Back to Journals » Journal of Inflammation Research » Volume 16
Targeting Ferroptosis in Bone-Related Diseases: Facts and Perspectives
Authors Chen H, Han Z, Wang Y, Su J, Lin Y, Cheng X, Liu W, He J, Fan Y, Chen L, Zuo H
Received 24 July 2023
Accepted for publication 12 October 2023
Published 18 October 2023 Volume 2023:16 Pages 4661—4677
DOI https://doi.org/10.2147/JIR.S432111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Haoran Chen,1,2,* Zhongyu Han,2,* Yi Wang,1,* Junyan Su,3 Yumeng Lin,4 Xuhua Cheng,1 Wen Liu,1 Jingyu He,5 Yiyue Fan,6 Liuyan Chen,2 Houdong Zuo1
1Department of Orthopaedics, Chengdu Xinhua Hospital, Chengdu, 610000, People’s Republic of China; 2School of Medical and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, 610000, People’s Republic of China; 3Department of Orthopaedics, The First People’s Hospital of Longquanyi District, Chengdu, 610000, People’s Republic of China; 4School of Ophthalmology, Chengdu University of Traditional Chinese Medicine, Chengdu, 610000, People’s Republic of China; 5Sichuan Judicial and Police Officers Professional College, Deyang, 618000, People’s Republic of China; 6Affiliated Hospital of North Sichuan Medical College, Nanchong, 637000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Houdong Zuo, Department of Orthopaedics, Chengdu Xinhua Hospital, Chengdu, 610000, People’s Republic of China, Email [email protected] Liuyan Chen, School of Medical and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, 610000, People’s Republic of China, Email [email protected]
Abstract: Ferroptosis is a new cell fate decision discovered in recent years. Unlike apoptosis, autophagy or pyroptosis, ferroptosis is characterized by iron-dependent lipid peroxidation and mitochondrial morphological changes. Ferroptosis is involved in a variety of physiological and pathological processes. Since its discovery, ferroptosis has been increasingly studied concerning bone-related diseases. In this review, we focus on the latest research progress and prospects, summarize the regulatory mechanisms of ferroptosis, and discuss the role of ferroptosis in the pathogenesis of bone-related diseases, such as osteoporosis (OP), osteoarthritis (OA), rheumatoid arthritis (RA), and osteosarcoma (OS), as well as its therapeutic potential.
Keywords: ferroptosis, cell death, iron accumulation, lipid peroxidation, bone-related diseases
Introduction
Cell fate decision (CFD) is an important mechanism for multicellular organisms to maintain biological stability. Under physiological or pathological conditions, the body maintains homeostasis through different CFDs. Abnormal regulation of CFDs also contributes to a variety of diseases. Currently, known CFDs include apoptosis, necrosis, necroptosis, autophagy, pyroptosis, ferroptosis, cuproptosis, etc.1 In 2012, Dixon et al found that Ras-selective lethal (RSL) compounds RSL3 and Erastin can induce cell death in RAS mutant cell lines through a unique iron-dependent CFD and termed it Ferroptosis.2 Unlike other CFDs, ferroptosis cells morphologically exhibit distinctive mitochondrial structural abnormalities, including shrunken mitochondrial, reduced mitochondrial cristae, and increased mitochondrial membrane density.3 Ferroptosis is mainly due to excessive accumulation of intracellular iron and iron-dependent increase in reactive oxygen species (ROS) and lipid peroxidation, which eventually leads to cell membrane rupture, contents efflux and necrosis-like CFD.4 Therefore, various molecules and signals related to iron metabolism and peroxidation are critical for regulating ferroptosis.
