Back to Journals » Infection and Drug Resistance » Volume 16
Tetanus Following Canine Bite in Japan: A Case Report and Literature Review
Authors Hirai J , Mori N , Shibata Y, Asai N, Hagihara M, Mikamo H
Received 27 September 2023
Accepted for publication 29 November 2023
Published 4 December 2023 Volume 2023:16 Pages 7427—7434
DOI https://doi.org/10.2147/IDR.S442279
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jun Hirai,1,2 Nobuaki Mori,1,2 Yuichi Shibata,2 Nobuhiro Asai,1,2 Mao Hagihara,3 Hiroshige Mikamo1,2
1Department of Clinical Infectious Diseases, Aichi Medical University Hospital, Nagakute, Aichi, Japan; 2Department of Infection, Prevention, and Control, Aichi Medical University Hospital, Nagakute, Aichi, Japan; 3Department of Molecular Epidemiology and Biomedical Sciences, Aichi Medical University, Nagakute, Aichi, Japan
Correspondence: Jun Hirai, Department of Clinical Infectious Diseases, Aichi Medical University Hospital, 1-1, Yazako-karimata, Nagakute, Aichi, 480-1195, Japan, Tel +81-561-62-3311, Fax +81-561-76-2673, Email [email protected]
Background: The incidence of tetanus has significantly declined in developed countries owing to widespread vaccination efforts. However, it remains a threat worldwide, including in Japan, because of the sharp decline in antibody titers against tetanus in adults. Animal bites, including canine bites, are potential sources of tetanus infection. This case highlights the rarity of tetanus caused by canine bites and the need for continued vigilance for tetanus prevention. This case report and literature review aimed to shed light on the clinical course and outcomes of tetanus following a canine bite.
Case Presentation: A 46-year-old Japanese man with no medical history presented with symptoms of tetanus, such as difficulty in opening his mouth, 19 days after a canine bite on his right hand. He was born and brought up in Japan. He had never been vaccinated against tetanus. Despite washing the wound and receiving human tetanus immunoglobulin (HTIG) and a tetanus toxoid vaccine, the patient developed tetanus. After intravenous metronidazole and HTIG were administered, the symptoms improved gradually. The patient was discharged after a 12-day hospital stay.
Discussion: This is the first reported case of canine bite-induced tetanus in Japan, where tetanus toxoid vaccination is provided routinely. This case highlights the waning immunity in adults and the critical need for education on tetanus immunization, including catch-up immunization, particularly for adults and individuals in high-risk occupations. A review of the existing literature revealed only four cases of tetanus following canine bites between 1889 and 2018. All patients experienced symptom onset between 3 and 19 days post injury. Treatment typically involved HTIG, metronidazole, and toxoid administration. A higher risk of mortality is seen in unvaccinated individuals than in vaccinated individuals, highlighting the critical role of tetanus vaccination.
Conclusion: Physicians should consider canine bite-induced tetanus in the differential diagnosis when patients exhibit relevant symptoms.
Keywords: tetanus, Clostridium tetani, canine, bite, vaccine, Japan
Introduction
Tetanus is a serious neurological disease characterized by muscle rigidity and spasms.1 The incidence of tetanus has declined significantly in high-income countries owing to widespread vaccination efforts, with the World Health Organization reporting an impressive 90% reduction in global tetanus-related mortality between 1990 and 2015. However, tetanus remains a threat to all unvaccinated people, particularly in resource-limited countries.2 Clostridium tetani, an anaerobic gram-positive bacillus, the causative agent of tetanus, thrives in spore-producing environments and can enter through wounds, particularly puncture wounds.3 Among these, animal bites, including canine bites, are recognized as potential sources of tetanus infection.4
Clostridium tetani spores that enter the wound germinate and multiply in the anaerobic environment of the wound.5 Tetanus toxin spreads through hematogenous and lymphatic routes, resulting in peripheral motor, cranial, and sympathetic nerve depression and tetanus symptoms,5 of which muscle spasms and rigidity are the main symptoms. Cranial nerve symptoms include lockjaw, risus sardonicus (facial muscle spasms), laryngospasm, and dysphagia. Difficulty in opening the mouth is a common initial symptom. Stiffness and painful spasms in the limb, abdominal, and paraspinal muscle groups, as well as opisthotonos (posterior arch tension), may be observed. Sympathetic overactivity causes autonomic instability, and symptoms such as tachycardia, bradycardia, hypertension, hypotension, and hyperhidrosis are also observed. A unique feature of tetanus is preservation of consciousness. Clinically, this disease can be divided into three types: generalized, localized, and cephalic tetanus.5
Although we have a firm understanding of the pathophysiology of tetanus and its association with animal bites, specific insights into the clinical course, risk factors, and outcomes of tetanus after canine bites is limited owing to the small number of reported cases.6–9
The primary objective of this study was to present a comprehensive case report of a patient with canine-bite tetanus in Nagakute city, Aichi prefecture, Japan, in August 2023. By analyzing this case, we aimed to identify the clinical features, diagnostic challenges, and therapeutic considerations associated with tetanus after a canine bite. We also conducted a literature review to identify the characteristics of tetanus following canine bites. Through this study, we hope to emphasize the importance of maintaining vigilance in tetanus prevention, even in areas where the disease has become uncommon.
