Back to Journals » Cancer Management and Research » Volume 14
Technological Advancements in External Beam Radiation Therapy (EBRT): An Indispensable Tool for Cancer Treatment
Authors Koka K, Verma A, Dwarakanath BS, Papineni RVL
Received 30 November 2021
Accepted for publication 26 March 2022
Published 11 April 2022 Volume 2022:14 Pages 1421—1429
DOI https://doi.org/10.2147/CMAR.S351744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Seema Singh
Krishna Koka,1 Amit Verma,2 Bilikere S Dwarakanath,3 Rao VL Papineni2,4
1Penn State University, Pennsylvania, PA, USA; 2PACT & Health LLC, Branford, CT, USA; 3Central Research Facility, Sri Ramachandra Institute of Higher Education and Research Porur, Chennai, India; 4Department of Surgery, University of Kansas Medical Center, Kansas City, KS, USA
Correspondence: Rao VL Papineni; Amit Verma, PACT & Health LLC, Branford, CT, USA, Tel +1 832-687-4334 ; Tel +1-919-358-2644, Email [email protected]; [email protected]; [email protected]
Abstract: Recent technological advancements have increased the efficacy of radiotherapy, leading to effective management of cancer patients with enhanced patient survival and improved quality of life. Several important developments like multileaf collimator, integration of imaging techniques like positron emission tomography (PET) and computed tomography (CT), involvement of advanced dose calculation algorithms, and delivery techniques have increased tumor dose distribution and decreased normal tissue toxicity. Three-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiotherapy (IMRT), stereotactic radiotherapy, image-guided radiotherapy (IGT), and particle therapy have facilitated the planning procedures, accurate tumor delineation, and dose estimation for effective personalized treatment. In this review, we present the technological advancements in various types of EBRT methods and discuss their clinical utility and associated limitations. We also reveal novel approaches of using biocompatible yttrium oxide scintillator-photosensitizer complex (YSM) that can generate X-ray induced cytotoxic reactive oxygen species, facilitating X-ray activated photodynamic therapy (XPDT (external beam) and/or iXPDT (internal X-ray source)) and azido-derivatives of 2-deoxy-D-glucose (2-DG) as agents for site-specific radiation-induced DNA damage.
Keywords: radiotherapy, external beam, computed tomography, 2-deoxy-D-glucose, 2-DG, 2-azido-2-deoxy-D-glucose, 2-AZ-2-DG
Introduction
Radiation therapy (RT) is the widely used therapeutic modality for cancer treatment and is used either alone or in combination with other conventional modalities such as surgery, chemotherapy, immunotherapy etc.1,2 However, RT causes collateral damage to normal tissues/organs, compromising their functioning, thereby leading to multi-organ dysfunction and death.3,4 Patients with advanced tumors such as glioblastoma multiforme, pancreatic, and lung cancers are very difficult to treat and have very low survival rate.5 Recent technological advances have enabled the effectiveness of RT by achieving a higher degree of local tumor control and enhanced patient survival.5–7 RT is mainly divided into, external beam RT (EBRT), internal beam RT (IBRT) or brachytherapy, and systemic radioisotope therapy, based on the location of the radiation source.
External Beam Radiation Therapy (EBRT)
EBRT is a broad yet effective form of cancer treatment that is used in cases where there is more advanced local disease.8,9 EBRT induces DNA damage, cell cycle arrest, cytogenetic damage, apoptosis, and senescence in cancer cells, thereby blocking the ability of cancer cells to proliferate further and undergo death.6 While normal cells receive some radiation damage when radiation is used to treat cancer cells, normal cells are usually able to repair themselves faster than cancer cells.10 This results in the killing of cancer cells with minimized damage to normal cells. The main types of radiation used to treat cancer are widely used photon radiation (X-rays and gamma-rays) and electron beams, which have low penetration power and are useful in treating tumors close to a body surface. Particle radiation, such as proton and neutron beams, is very effective in treating deep-seated tumors one of the newer forms of particle beam radiation, has allowed for better dose distribution in deep-seated tumors. The relative biological effectiveness (RBE) of radiation refers to the effectiveness of its cell killing (RBE). This effectiveness is dependent upon LET, fractionation rate, total dose, and radio-sensitivity of the targeted tissues and cells.11 Radiation with low LET (X-rays and gamma-rays) results in relatively small amounts of energy being deposited. Alternatively, radiation with high LET (neutrons and protons) results in higher energy deposits in the targeted areas. The indirect DNA damage that results from radiation is due to ionization or excitation of the cell’s water components.12 Most of the cell killing in cancer and normal cells results from double-strand DNA breaks, which are irreparable. While single-strand DNA breaks exist, these are less responsible for cell deaths. The main goal of radiation therapy is to deprive cancer cells of their multiplication potential as well as to eventually kill the cancer cells. Once a cancer cells DNA has been damaged beyond repair, the cells stop dividing and die. There is potential to improve radiation therapy via identification of the importance of radiation-induced cell death and of the further mechanisms that are involved.