Ferroptosis has been explored to play important roles in different diseases such as cancers, cardiovascular diseases, and kidney diseases.5–7 Recent studies have shown that ferroptosis is closely related to bone-related diseases, but the underlying mechanisms still need to be further studied.8 In the following sections, we focus on the relevant mechanism of ferroptosis and the role of ferroptosis in different bone-related diseases, such as osteoporosis (OP), osteoarthritis (OA), rheumatoid arthritis (RA), and osteosarcoma (OS), aiming to provide a theoretical basis for further research on the pathogenesis and treatment of ferroptosis in bone-related diseases.
Inducers of Ferroptosis
Iron overload is necessary for the occurrence of ferroptosis. Iron is an essential trace element for the human body and participates in a variety of life processes, such as nucleic acid metabolism, cell cycle and enzyme synthesis, etc. Dietary sources of iron mainly include heme iron and non-heme iron. Non-heme iron can be derived from iron ions or ferritin, etc.9 Iron ions are usually obtained from food in ferrous (Fe2+) or ferric (Fe3+) form. Fe3+ is reduced to Fe2+ by different ferrireductases in the intestine such as duodenal cytochrome B (DCYTB), and then Fe2+ enters enterocytes via divalent metal transporter 1 (DMT1) on the apical membrane.10 Ferritin iron and heme iron can enter enterocytes via ferritin receptor and CD91, respectively.11 In enterocytes, Fe2+ not involved in biological processes can be bound to ferritin for storage.12 As required, Fe2+ can leave enterocytes via ferroportin 1 (FPN1) and be oxidized to Fe3+ by ferroxidase hephaestin (HEPH) for transport.9,13 Transferrin (TF) produced from the liver is responsible for transporting Fe3+, and one TF can bind two Fe3+.14 Fe3+-TF transports to target cells and binds to its receptor transferrin receptor (TFR1), then subsequently transports Fe3+ to endosomes.15 In the acidic environment of endosomes, Fe3+ is reduced to Fe2+ by metalloreductases such as six transmembrane epithelial antigen of the prostate 3 (STEAP3) and subsequently transported into the cytoplasm via DMT1.16
Intracellular Fe2+ needs to be strictly regulated. Intracellular Fe2+ deficiency restricts various biological processes. Intracellular Fe2+ excess leads to the Fenton reaction-excess Fe2+ reacts with hydrogen peroxide (H2O2).17 Fenton reaction generates ROS, and iron overload and ROS accumulation can lead to ferroptosis.18 Excess Fe2+ also promotes lipid peroxidation by participating in the catalytic subunit of lipoxygenase (LOX).10,17 Excess intracellular Fe2+ can be oxidized to Fe3+ by ferritin and stored as ferritin heavy chain 1 (FTH1) or ferritin light chain (FTL).19 This iron-binding ferritin can be degraded by nuclear receptor coactivator 4 (NCOA4) and release free iron.20 Cells can also expel excess Fe2+ via FPN1. FPN1 and its regulator hepcidin are essential for iron regulation, and FPN1 is currently the only iron export pathway.21 Abnormal expression or function of these proteins can lead to increased intracellular labile iron (Figure 1).