Case Report
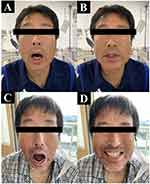
A 46-year-old Japanese man with no specific medical history presented to the emergency room complaining of perioral pain, neck and shoulder stiffness, and difficulty opening his mouth, which started two days before admission. He had no history of trauma or drug use but had experienced canine bite injuries on his right hand 3 weeks prior (Figure 1A and B). He was asked by a friend to look after a pet canine with rabies immunization for a couple of days. When he first met that canine, he tried to touch the canine’s head, but he startled the canine and the canine bit his right hand. The patient had not been vaccinated against tetanus. He washed his right hand immediately after being bitten by the canine. He received 250 units of human tetanus immunoglobulin (HTIG) intramuscularly and the tetanus toxoid vaccine subcutaneously the following day at a hospital near his home. The use of HTIG to neutralize unbound tetanus toxin is associated with improved survival and is considered the standard care to prevent tetanus after an injury. The United States Centers for Disease Control and Prevention recommends a single dose of HTIG intramuscularly in addition to wound management, antimicrobial therapy, and tetanus toxoid vaccination.10 On admission, the patient was conscious. Although he did not have difficulty speaking, he complained of difficulty swallowing solid foods. His blood pressure, heart rate, body temperature, and respiratory rate were 137/85 mmHg, 78 beats/min, 36.4 °C, and 16 breaths/min, respectively. Physical examination revealed hypertonicity, particularly of the left upper limb. He could open his mouth to a maximum of 1.5 fingers width (Figure 2A), and there were no breathing difficulties. In addition, we found that the patient experienced laughter spasms (Figure 2B). Manual muscle testing of both upper limbs revealed a grade 4 result (implying that the patient could complete the full range of motion [movement] against gravity while the practitioner applies moderate resistance), with no evidence of weakness in the lower limbs. Trigeminal paralysis (mandibular area) was observed, but the sensation was normal. Cardiovascular, respiratory, and abdominal examination results were normal. The wound on the right hand had healed; therefore, no additional cleaning or treatment of the patient’s right hand was required. Blood tests showed normal white blood cell count (6700 cells/mm3, normal range: 3300–8600 cells/mm3) and biochemical blood test results, except for a slight elevation in C-reactive protein (1.64 mg/dL, normal range: ≤0.04 mg/dL). Urine test results were normal. The differential diagnoses were tetany due to hypocalcemia, rabies, or Strychnine poisoning. These differential diagnoses were ruled out because the serum calcium levels were normal (9.0 mg/dL, normal range: 8.8–10.1 mg/dL); the patient did not have symptoms specific to rabies, such as mydriasis, increased salivation, and hydrophobia; and there was no definite history of poisoning.
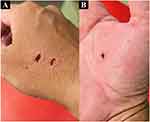 |
Figure 1 Right-hand wound caused by canine bite (A and B). |
Based on the clinical findings, intravenous antibiotic metronidazole (500 mg every 8 h) was initiated, and 1500 units of HTIG was also injected into the deltoid muscle of the right upper arm following the diagnosis of generalized tetanus.