EBRT includes 3-dimensional conformal radiotherapy, intensity-modulated radiotherapy, stereotactic radiotherapy, image-guided radiotherapy, volumetric modulated arc radiotherapy, proton and heavy ion beam therapy, etc, with each individual modality having a specific advancements and adaptation for the tumor type and spatio-temporal dynamics. The technological advancements have been significantly demonstrated in the area of computer-based imaging and analysis algorithms, dose calculation methods, and delivery techniques, providing increased tumor dose distribution, better delineation, and decreased normal tissue irradiation. In the present review we discuss the various types of technological advancements in EBRT techniques used for treating different types of cancers to achieve therapeutic gain; besides discussing their associated advantages and disadvantages (Table 1).
 |
Table 1 Different Types of External Beam Radiation Therapy (EBRT) Techniques and Associated Advantages and Disadvantages |
3 Dimensional Conformal Radiotherapy (3DCRT)
3D Conformal radiotherapy is a form of EBRT that uses 3D treatment planning, multiple cross-firing with carefully shaped fixed fields is 3D conformal radiotherapy (3DCRT).13 3DCRT is an improvement on 2D imaging as it uses CT imaging instead of X-ray imaging, allowing accurate tumor localization and showing the critical normal organ structures in the imaging, facilitating the optimal beam placement and shielding to be used. The widest use of 3DCRT for the treatment of brain tumors, breast cancer, gastrointestinal cancer, lung cancer and gynecologic malignancies etc. 3DCRT and intensity-modulated radiotherapy (IMRT) has been demonstrated to improve short-term response rate, reduce mouth dryness and parotid gland injury, thereby enhancing the quality of life, and promote the prognosis of patients with nasopharyngeal carcinoma.14 Contrarily, patients with endometrial cancer showed higher incidence of GI-toxicities post 3DCRT treatment compared to IMRT-treated patients.15 Thus, there are some difficulties associated with 3DCRT related to performing correct quality procedures, positioning, imaging, contouring, dosimetry, follow-up, and dose delivery.
Intensity Modulated Radiotherapy (IMRT)
IMRT has a unique feature of regulating the intensity of each beam in order to determine the target and surrounding organs shape through computer-based optimization algorithm assistance. Multiple beams for radiation are used in order to allow for multiple areas of varying radiation intensity. IMRT has been successfully used in head and neck, prostate, breast, lung, brain, gynecologic, and GI cancers, with minimal toxicities to the adjacent tissues/ organs compared to the target volume.13,16 Recent clinical data in whole breast irradiation, partial breast irradiation, and ultra-hypofractionated have suggested IMRT safety, efficacy and improved local recurrence, including reduced acute/ late toxicity.17 IMRT has been demonstrated to be effective for dose escalation studies in advanced floor-of-the mouth tumors in combination with stereotactic hypofractionated boost. Studies in patients where the surgical removal of tumors is not possible IMRT has been frequently used for treatment viz, non-small cell lung carcinoma, improving the targeting of tumor volume without increasing doses to organs at risk (OARs), besides reducing dysphagia.18 In a study IMRT decreases the heart and ipsilateral lung irradiation, increasing the target irradiation volumes in breast cancer patients.19 Although IMRT provides a high conformity and high precision, it harbors high cost, involves, high complexity and time-consuming procedures (dosimetry and treatment QA).20 IMRT is prone to geometrical errors, compared to 3DCRT due to higher dose conformity indices, including the considerations in patient immobilization for determining the treatment margin and day-to-day changes in the target volumes of some cancers such as prostate, cervix, bladder, and rectum.20 Several newer developments, such incorporation of Magnetic resonance imaging (MRI), Positron emission tomography (PET), and single-photon emission computed tomography (SPECT) has enabled the in-homogenous dose distribution (dose painting). Automation in patient positioning and dosimetry QA procedures have enabled accurate dose delivery.