Iron-dependent lipid peroxidation is another important process of ferroptosis. Phospholipid (PL) is one of the main components of cell membranes. PLs can bind different fatty acyl chains to their sn1 and sn2 sites to increase their diversity.22 Polyunsaturated fatty acyl (PUFA) can combine with the sn2 site of PL to form PUFA-PLs after being catalyzed by acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3).23 ACSL4 or LPCAT3 knockout results in a marked reduction in PUFA-PLs production.24,25 PUFA-PLs can increase the fluidity of cell membranes and maintain the normal physiological function of cells.26 However, PUFA-PLs are also important substrates of lipid peroxidation. PUFAs contain bis-allylic, which can be easily stripped a hydrogen atom by strong oxidants and form a phospholipid radical (PL•), and subsequently bind to an oxygen molecule to form a phospholipid peroxyl radical (PLOO).27 Importantly, PLOO• can rob a hydrogen atom from the bis-allylic of another PUFA-PL, leading to the formation of PUFA-PL-OOH and the formation of another PL.28 The continuous production and accumulation of PUFA-PL-OOH driven by the labile iron pool and ROS will disrupt the integrity of the cell membrane and lead to ferroptosis.29 LOXs can also drive PUFA-PLs peroxidation and promote ferroptosis.29
Inhibitors of Ferroptosis
Under physiological conditions, cells can neutralize lipid peroxidation in time to prevent overload. Glutathione peroxidase 4 (GPX4) belongs to the glutathione peroxidase (GPXs) family and is the main detoxifying enzyme of PL-OOH. GPX4 can convert PL-OOH to non-toxic phospholipid alcohol (PL-OH) and simultaneously convert glutathione (GSH) to glutathione disulfide (GSSG).30 GSH is an important cofactor for the lipid peroxidation detoxification of GPX4, which is inhibited when GSH biosynthesis is reduced.31 GSH is composed of glutamate, glycine and cysteine, of which cysteine needs to be ingested extracellular and is the rate-limiting precursor for GSH biosynthesis.32 Intracellular cysteine acquisition depends on cystine/glutamate reverse transporter (System XC-), whose core components are light chain subunit solute carrier family 7 member 11 (SLC7A11) and heavy chain subunit solute carrier family 3 member 2 (SLC3A2). SLC7A11 can exchange intracellular glutamate and extracellular cystine in a 1:1 ratio.33 Cystine that enters cells can be reduced to cysteine and participate in GSH synthesis.34 Up to this, GSH biosynthesis and GSH-dependent PL-OOH detoxification of GPX4 constitute the main ferroptosis defense system: SLC7A11-GSH-GPX4 axis and inhibition of SLC7A11-GSH-GPX4 axis will lead to ferroptosis. For example, Dixon et al found that RSL3 and Erastin lead to intracellular PL-OOH overload and ferroptosis by inhibiting GPX4 and system XC-, respectively.2
In addition to SLC7A11-GSH-GPX4 axis, cells are able to inhibit ferroptosis through parallel pathways. Ubiquitin (UQ, coenzyme Q10, CoQ10) is a lipophilic molecule that is widely presented in various cells. CoQ10 is mainly synthesized in mitochondria, has redox activity, and is participated in electron transport in the mitochondrial respiratory chain.35 CoQ10 can be cycled in both oxidized and partially/fully reduced states, and its fully reduced form ubiquinol (CoQ10H2) has strong antioxidant properties, which can consume lipophilic free radicals and inhibit ferroptosis. Ferroptosis suppressor protein 1 (FSP1) is a ubiquitin reductase that can deplete NAD(P)H and reduce CoQ10 to CoQ10H2.36 FSP1 structurally has a characteristic N-terminal canonical myristoylation motif, which may aid in the localization of FSP1 to the plasma membrane.36 Alterations in this motif can lead to aberrant FSP1 plasma membrane targeting and abnormal FSP1 function. FSP1-CoQ10H2 axis forms a ferroptosis defense system parallel to SLC7A11-GSH-GPX4 axis by continuously reducing CoQ10 to CoQ10H2 and directly consuming lipid free radicals. Bersuker et al demonstrated that FSP1 is parallel to GXP4 to defend ferroptosis. FSP1 KO cells showed no change in GSH levels but increased lipid peroxidation. GPX4 KO H460 cells could maintain normal growth through FSP1, while GPX4 KO/FSP1 KO H460 cells could induce severe ferroptosis.37 Doll et al found that FSP1 overexpression attenuated GPX4 KO-induced lipid peroxidation.38
GTP cyclohydrolase-1 (GCH1)-tetrahydrobiopterin (BH4) is another ferroptosis defense axis. BH4 is an important cofactor with strong antioxidant properties, which can directly reduce lipid peroxidation and promote the synthesis of CoQ10.39 GCH1 is the rate-limiting enzyme that catalyzes GTP to BH4.40 Several studies have demonstrated that GCH1-BH4 axis prevents ferroptosis through lipid remodeling (Figure 2).39,41–43