Although the patient’s wound was classified as category III exposure according to the World Health Organization rabies exposure categories,10 we did not administer anti-rabies vaccine to the patient because the dog that bit the patient had already been vaccinated against rabies, and no human case of rabies has been reported in Japan since 1956. In addition, no outbreak of rabies in animals has occurred since 1957, when there was an outbreak in cats. Currently, Japan is a rabies-free country.
Surgical debridement was not performed as the wound on his right hand had healed. Subsequently, the patient’s condition improved gradually. The perioral, neck, and shoulder pain disappeared on hospitalization day 5, and the patient started opening his mouth 7 days after starting treatment (Figure 2C and D). His clinical course was good without exacerbation, and the patient was able to eat and drink without difficulty on day 8 after hospitalization. The patient received metronidazole intravenously for 10 days. The patient was discharged after a 12-day hospital stay (Figure 3). The patient was informed of the importance of vaccination for tetanus prevention and completed a primary series of tetanus toxoid vaccinations after discharge. The canine that bit his hand did not show any signs of illness for 4 weeks during the period in which it was kept in his care. Therefore, we thought it unlikely that the canine had tetanus or rabies.
 |
Figure 3 The clinical course of the present case. The map shows the place where a 46-year-old man with tetanus after a canine bite was treated in August to September 2023. |
Discussion
We report a rare case of tetanus caused by a canine bite. In this case, the patient developed tetanus 21 days after a canine bite, even though the wound was washed with tap water, and was administered 250 units of HTIG and tetanus vaccine the day after exposure. This case illustrates that even when HTIG and tetanus vaccines are administered immediately after exposure, the potential for tetanus should always be considered. In the case of canine bites, we focused on preventing rabies. However, the possibility of tetanus should be considered.
In Japan, tetanus toxoid was introduced in 1952, and the combination of tetanus toxoid, diphtheria toxoid, and pertussis vaccine (DPT) became routine in 1968.11 However, it appears that many communities were reluctant to vaccinate with the DPT vaccine for fear of adverse reactions. In Japan, approximately 100 people develop tetanus each year, with 5–9 of these cases resulting in fatalities.12 Notably, in Japan, all cases of tetanus have been reported to the government since 1947, and there has been no reported case of tetanus caused by canine bites. To the best of our knowledge, the present case is the first report of tetanus caused by a canine bite in Japan. Furthermore, in addition to routine tetanus immunization, tetanus toxoids in 10-year intervals throughout life are also generally recommended.13 Despite these recommendations, immunity to tetanus continues to wane among adults, including in Japan.14,15 A 2018 study in Japan clarified a sharp decline in antibody titers to tetanus in people, particularly those over the age of 50 years.16 Therefore, to avoid cases such as the present one, it is important to educate people over 50 years about tetanus vaccination, as well as those responsible for keeping canines, and high-risk occupations such as veterinarians, zookeepers, and public health workers in Japan.
Notably, the patient developed tetanus even though the tetanus toxoid-containing vaccine and HTIG were administered the day after the canine bite. We assume that this may be because he did not receive a series of tetanus vaccinations in his childhood or that the amount of HTIG given the day after the canine bite may have been lower than the tetanus toxin, although the recommended amount (250 units) for tetanus prophylaxis was administered intramuscularly. Also, he may have failed to develop antibodies despite being vaccinated after exposure and did not have sufficient anti-tetanus toxin IgG titers to prevent the development of tetanus. However, we did not measure the actual blood levels of tetanus toxin IgG titers. Another reason may be that the memory response was not sufficient to protect against tetanus because of insufficient antibody levels for a protective threshold. Memory B cells require 3–4 days to generate antibody titers after tetanus vaccination.17 Moreover, the bite site may not have been adequately cleaned. In addition, some case reports have described occurrence of tetanus in patients who had an appropriate vaccination history and antibody titers to prevent the disease.18–20 Therefore, physicians must carefully observe patients during the incubation period of tetanus, even after treatment at the trauma site and administration of the tetanus vaccine and HTIG.