Volumetric Modulated Arc Therapy (VMAT)
VMAT provides a full 360° of beam directions with the entire dose volume delivered in a single rotation by regulating dose-rate and multileaf collimator (MLC). The MLC requires regulation because they affect treatment time through increasing the number of beam directions and increasing the monitor units (MU). This rotational method results in differing dose distributions to different parts of the tumor. VMAT is better at sparing organ risk than is IMRT due to this differing dose distribution, which results in lower dosages to the surrounding tissue. VMAT also has a lower risk of secondary malignancy than does IMRT due to its use of fewer MU. Still, this lower risk may be counteracted by the increase in the normal tissue volume that receives low-dose wash. VMAT treatment shows a lower risk of OAR irradiation and has better homogeneity compared to IMRT, thus playing a significant role in uncomfortable immobilization, such as head and neck cancers. VMAT with simultaneous integrated boost (SIB) technique has been shown to achieve high local tumor control and increased survival and well-tolerated toxicities in non-small cell lung cancer (NSCLC) patients who are not fit for standard definitive therapy.21 Further, VMAT has been utilized in planning and clinical outcome studies in tumors like prostate, gastrointestinal, gynecological, head and neck thoracic, central nervous system, and breast tumors.22 The potential risk associated with VMAT is the increase in the low dose radiation to the surrounding tissues and organs, with a greater chance of having secondary malignancies. Therefore, patient follow-up for a longer period is recommended to estimate the chances of having secondary malignancies.
Image Guided Radiotherapy (IGRT)
IGRT is the direct integration of on-board imaging and the recent development of the MR-Linac. The technique has increased awareness of the geometrical uncertainty of treatment due to organ motion, treatment response, and patient setup. It has been made clear that efficient processing of image information is essential for radiotherapy. Any imprecision in patient positioning and anatomic alterations can be corrected promptly during IGRT. IGRT has been widely used for prostate, lung and head and neck cancers and allows significant reduction in set-up margins resulting in reduced toxicities in sites with demonstrable, quantifiable, and correctable inter- and/ or intra- fraction motion (lung, prostate, and head and neck cancer).23 The pitfalls associated with IGRT include uncertainties in target volume delineation, image quality, longer acquisition times, high intra-fractional errors, and extra-dose delivery during daily imaging. Adaptive radiotherapy overcomes these limitations, where changes are made to the original radiation treatment plan during the course of hypo-fractionated radiotherapy based on changes in anatomical and tumor features/ biology.
Stereotactic Body Radiation Therapy (SBRT)
SBRT is a new EBRT technique that is made possible by improved radiation accuracy and improved therapeutic ratios for many tumor sites.24 This technique delivers precise and very high individual doses of radiation by focusing multiple radiation beams on the target form different directions. This delivery occurs over a few treatment fractions to remove primary and oligometastatic tumors that are small, well-defined, and found at different locations in the body.25 The high radiation dose results in a likelihood of damage to any tissue immediately adjacent to the target. Although this likelihood exists, the small and non-eloquent amount of normal tissue makes clinically significant toxicity low. SBRT can be used in many types of tumors, including prostate, head and neck, spinal, renal, oligo metastases, and pancreatic. This technique attempts to take advantage of the potentially low alpha/beta of prostate cancer.26 The technique attempts to do this by delivering greater doses per fraction to the prostate. In whole-gland boost therapy, SBRT is used as a method of delivering a boost of radiation to the prostate.27 This is accomplished after patients with intermediate and high risk disease undergo more regional radiotherapy investigation. SBRT has also been researched as a way of focally escalating the dose delivered to the dominant intra-prostatic lesion.28 Furthermore, the technique has been shown to be effective in the treatment of early non-small cell lung cancer for patients that were unfit for surgery.29 However, several disadvantages of SBRT in NSLC patients are radiation pneumonitis, skin toxicity, odynophagia, rib fracture pain, and nerve injury.