Regulation of Ferroptosis
p53 Pathway
p53 is an important tumor suppressor gene involved in various CFDs such as apoptosis, senescence, mitotic catastrophe and ferroptosis.1 p53 plays a dual role in ferroptosis, possibly related to the level of stress. p53 promotes cell survival under low levels of stress and induces various cell fate decisions such as ferroptosis under high levels of stress. SLC7A11 is a target of p53, and several studies have shown that p53 can inhibit SLC7A11 expression and GSH biosynthesis and promote ferroptosis, which is an effective way to induce tumor cell death.44–46 The acetylation-defective mutant of p53, p533KR, which has three arginine residues K117R, K161R and K162R, can precisely inhibit SLC711A and do not involve in other CFDs.47 p53 can also promote the expression of arachidonate lipoxygenase 15 (ALOX15) and induce ACSL4-independent lipid peroxidation through p53-spermidine/spermine N1-acetyltransferase 1 (SAT1)-ALOX15 axis.48 p53 can also promote the transcription of glutaminase 2 (GLS2) and promote ferroptosis.49 GLS2 is involved in glutaminolysis, converting glutamate to α-ketoglutarate and increasing lipid ROS. GLS2 also enhanced GSH and antioxidant functions, but it was not sufficient to counteract the promotion of lipid ROS.50
p53 exerts an anti-ferroptosis effect through p21 promoting and dipeptidyl peptidase-4 (DDP4) reducing.51 p21 regulates the cell cycle and is involved in CFDs such as senescence and mitotic catastrophe.1 p53 can promote p21 transcription, increase GSH production, and delay ferroptosis.52 p53 can bind to DDP4 and promote nuclear accumulation, inhibiting its function. p53 inhibition can lead DDP4 inter the plasma membrane and combine with NADPH oxidase 1 (NOX1) to promote lipid peroxidation and ferroptosis.53
Keap1-Nrf2-HO-1 Pathway
Nuclear factor E2-related factor 2 (Nrf2) is an important transcription factor for cellular antioxidant regulation, and Kelch-like ECH-associated protein 1 (Keap1) is a major negative regulator of Nrf2.54 Under physiological conditions, Keap1 binds and inactivates Nrf2, maintaining intracellular Nrf2 at low levels. Under oxidative stress conditions or when Keap1 is artificially inhibited (such as p62), Nrf2 is released from Keap1.55 Nrf2 then translocates to the nucleus and binds to the antioxidant response element (ARE), activating various downstream antioxidant pathways to maintain intracellular redox homeostasis. Nrf2 regulates ferroptosis at multiple steps. Nrf2 controls FTL/FTH1 expression and regulates intracellular free iron concentration.56 SLC7A11 is also regulated by Nrf2, which can promote cystine uptake and GSH synthesis.57 Nrf2 also promotes ferroptosis SLC7A11-GSH-GPX4 defense axis by inducing GPX4 synthesis.58 Nrf2 promotes NADPH regeneration and plays an antioxidant role.59
Heme oxygenase-1 (HO-1), as one of the key downstream of Nrf2, possesses some features of Nrf2, and Nrf2-HO-1 axis can promote the production of GPX4 and NADPH.60 However, HO-1 can degrade hemosiderin to biliverdin, CO, and Fe2+, resulting in an increase in free iron and may promote ferroptosis.61 Tang et al found that elevated HO-1 promoted ferroptosis in retinal pigment epithelium cells.62
AMPK Pathway
AMP-activated protein kinase (AMPK) is an important sensor and regulator of cellular energy balance. AMPK can sense the intracellular AMP-ATP ratio and is activated in response to the elevation of AMP.63 Activated AMPK promotes complex downstream pathways, reducing ATP consumption and increasing ATP production to restore AMP-ATP balance and maintain cell physiological function. AMPK also regulates mitochondrial biosynthesis, dynamics, and mitophagy.64 Due to the complexity and phosphorylation activation level of the downstream targets, AMPK may promote or inhibit ferroptosis. Song et al found that AMPK promotes phosphorylation of downstream BECN1 (beclin 1) at S90 and S93, which combines with SLC7A11 to inhibit system XC- and promote ferroptosis.65 AMPK can also inhibit stearoyl-CoA desaturase-1 (SCD1), an enzyme that catalyzes the production of monounsaturated fatty acyl (MUFA), which can replace PUFA and inhibit ferroptosis.66,67 Several studies have shown that AMPK can induce the activation and nuclear translocation of Nrf2, promote cellular antioxidant function and inhibit ferroptosis.68,69 Lee et al found that AMPK activation inhibited acetyl-CoA carboxylases 1 (ACC1).70 ACC1 is involved in de novo lipid synthesis, and its inhibition reduces PUFA biosynthesis and inhibits ferroptosis.71 Contrary conclusions confirm the complexity of AMPK reticular pathways. Based on the importance of AMPK in mitochondria, further studies are needed to explore the specific role of AMPK in ferroptosis.