Tetanus, a potentially fatal condition, is linked to various risk factors.21 The primary entry point for tetanus bacteria is wounds, including deep punctures, cuts, burns, animal bites, or any skin break. Even seemingly minor wounds, if exposed to tetanus spores, pose an infection risk. Contact with objects carrying Clostridium tetani spores heightens this risk, with rusty items often implicated due to potential spore contamination. Inadequate immunization amplifies susceptibility to tetanus infection. Tetanus vaccines, integral to childhood immunizations, necessitate periodic booster shots to maintain immunity. Advanced age may increase vulnerability to tetanus owing to waning immunity or missed boosters. Intravenous drug use also raises the risk of tetanus, especially if the equipment carries tetanus spores. Prevention measures for tetanus include up-to-date vaccinations, particularly those including tetanus toxoids, and stringent hygiene practices to curtail contamination risk.22 In case of uncertain vaccination status or lack of a booster in a decade, one should seek prompt medical advice to ascertain the need for a tetanus booster. Timely interventions and preventive measures are critical defenses against tetanus infection.22
A literature review based on the PubMed database revealed only four tetanus cases due to canine bites between 1889 and 2018 (Table 1).6–9 One tetanus case related to canine bite was excluded from this review because information on age, sex, incubation period, treatment, and prognosis was unavailable.23 Including the present case, the range of onset days from injury was 3 to 19 days, which is the typical incubation period of tetanus.24 All four tetanus cases in the 2000s received HTIG, metronidazole, and toxoid as initial treatment. HTIG is the antitoxin of choice to neutralize unbound toxins. The amount of immunoglobulin administered to patients with tetanus varied from 1500 to 3000 to 5000 units (Table 1). There is insufficient data on the optimal dosage, but United States guidelines suggest that a single dose of 500 international units (IU) of HTIG is as effective as the previously recommended dose range of 3000–6000 IU. One unvaccinated patient died 15 days after onset due to multi-organ failure, suggesting the importance of basic immunity using vaccines. In addition, one patient developed tetanus despite having a protective anti-tetanus antibody level (0.22 U/mL), indicating that tetanus can develop even with adequate antibody titers and that the presence of a protective anti-tetanus antibody level should not be used to exclude the diagnosis of tetanus.7 Furthermore, a case of localized tetanus that progressed to generalized tetanus despite administration of the recommended 250 units of HTIG after exposure was reported, suggesting that the HTIG dose may have been too low.25 In addition to determining the appropriate amount of HTIG for protecting against tetanus, further research is needed to determine the true protective antibody titer required to prevent disease onset and to investigate whether the tetanus antibody function is uniform.
 |
Table 1 Literature Published in Relation to Tetanus Caused by Canine Bites Between 1889 and 2023 in English, Including the Present case6–9 |
A previous study reported no single case of tetanus from an animal bite after analysis of 8697 tetanus cases over a 14-year period;26 the mortality rate among non-neonates was 40.18%. Being male, having an incubation period of at least 96 hours prior to the onset of illness, and not having fever were associated with a lower mortality rate. Notably, the study also showed a very low mortality rate (2.14%) among 2100 patients without seizures. The reason for the better outcome in the present case without a history of tetanus immunization may also have been the fact that the patient met all these low-mortality factors.
In the present case, we could not explain why the patient developed tetanus despite the administration of HTIG and tetanus vaccine. To date, only a few cases of tetanus caused by canine bites have been reported. Further investigation is needed to elucidate the risk factors for tetanus development after canine bites.
Conclusions
We described a case of tetanus that developed after a canine bite. Our case highlights that even if HTIG and the tetanus vaccine are administered immediately after exposure, in addition to wound treatment, the possibility of tetanus should always be considered.
Abbreviations
HTIG, human tetanus immunoglobulin; DPT, tetanus toxoid, diphtheria toxoid, and pertussis vaccine.
Data Sharing Statement
The clinical data are available from the corresponding author upon reasonable request.
Ethics and Consent
Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. The present case report does not require ethics committee approval based on the Japanese Ethical Guidelines for Clinical Research to publish case details.
Acknowledgments
We thank Editage for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, in study conceptualization, design, and execution; data acquisition, analysis, and interpretation; or in all these areas. All authors took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
The authors and co-workers did not receive any specific funding.
Disclosure
The authors report no conflicts of interest with regards to this work.