Particle Therapy
Particle radiation like proton beams, one of the newer forms of particle beam radiation, has allowed for better dose distribution in deep-seated tumors, due to Bragg’s peak (a unique absorption profile in tissues).30 Bragg’s peak allows for deposition of maximum destructive energy at the site of the tumor while also minimizing the damage done to healthy tissues along the beam’s path when using proton beams.31 This makes proton beams special in clinical use when applied to pediatric and adult tumors near critical structures, including the spinal cord and skull base tumors.32,33 Proton irradiation has achieved a good local tumor control with minimal normal tissue toxicity in patients for stage II–III NSCLC, prostate carcinoma, chordoma and hepatocellular carcinoma.34 Proton beams are critical here because maximal normal tissue sparing is observed. The planning for proton beam therapy includes patient immobilization, imaging, and modeling of the planned dose distributions. Another type of beam used are neutron beams, high LET (linear energy transfer) beams that can cause more DNA damage than photons.35 Boron neutron capture therapy (BNCT) utilizes boron-10 target drug that accumulates preferentially into the tumors and post neutron irradiation yields lithium-7 and an alpha particle, killing cancer cells specifically and sparing the normal tissue. BNCT has been clinically used in glioblastoma multiforme, meningioma, head and neck cancers, lung cancer, breast cancers, hepatocellular carcinoma, and pediatric cancers etc.36 Carbon ion radio-therapy (CIRT), has very high radiobiological effect compared to proton and neutron therapy, and is limited to very few centers worldwide. CIRT has been implicated in variety of cancers such as intracranial, head and neck, gastrointestinal tract, prostate, and genitourinary cancers etc.36 CIRT has shown a potential benefit in combination with immunotherapy, due to high anti-tumor immunogenic response elicited by the combinatorial treatment compared to either of the treatment alone. There are limitations associated with CIRT linked to precise dose calculation, treatment proficiency, and to the treatment positions available.37 Particle radiation has a higher biological effectiveness and is very effective in radio-resistant cancers, including sarcomas, renal cell carcinomas, melanomas, and glioblastoma. While particle radiation may be more effective, it has innate limitations:38 the production of particle radiation therapy is much more expensive than the production of photons, and has more logistical requirements. Also several advancements in beam production systems (accelerators), beam transport systems including gantries and beam delivery systems are underway to overcome the above mentioned limitations, thereby accelerating the widespread use of particle therapy in developed and developing countries.
Photodynamic Therapy (PDT)
PDT is a minimally invasive procedure, used for treating various types of cancers such as leukemia, carcinomas, and precancerous lesions. PDT utilizes photosensitizing drugs that accumulate selectively into the tumor tissue, and are activated by exposure to intense non-thermal (visible) light sources of a specific wavelength. The interaction of photosensitizers with light, elicits a photodynamic reaction mediated by highly reactive singlet oxygen species leading to macromolecular damage (DNA, RNA, lipids, and proteins) and cancer cell death.39 The anti-cancer efficacy of photosensitizers like hematoporphyrin is well established for the treatment of a variety of hematological malignancies and solid tumors, including its utility in fluorescence-based cancer diagnostics.40 The hematoporphyrin derivative, Photofrin® (porfimer sodium) is approved by the US-FDA for treatment of esophageal, non-small cell lung cancer and Barrett's esophagus patients. Photosensitizers like 1,5-aminolevulinic acid (ALA), methyl aminolevulinate (MAL), hexaminolevulinate (HAL), etc has received FDA approval for PDT applications. However, non-porphyrin photosensitizers like anthraquinonoid and curcuminoid are still under consideration for approval and clinical testing.41 The PDT uses laser and non-laser light sources for activation of photosensitizers, which can be directed through fiber optics to deliver light to the tumor tissue inside the body via fiber optic devices like cylindrical fibers, balloon catheters, and lens fiber optics. Photofrin is activated through fiber optics attached to a 630-nm emitting laser, Visudyne (QLT) by a 690-nm emitting laser and 5-ALA by Blue U (a non-laser light source).42 Light–emitting diodes (LEDs) are used to treat surface tumors like melanomas.43 Further, the anti-cancer efficacy of PDT is determined by several factors determine the success of PDT such as the uptake and localization of the photosensitizer, the method of light delivery, spatio-temporal organization and location of tumors, singlet and triplet quantum yields, and associated side-effects.44 However, the success of PDT, is limited and considerable efforts are being made to enhance yield singlets and triplets by photosensitizer and light delivery systems are underway. Recently, we characterized nano-scintillators emitting light that activates paired photosensitizers in photodynamic therapy (PDT) that improves the prowess of PDT in deep-seated tumor treatment.45 Yttrium oxide nanoparticle construct doped with Europium (YOE) and coated with a silica layer entrapping methylene blue (MB) as the photosensitizer was fabricated.45 The nanoconstruct (YSM, for YOE, silica, MB) was decorated with polyethylene glycol (PEG) to make it biocompatible and characterized extensively for physio-chemical parameters. Transmission electron microscopy (TEM) revealed a resultant average nanoparticle diameter of 24.1 ± 5.7 