating the AKT signaling pathway.
The Expression of TGIF1 Was Significantly Correlated with M and T Stages of Gastric Cancer
Finally, we analyzed the clinical significance of TGIF1 in stomach adenocarcinoma (STAD) using LinkedOmics database on the TCGA dataset (www.linkedomics.org/).17 As shown in Table 1, TGIF1 was significantly correlated with histological type of gastric cancer patients. Importantly, TGIF1 was also associated with the M stage and T stage of gastric cancer patients, suggesting that TGIF1 might be involved in the metastasis of gastric cancer. However, TGIF1 was not associated with the prognosis of gastric cancer patients.
 |
Table 1 Clinical Significance of TGIF1 Was Obtained Using LinkedOmics Based Analysis |
Discussion
Due to the lack of early specific symptoms, gastric cancer is difficult to diagnose, so the mortality rate remains high. The discovery of targets in the diagnosis and treatment of gastric cancer provides great help for the diagnosis and treatment of gastric cancer.18,19 TGIF1 (TG-interacting Factor 1) is a member of the TALE (three-amino-acid-loop-extension superclass) superfamily, named for its conserved DNA binding sequence and its specific binding with TGFP. It has been shown that TGIF1 plays a major role in transcriptional inhibition,20 and its specific binding sequence is CTGTCAA. In the latest study, TGIF1 was found to be involved in the progression of gastric carcinoma as a downstream target of XTP8 stimulates migration and invasion through interacting with TGIF1.21 In this study, si-TGIF1 was transfected to reduce the expression of TGIF1 in gastric cancer cells. The proliferation, invasion, migration, and apoptosis of gastric cancer cells were detected; the results showed that inhibition of TGIF1 expression can significantly reduce the growth activity and promote apoptosis of gastric cancer cells.
In order to study the mechanism of the inhibitory effect of si-TGIF1 on gastric cancer cells, we analyzed the expression of MMP-2, a cell migration marker, by gelatinase. The high expression of MMP-2 plays an important role in tumor invasion and metastasis.22 In breast cancer, up-regulating the expression of MMP-2 can significantly promote the invasion and metastasis of breast cancer;23 in lung cancer, the expression of MMP2 in high metastatic potential lung cancer cells was significantly higher than that in low metastatic potential lung cancer cells. Interference with the expression of MMP2 can significantly inhibit the metastasis of lung cancer.24 Our results showed that knockdown of TGIF1 can down-regulate the expression of MMP2.
Bcl2 and Bax are the two key factors in apoptosis, which are the regulatory genes that inhibit and promote apoptosis.25 The ratio of Bax/Bcl2 can be used to determine whether the cells are in apoptosis or not.26 Among most of the factors involved in the complex process of apoptosis, Caspase enzyme cascade reaction is the core, and Caspase 3 usually exists in the form of inactive proenzyme. Caspase 3, when activated, can catalyze the cleavage of a series of key intracellular proteases downstream, leading to the damage of cell structure and function.27 So we detected the expression of apoptosis-related proteins Bax, Bcl2, and Caspase3. The results of Western blot showed that TGIF1 knockdown increased the expression of Bax and Caspase3, and decreased the expression of Bcl2. Furthermore, we evaluated the AKT signaling pathway, the results indicated that the levels of the phosphorylated form of AKT and Cyclin D1 were significantly decreased by si-TGIF1. Akt is a key factor in PI3K/Akt pathway. It promotes the growth, metastasis and inhibits apoptosis of tumor cells by phosphorylating a variety of substrates, such as mTOR, P21Cipl/WAF1, GSK3, TSC2 and FOXO family. These data prove that TGIF1 can affect the development of gastric cancer cells via the AKT signaling pathway, suggesting that TGIF1 is a potential target for gastric cancer treatment. In addition, we predict that TGIF1 affects the AKT pathway through specific downstream targets, which will be the focus of our future research.
Finally, we found that the expression of TGIF1 was significantly correlated with histological type, M and T stages, but not OS of gastric cancer through LinkedOmics based analysis. It has been found that the expression of TGIF1 is increased in oral squamous cell carcinoma (OSCC), and is related to cell differentiation, vascular invasion, pathological stage, lymphatic invasion, and overall survival.28,29 In addition, TGIF1 overexpression is associated with poor prognosis of upper urinary tract urothelial carcinoma and acute myeloid leukemia.7,13 The clinical significance of TGIF1 in gastric cancer needs to be further verified by clinical samples.
Conclusion
TGIF1 knockdown inhibited the proliferation, migration, and invasion and promoted apoptosis of gastric cancer cells via the AKT signaling pathway. Our results enriched our understanding of the mechanism of gastric cancer progression and revealed that siRNA of TGIF1 can be considered potential gastric cancer inhibitors in therapy.
Funding
This research was funded by Science and Technology Project of Hebei Education Department, China (Grant No. QN2020117, study on Rab23 about the mechanism of action and its self-regulation in hepatocellular carcinoma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Deng J, Liang H, Wang D, et al. Investigation of the recurrence patterns of gastric cancer following a curative resection. Surg Today. 2011;41(2):210–215. doi:10.1007/s00595-009-4251-y
3. Soroush A. Surgical outcome in patients with gastrointestinal malignancies; a report from a large referral hospital, 2008–2010. Middle East J Dig Dis. 2013;5(4):201–208.