Hippo Pathway
Studies have shown that increasing the density of individual cells that are sensitive to ferroptosis increases their ferroptosis resistance, and this alteration is closely related to cell–cell contact and activation of Hippo pathway.72–74 Hippo pathway is regulated by cell–cell contact, physical and biochemical signals, and controls a variety of biological processes such as cell proliferation, CFDs, and organ size.75 Hippo pathway consists of several core components, which constitute signal modules to regulate the downstream effector transcription coregulators Yes-associated protein 1 (YAP)/WW domain-containing transcription regulator protein 1 (TAZ).76 Hippo pathway inhibition leads to the dephosphorylation and nuclear translocation of YAP/TAZ, which subsequently binds to TEA-domain transcription factor (TEAD) and promotes cell proliferation; high cell density activates Hippo pathway, leading to phosphorylation and inhibition of YAP/TAZ, inhibiting cell proliferation, promoting apoptosis, and controlling organ size.77 Several stages of ferroptosis, such as TFR1, ACSL4 and NOX2/4, are regulated by Hippo pathway.74,78–80 Studies have shown that overexpression of E-cadherin in mesothelioma and renal cell carcinoma mediates cell–cell contact and neurofibromatosis 2 (NF2) activation, leading to Hippo pathway activation and YAP/TAZ inhibition, promoting ferroptosis.72,81 Therefore, YAP/TAZ-TEAD complex is an important target for regulating ferroptosis. Furthermore, the role of specific components in the core cascade of Hippo pathway in ferroptosis remains to be elucidated.
PI3K-Akt-mTOR Pathway
Phosphatidylinositol-3 kinase (PI3K)-protein kinase B (Akt)-mammalian target of rapamycin (mTOR) pathway plays an important role in cell survival, proliferation and other physiological processes.82 PI3K-Akt-mTOR pathway is frequently abnormal in cancer, and activating mutations in PI3K or inactivation of tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) can confer ferroptosis resistance in tumor cells.27,83 Further studies showed that PI3K-Akt-mTOR pathway inhibited lipid peroxidation and promoted GPX4 expression. Sterol regulatory element-binding protein 1 (SREBP-1) is a key transcription factor involved in lipogenesis, and SCD1 is its important target. PI3K-Akt-mTOR pathway promotes SREBP-1 transcription and SCD1 expression, leading to an increased MUFA and inhibition of PUFA-PL-OOH.84–86 In addition, Zhang et al showed that mTORC1 can activate eukaryotic initiation factor 4E (eIF4E)-binding proteins (4EBPs) to promote GPX4 synthesis.87 Taken together, targeting PI3K-Akt-mTOR pathway or SCD1 will contribute to the regulation of ferroptosis (Table 1 and Figure 2).