nm, and dynamic light scattering (DLS) a hydrodynamic diameter of 135.3 ± 17.9 nm. This nanoconstruct was able to generate singlet oxygen-reactive oxygen species upon irradiation, and induced cytotoxicity in pancreatic cancer cells (Panc02 cells) post-irradiation (8 Gy). Since 8 Gy is within the range of a clinically applicable dose used routinely in stereotactic radiotherapy regimens, our results verified the potential of YSM to be used as scintillator-photosensitizer complexes for x-ray activated photodynamic therapy.45 Finally, the biodistribution of YSM nanoconstructs in vivo in C57BL6 mice harboring subcutaneous Panc02 tumors showed YSM accumulation in tumors.45 A versatile, biocompatible Yttrium oxide scintillator-photosensitizer complex (YSM) that can generate x-ray induced cytotoxic reactive oxygen species, facilitating x-ray activated photodynamic therapy (XPDT (external beam) and/or iXPDT (internal x-ray source)). Thus, use of YSM nano-construct appears to be a potential technological advancement in PDT, thereby enhancing efficacy and overall survival in cancer patients.45
A Novel Approach for Enhancing the Efficacy of EBRT
Recently, the National Cancer Institute (NCI), USA, has initiated the program encouraging development of technology in combinatorial treatment, using external radiation (X-rays or particle beam used for radiotherapy) for local drug activation or release systemically or intra-tumorally delivered therapeutics, including high-atomic number elements that emit auger electrons.46 This combinatorial approach would, deliver safe doses of external radiation dose to locally activate or release the therapeutic agent, and prevent adverse toxicities in the normal tissue. Such a strategy could extend the range of PDT to deep-seated tumors that are currently intractable with existing PDT. Several studies demonstrated that X-ray exposure triggers the release of singlet oxygen that disintegrates the liposome carrier and releases the gold nanoparticle and verteporfin cargo. Several other radiosensitive polymeric carriers have been reported for sustained release of chemotherapeutics.47,48 Recently, polymer poly (ε-caprolactone-b-ethylene glycol) (PCL-PEO) micellar system with Chlorin e6 (Ce6) photosensitizer and chemotherapeutic drug, showed that there is a sustained release of Doxorubicin (Dox), triggered by ionizing radiation-induced activation of Ce6 which destroys the micelle and releases DOX. Interestingly, in this study the sustained release was not observed with other chemotherapeutic drugs such as paclitaxel (PTX) and docetaxel (DTX), suggesting detailed understanding of the mechanism of radiation-induced drug release from the PCL-PEO is warranted.49
Our recent work on azido-DNA-model systems showed that radiation-produced electrons can be converted to highly damaging aminyl radicals (RNH•) which do have the potential to cause strand breaks.50 Experimental studies in our labs (both in vitro and in vivo) have shown that 2-deoxy-D-glucose (2-DG), a glucose mimic and glycolytic inhibitor, enhances radiation-induced damage selectively in tumor cells while protecting normal cells and against infection. Thereby suggesting that 2-DG can be used as a differential radiomodifier to improve the efficacy of cancer radiotherapy. Based on these experimental results and data mining models, we show that the azido derivative of 2DG; 2-Azido-2-deoxy-D-glucose (2-A Z-2-DG) and other derivatives have unique azido chemistries.51 The neutral aminyl radical produced from 2-AZ-2-DG under a reductive environment undergoes facile conformational change from the stable chair form to the reactive boat form.52 Subsequently, this aminyl radical causes H-atom abstraction from C5 of the sugar moiety via a proton-coupled electron transfer process. Further, a promise as multivalent drug to augment multipronged cellular damage including site-specific DNA radiation damage, and also counter infections persisting in several cancer cases. Our long range goals are to harness these unique chemistries in gold based nanotools viz: IRaGAZ (Ionizing radiation + Gold nanoparticles + Azido-2-DG) for X-PDT (X-ray induced photodynamic therapy) (Scheme 1).
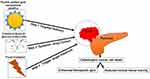 |
Scheme 1 IRaGAZ (Ionizing radiation + Gold nanoparticles + Azido-2-DG). Note: A novel three stage nanotechnology- chemo-radiation combinatorial approach in enhancing therapeutic efficacy. |
Conclusion
In conclusion, EBRT has technologically improved significantly, related to the in-depth planning and treatment, with main focus of each method to image and delineate accurate tumor volume, irradiate maximum target volume, sparing the surrounding normal tissue. Image-guided radiotherapy, stereotactic radiotherapy, and particle therapy which involve the combination of CT, PET have revolutionized the treatment strategies, leading to enhanced patient survival and therapeutic gain. Recently single dose ultrahigh dose rate irradiation (> 40 Gy per second) called FASH radiotherapy has been introduced that is purported to spare the normal tissue from toxicity without compromising tumor response thereby achieving an enhanced therapeutic gain. Although the underlying biological mechanisms have not been completely elucidated, induction of hypoxia in the normal tissue and a modified immune response has been suggested to contribute to the normal tissue sparing and overall FLASH effect.55 In addition adaptive radiation therapy viz. modifying treatment plan during the course of the treatment, and radiomics that uses advanced mathematical analysis to enhance the existing data for clinicians is becoming popular in clinical practice.53,54 Involvement of advanced robotics and artificial intelligence will enhance the utilization of EBRT for effective management of refractory deep seated tumors.