References
1. Fields B, Guerin CS, Justice SB. Don’t be a stiff: a review article on the management of tetanus. Adv Emerg Nurs J. 2021;43:10–20. doi:10.1097/TME.0000000000000333
2. World Health Organization. Electronic address: [email protected]. Tetanus vaccines: WHO position paper, February 2017 - Recommendations. Vaccine. 2018;36:3573–3575. doi:10.1016/j.vaccine.2017.02.034
3. Campbell JI, Hien TT, Loan HT, et al. Microbiologic characterization and antimicrobial susceptibility of Clostridium tetani isolated from wounds of patients with clinically diagnosed tetanus. Am J Trop Med Hyg. 2009;80:827–831. doi:10.4269/ajtmh.2009.80.827
4. Kobayashi T, Tsurukiri J, Hoshiai A, Konishi H, Arai T. Domestic animal bites in infants: potential risk of fatal maltreatment. Pediatr Int. 2019;61:1175–1176. doi:10.1111/ped.13974
5. Yen LM, Thwaites CL. Tetanus. Lancet. 2019;393:1657–1668. doi:10.1016/S0140-6736(18)33131-3
6. Fink GH. Case of tetanus following dog bite. Treatment-recovery. Ind Med Gaz. 1889;24:142.
7. Beltran A, Go E, Haq M, et al. A case of clinical tetanus in a patient with protective antitetanus antibody level. South Med J. 2007;100:83. doi:10.1097/SMJ.0b013e31802e2717
8. Radjou A, Hanifah M, Govindaraj V. Tetanus following dog bite. Indian J Community Med. 2012;37:200–201. doi:10.4103/0970-0218.99933
9. Moynan D, O’Riordan R, O’Connor R, Merry C. Tetanus - A rare but real threat. IDCases. 2018;12:16–17. doi:10.1016/j.idcr.2018.02.004
10. Centers for Disease Control and Prevention. Tetanus - Wound Management. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html#medical.
11. Kimura M, Hikino N. Results with a new DTP vaccine in Japan. Dev Biol Stand. 1985;61:545–561.
12. Tetanus. Available from: https://www.niid.go.jp/niid/ja/kansennohanashi/466-tetanis-info.html.
13. Kim DK, Hunter P. Advisory committee on immunization practices. Recommended adult immunization schedule, United States, 2019. Ann Intern Med. 2019;170:182–192. doi:10.7326/M18-3600
14. McQuillan GM, Kruszon-Moran D, Deforest A, Chu SY, Wharton M. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med. 2002;136:660–666. doi:10.7326/0003-4819-136-9-200205070-00008
15. Wagner KS, White JM, Andrews NJ, et al. Immunity to tetanus and diphtheria in the UK in 2009. Vaccine. 2012;30:7111–7117. doi:10.1016/j.vaccine.2012.09.029
16. Tetanus antibody carriage by age; 2018. Available from: https://www.niid.go.jp/niid/images/epi/yosoku/2018/Seroprevalence/t2018serum.pdf.
17. Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi:10.1038/s41577-020-00479-7
18. Vollman KE, Acquisto NM, Bodkin RP. A case of tetanus infection in an adult with a protective tetanus antibody level. Am J Emerg Med. 2014;32:
19. Tharu B, Ibrahim S, Shah M, Basnet S, Park T. An unusual case of evolving localized tetanus despite prior immunization and protective antibody titer. Cureus. 2020;12:e9498. doi:10.7759/cureus.9498
20. Akane Y, Tsugawa T, Hori T, et al. Tetanus in a partially immunized child. J Infect Chemother. 2018;24:980–982. doi:10.1016/j.jiac.2018.05.002
21. Finkelstein P, Teisch L, Allen CJ, Ruiz G. Tetanus: a potential public health threat in times of disaster. Prehosp Disaster Med. 2017;32:339–342. doi:10.1017/S1049023X17000012
22. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2018;67:1–44.
23. Qaderi S, Qaderi F, Tarki FE, et al. Generalized, non-neonatial tetanus is a highly fatal disease in Afghanistan: a case series study. Int J Infect Dis. 2021;103:568–572. doi:10.1016/j.ijid.2020.12.019
24. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. Tetanus. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html.
25. Licindo D, Putra EM, Rombetasik M, Lim H. Progressive localized tetanus in patient with inadequate human tetanus immunoglobulin therapy. IDCases. 2021;24:e01147. doi:10.1016/j.idcr.2021.e01147
26. Patel JC, Mehta BC. Tetanus: study of 8697 cases. Indian J Med Sci. 1999;53:393–401.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.