4. Abubakar II, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi:10.1016/S0140-6736(14)61682-2
5. Lee BK, Shen W, Lee J, et al. Tgif1 counterbalances the activity of core pluripotency factors in mouse embryonic stem cells. Cell Rep. 2015;13(1):52–60. doi:10.1016/j.celrep.2015.08.067
6. Taniguchi K, Anderson AE, Sutherland AE, et al. Loss of Tgif function causes holoprosencephaly by disrupting the SHH signaling pathway. PLoS Genet. 2012;8(2):e1002524. doi:10.1371/journal.pgen.1002524
7. Hamid R, Brandt SJ. Transforming growth-interacting factor (TGIF) regulates proliferation and differentiation of human myeloid leukemia cells. Mol Oncol. 2009;3(5–6):451–463. doi:10.1016/j.molonc.2009.07.004
8. Mukherjee K, Bürglin TR. Comprehensive analysis of animal TALE homeobox genes: new conserved motifs and cases of accelerated evolution. J Mol Evol. 2007;65(2):137–153. doi:10.1007/s00239-006-0023-0
9. Bertolino E, Reimund B, Wildt-Perinic D, et al. A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. J Biol Chem. 1995;270(52):31178–31188. doi:10.1074/jbc.270.52.31178
10. Sharma M, Sun Z. 5ʹTG3ʹ interacting factor interacts with Sin3A and represses AR-mediated transcription. Mol Endocrinol. 2001;15(11):1918–1928.
11. Chen CP, Chern S-R, Du S-H, et al. Molecular diagnosis of a novel heterozygous 268C–>T (R90C) mutation in TGIF gene in a fetus with holoprosencephaly and premaxillary agenesis. Prenat Diagn. 2002;22(1):5–7. doi:10.1002/pd.202
12. El-Jaick KB, Powers SE, Bartholin L, et al. Functional analysis of mutations in TGIF associated with holoprosencephaly. Mol Genet Metab. 2007;90(1):97–111. doi:10.1016/j.ymgme.2006.07.011
13. Yeh BW, Wu W-J, Li W-M, et al. Overexpression of TG-interacting factor is associated with worse prognosis in upper urinary tract urothelial carcinoma. Am J Pathol. 2012;181(3):1044–1055. doi:10.1016/j.ajpath.2012.05.024
14. Liu ZM, Tseng J, Hong D-Y, et al. Suppression of TG-interacting factor sensitizes arsenic trioxide-induced apoptosis in human hepatocellular carcinoma cells. Biochem J. 2011;438(2):349–358. doi:10.1042/BJ20101653
15. Wang Y, Wang H, Gao H, et al. Elevated expression of TGIF is involved in lung carcinogenesis. Tumour Biol. 2015;36(12):9223–9231. doi:10.1007/s13277-015-3615-8
16. Ying J, Xu Q, Liu B, et al. The expression of the PI3K/AKT/mTOR pathway in gastric cancer and its role in gastric cancer prognosis. Onco Targets Ther. 2015;8:2427–2433. doi:10.2147/OTT.S88592
17. Vasaikar SV, Straub P, Wang J, et al. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46(D1):D956–d963. doi:10.1093/nar/gkx1090
18. Fang J, Wang H, Liu Y, et al. High KRT8 expression promotes tumor progression and metastasis of gastric cancer. Cancer Sci. 2017;108(2):178–186. doi:10.1111/cas.13120
19. Gekas C, D’Altri T, Aligué R, et al. β-Catenin is required for T-cell leukemia initiation and MYC transcription downstream of Notch1. Leukemia. 2016;30(10):2002–2010. doi:10.1038/leu.2016.106
20. Bürglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25(21):4173–4180. doi:10.1093/nar/25.21.4173
21. Jia K, Wen Q-H, Zhao X, et al. XTP8 stimulates migration and invasion of gastric carcinoma through interacting with TGIF1. Eur Rev Med Pharmacol Sci. 2020;24(5):2412–2420. doi:10.26355/eurrev_202003_20508
22. Kurihara Y, Hatori M, Ando Y, et al. Inhibition of cyclooxygenase-2 suppresses the invasiveness of oral squamous cell carcinoma cell lines via down-regulation of matrix metalloproteinase-2 production and activation. Clin Exp Metastasis. 2009;26(5):425–432. doi:10.1007/s10585-009-9241-3
23. Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis. 2005;22(3):237–246. doi:10.1007/s10585-005-8115-6
24. Zeng Q, Li S, Zhou Y, et al. Interleukin-32 contributes to invasion and metastasis of primary lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases 2 and 9 expression. Cytokine. 2014;65(1):24–32. doi:10.1016/j.cyto.2013.09.017
25. Tsujimoto Y, Cossman J, Jaffe E, et al. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228(4706):1440–1443. doi:10.1126/science.3874430
26. Yang HL, Kuo Y-H, Tsai C-T, et al. Anti-metastatic activities of Antrodia camphorata against human breast cancer cells mediated through suppression of the MAPK signaling pathway. Food Chem Toxicol. 2011;49(1):290–298. doi:10.1016/j.fct.2010.10.031
27. Kuranaga E. Caspase signaling in animal development. Dev Growth Differ. 2011;53(2):137–148. doi:10.1111/j.1440-169X.2010.01237.x
28. Libório TN, Ferreira EN, Aquino Xavier FC, et al. TGIF1 splicing variant 8 is overexpressed in oral squamous cell carcinoma and is related to pathologic and clinical behavior. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(5):614–625. doi:10.1016/j.oooo.2013.07.014
29. Matizonkas-Antonio LF, Libório TN, Aquino Xavier FC, et al. Detection of TGIF1 homeobox gene in oral squamous cell carcinoma according to histologic grading. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):218–224. doi:10.1016/j.tripleo.2010.10.003
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.