Acknowledgment
RVLP acknowledges NCI-SBIR for the support, grant# [NCI 75N91019C000043 and 75N91019C000016].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no conflicts of interest in the work related to this submission.
References
1. Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and Radiation Therapy: current Advances and Future Directions. Int J Med Sci. 2012;9(3):193–199. doi:10.7150/ijms.3635
2. Orth M, Lauber K, Niyazi M, et al. Current concepts in clinical radiation oncology. Radiat Environ Biophys. 2014;53(1):1–29. doi:10.1007/s00411-013-0497-2
3. Barnett GC, West CML, Dunning AM, et al. Normal tissue reactions to radiotherapy. Nat Rev Cancer. 2009;9(2):134–142. doi:10.1038/nrc2587
4. Prasanna PGS, Stone HB, Wong RS, et al. Normal tissue protection for improving radiotherapy: where are the Gaps? Transl Cancer Res. 2012;1(1). doi:10.21037/372
5. Lee TF, Yang J, Huang EY, Lee CC, Chan MF, Liu A. Technical Advancement of Radiation Therapy. Biomed Res Int. 2014;2014:e797412. doi:10.1155/2014/797412
6. Garibaldi C, Jereczek-Fossa BA, Marvaso G, et al. Recent advances in radiation oncology. Ecancermedicalscience. 2017;11:785. doi:10.3332/ecancer.2017.785
7. Tseng M, Ho F, Leong YH, et al. Emerging radiotherapy technologies and trends in nasopharyngeal cancer. Cancer Commun. 2020;40(9):395–405. doi:10.1002/cac2.12082
8. Shirato H, Le QT, Kobashi K, et al. Selection of external beam radiotherapy approaches for precise and accurate cancer treatment. J Radiat Res. 2018;59(Suppl 1):i2–i10. doi:10.1093/jrr/rrx092
9. Tam S, Amit M, Boonsripitayanon M, et al. Adjuvant External Beam Radiotherapy in Locally Advanced Differentiated Thyroid Cancer. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1244–1251. doi:10.1001/jamaoto.2017.2077
10. Mladenov E, Magin S, Soni A, Iliakis G, Double-Strand Break DNA. Repair as Determinant of Cellular Radiosensitivity to Killing and Target in Radiation Therapy. Front Oncol. 2013;3:113. doi:10.3389/fonc.2013.00113
11. Suman S, Kumar S, Moon BH, et al. Relative Biological Effectiveness of Energetic Heavy Ions for Intestinal Tumorigenesis Shows Male Preponderance and Radiation Type and Energy Dependence in APC1638N/+ Mice. Int J Radiation Oncol. 2016;95(1):131–138. doi:10.1016/j.ijrobp.2015.10.057
12. Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiotherapy Oncol. 2013;108(3):362–369. doi:10.1016/j.radonc.2013.06.013
13. Department of Radiation Oncology, University of Health Sciences, Okmeydani Training and Research Hospital, Istanbul, Turkey, Kandemir Gursel O. Recent Technological Advances in Radiotherapy. Eur Arch Med Res. 2018;34(Suppl1):55–60. doi:10.5152/eamr.2018.69775.
14. Chen JLY, Huang YS, Kuo SH, et al. Intensity-modulated radiation therapy achieves better local control compared to three-dimensional conformal radiation therapy for T4-stage nasopharyngeal carcinoma. Oncotarget. 2016;8(8):14068–14077. doi:10.18632/oncotarget.12736
15. Onal C, Yuce Sari S, Yavas G, et al. Outcome and safety analysis of endometrial cancer patients treated with postoperative 3D-conformal radiotherapy or intensity modulated radiotherapy. Acta Oncol. 2021:1–7. doi:10.1080/0284186X.2021.1926537.
16. Taylor A, Powell MEB. Intensity-modulated radiotherapy—what is it? Cancer Imaging. 2004;4(2):68–73. doi:10.1102/1470-7330.2004.0003
17. Jones BM, Green S. Modern radiation techniques in early stage breast cancer for the breast radiologist. Clin Imaging. 2021;80:19–25. doi:10.1016/j.clinimag.2021.06.035
18. Guillemin F, Berger L, Lapeyre M, Bellière-Calandry A. [Dosimetric and toxicity comparison of IMRT and 3D-CRT of non-small cell lung cancer]. Cancer Radiother. 2021. doi:10.1016/j.canrad.2021.03.001
19. Mansouri K, Rastegari-Pouyani M, Ghanbri-Movahed M, Safarzadeh M, Kiani S, Ghanbari-Movahed Z. Can a metabolism-targeted therapeutic intervention successfully subjugate SARS-COV-2? A scientific rational. Biomed Pharmacother. 2020;131:110694. doi:10.1016/j.biopha.2020.110694
20. Cheung K. Intensity modulated radiotherapy: advantages, limitations and future developments. Biomed Imaging Interv J. 2006;2(1):e19. doi:10.2349/biij.2.1.e19
21. Shen J, Yang D, Chen M, et al. Hypofractionated Volumetric-Modulated Arc Radiotherapy for Patients With Non-Small-Cell Lung Cancer Not Suitable for Surgery or Conventional Chemoradiotherapy or SBRT. Front Oncol. 2021;11:644852. doi:10.3389/fonc.2021.644852
22. Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011;84(1007):967–996. doi:10.1259/bjr/22373346
23. Gupta T, Narayan CA. Image-guided radiation therapy: physician’s perspectives. J Med Phys. 2012;37(4):174–182. doi:10.4103/0971-6203.103602
24. Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007;30(6):637–644. doi:10.1097/COC.0b013e3180ca7cb1
25. Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist. 2012;17(8):1100–1107. doi:10.1634/theoncologist.2012-0092
26. Ge X, Zhu H, Dai W, Sun X. Stereotactic body radiotherapy in the era of radiotherapy with immunotherapy. J Thorac Dis. 2016;8(11):2968–2970. doi:10.21037/jtd.2016.11.16
27. McDonald AM, Dobelbower MC, Yang ES, et al. Prostate Stereotactic Body Radiation Therapy With a Focal Simultaneous Integrated Boost: acute Toxicity and Dosimetry Results From a Prospective Trial. Adv Radiat Oncol. 2018;4(1):90–95. doi:10.1016/j.adro.2018.09.007
28. Kim YJ, Yoon KJ, Kim YS. Simultaneous integrated boost with stereotactic radiotherapy for dominant intraprostatic lesion of localized prostate cancer: a dosimetric planning study. Sci Rep. 2020;10(1):14713. doi:10.1038/s41598-020-71715-2
29. Chua GWY, Chua KLM. Which patients benefit most from stereotactic body radiotherapy or surgery in medically operable non‐small cell lung cancer? An in‐depth look at patient characteristics on both sides of the debate. Thoracic Cancer. 2019;10(10):1857. doi:10.1111/1759-7714.13160
30. Musielak M, Suchorska WM, Fundowicz M, Milecki P, Malicki J. Future Perspectives of Proton Therapy in Minimizing the Toxicity of Breast Cancer Radiotherapy. J Pers Med. 2021;11(5):410. doi:10.3390/jpm11050410
31. Flatten V, Baumann KS, Weber U, Engenhart-Cabillic R, Zink K. Quantification of the dependencies of the Bragg peak degradation due to lung tissue in proton therapy on a CT-based lung tumor phantom. Phys Med Biol. 2019;64(15):155005. doi:10.1088/1361-6560/ab2611
32. Athar BS, Bednarz B, Seco J, Hancox C, Paganetti H. Comparison of out-of-field photon doses in 6 MV IMRT and neutron doses in proton therapy for adult and pediatric patients. Phys Med Biol. 2010;55(10):2879–2891. doi:10.1088/0031-9155/55/10/006
33. Demizu Y, Mizumoto M, Onoe T, et al. Proton beam therapy for bone sarcomas of the skull base and spine: a retrospective nationwide multicenter study in Japan. Cancer Sci. 2017;108(5):972–977. doi:10.1111/cas.13192
34. Jiang GL. Particle therapy for cancers: a new weapon in radiation therapy. Front Med. 2012;6(2):165–172. doi:10.1007/s11684-012-0196-4
35. Kinashi Y, Yokomizo N, Takahashi S, Double-strand Breaks DNA. Induced byFractionated Neutron Beam Irradiation for Boron Neutron Capture Therapy. Anticancer Res. 2017;37(4):1681–1685. doi:10.21873/anticanres.11499
36. Malouff TD, Mahajan A, Krishnan S, Beltran C, Seneviratne DS, Trifiletti DM. Carbon Ion Therapy: a Modern Review of an Emerging Technology. Front Oncol. 2020;1:10.
37. Farr JB, Flanz JB, Gerbershagen A, Moyers MF. New horizons in particle therapy systems. Med Phys. 2018;45(11):e953–e983. doi:10.1002/mp.13193
38. Dell’Oro M, Short M, Wilson P, Bezak E. Clinical Limitations of Photon, Proton and Carbon Ion Therapy for Pancreatic Cancer. Cancers. 2020;12(1):163. doi:10.3390/cancers12010163
39. Mang TS. Lasers and light sources for PDT: past, present and future. Photodiagnosis Photodyn Ther. 2004;1(1):43–48. doi:10.1016/S1572-1000(04)00012-2
40. Wilson BC. Photodynamic therapy for cancer: principles. Can J Gastroenterol. 2002;16(6):393–396. doi:10.1155/2002/743109
41. Ormond AB, Freeman HS. Dye Sensitizers for Photodynamic Therapy. Materials. 2013;6(3):817–840. doi:10.3390/ma6030817
42. Marcus KJ, Haas-Kogan D. 8 - Pediatric Radiation Oncology. In: Orkin SH, Fisher DE, Look AT, Lux SE, Ginsburg D, Nathan DG, editors. Oncology of Infancy and Childhood. 2009:241–255. doi:10.1016/B978-1-4160-3431-5.00008-X
43. Capella MAM, Capella LS. A light in multidrug resistance: photodynamic treatment of multidrug-resistant tumors. J Biomed Sci. 2003;10(4):361–366. doi:10.1007/BF02256427
44. Gupta S, Dwarakanath BS, Chaudhury NK, Mishra AK, Muralidhar K, Jain V. In vitro and in vivo targeted delivery of photosensitizers to the tumor cells for enhanced photodynamic effects. J Cancer Res Ther. 2011;7(3):314–324. doi:10.4103/0973-1482.87035
45. Papineni RV, Krishnan S, Pulickel A, Sahin O. Abstract 3057: development of radiation-triggered phosphor platform for localized activation in combinatory cancer treatment. Cancer Res. 2021;81(13 Supplement):3057.
46. NIH/NCI 399 - Combinatory Treatment Utilizing Radiation to Locally Activate Systemically Delivered Therapeutics | NCI: SBIR & STTR. Available from: https://sbir.cancer.gov/funding/contracts/399.
47. Deng W, Chen W, Clement S, et al. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat Commun. 2018;9(1):2713. doi:10.1038/s41467-018-05118-3
48. Ma N, Xu H, An L, Li J, Sun Z, Zhang X. Radiation-sensitive diselenide block co-polymer micellar aggregates: toward the combination of radiotherapy and chemotherapy. Langmuir. 2011;27(10):5874–5878. doi:10.1021/la2009682
49. Liu H, Laan AC, Plomp J, et al. Ionizing Radiation-Induced Release from Poly(ε-caprolactone-b-ethylene glycol) Micelles. ACS Appl Polym Mater. 2021;3(2):968–975. doi:10.1021/acsapm.0c01258
50. Papineni R, Adhikary A. Abstract 482: multiple chemical action cancer therapeutics. Cancer Res. 2020;80(16 Supplement):482.
51. Verma A, Adhikary A, Woloschak G, Dwarakanath BS, Papineni RVL. A combinatorial approach of a polypharmacological adjuvant 2-deoxy-D-glucose with low dose radiation therapy to quell the cytokine storm in COVID-19 management. Int J Radiat Biol. 2020;96(11):1323–1328. doi:10.1080/09553002.2020.1818865
52. Papineni RV, Adhikary A, Tandon R, Mitra D. Abstract 713: inhibition of pseudo SARS-CoV-2 binding activity of a anti-cancer polypharmacological agent analogs. Cancer Res. 2021;81(13Supplement):713. doi:10.1158/1538-7445.AM2021-713
53. Glide-Hurst CK, Lee P, Yock AD, et al. Adaptive Radiation Therapy (ART) Strategies and Technical Considerations: a State of the ART Review From NRG Oncology. Int J Radiat Oncol Biol Phys. 2021;109(4):1054–1075. doi:10.1016/j.ijrobp.2020.10.021
54. van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging. 2020;11(1):91. doi:10.1186/s13244-020-00887-2
55. Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold?. Front Oncol. 2020;9:1563. doi:10.3389/fonc.2019.01563
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