 |
Table 1 Several Regulators of Ferroptosis |
Ferroptosis and Osteoporosis
Bone is an important tissue of the human body, which plays roles in the movement, protection of internal organs and endocrine regulation, etc.88 Bone cells are mainly composed of osteocytes, osteoblasts (OB), and osteoclasts (OC).89 Osteocytes are the major cells of bones, and more than 90% of adult bone cells are osteocytes.90 OBs, which are derived from mesenchymal stem cells (MSC), are cells that produce bone matrix and dominate bone-forming.91 Osteocytes can considered to be fully differentiated OBs.92 OCs are derived from monocyte/macrophage lineage of hematopoietic cells and contain abundant mitochondria and lysosomes and can lead to bone-resorbing.93 The homeostasis of OCs and OBs promotes the balance of bone-resorbing and bone-forming, promotes bone-remodeling, and makes bone a dynamic tissue to maintain its normal function.94
Osteoporosis (OP) is a common metabolic bone disease. Due to abnormal OBs-OCs homeostasis, bone-forming is weakened and bone-resorbing is enhanced, resulting in decreased bone mass, increased bone brittleness, and poor healing ability after fracture.95 According to the causes, OP is divided into primary, secondary and idiopathic. Primary OP mainly includes senile OP (SOP) and postmenopausal OP (PMOP), while secondary OP is usually diabetic OP (DOP) or glucocorticoid-induced OP (GIOP).96 Many studies indicate an association between ferroptosis and OP. An in vitro study confirmed the inhibitory effect of iron on bone-remodeling. Co-cultured iron-containing body fluids with hydroxyapatite crystals, the calcium concentration of hydroxyapatite significantly decreased as the iron concentration increased.97 In addition, iron overload produces a large number of ROS through the Fenton reaction, which affects bone metabolism and has different effects on OBs, OCs and osteocytes.98
Bone marrow-derived mesenchymal stem cells (BMSCs) are an important source of OBs. BMSCs are stem cells with the ability to differentiate into OBs, chondrocytes, and adipocytes, and other cells.99 Runt-related transcription factor 2 (Runx2) is an important transcription factor regulating BMSCs differentiation into OBs. Activation of Runx2 and expression of osteocalcin (OCN) and alkaline phosphatase (ALP) promote osteogenic differentiation.100 Iron overload increased the expression of ferritin in BMSCs in an iron dose-dependent manner, decreased the expression of Runx2, OCN, and ALP, and inhibited the osteogenic differentiation of BMSCs.101 Lan et al found that quercetin inhibited BMSCs ferroptosis and promoted the expression of Runx2 and ALP and osteogenic differentiation by inhibiting the PI3K-Akt-mTOR pathway.102 For mature osteoblasts, ferroptosis can lead to phenotypic and functional inhibition of OBs, which has been demonstrated in vivo and in vitro.103,104 Several studies showed the ability of Nrf2-GPX4, Nrf2-HO-1, AMPK-SIRT1 and other pathways to regulate OB ferroptosis and promote bone-forming.105–107 Tian et al found that ferric ion treatment could promote the increase of ferroptosis indicator NOX4 in MC3T3-E1 cells and also apoptosis indicators: increased caspase-3 and Bax and decreased Bcl-2.108
Osteoclast differentiation requires the activation of the receptor activator of the nuclear factor-κB (RANK)-RANKL pathway. RANKL is produced by OBs and binds to RANK on the surface of osteoclast precursor cells, promotes the differentiation of osteoclast precursor cells into OCs, and also inhibits OCs apoptosis.109 Studies have indicated that iron overload can promote RANKL expression and promote osteoclast differentiation.110,111 Ni et al found that, due to the decreased activity of aconitase, RANKL activation can lead to the increase of TFR1 and the NOCA4-induced ferritinophagy of FTH1, increase the free iron content in OCs and Fenton reaction, which may be one of the regulations to inhibit the overproduction of OCs.112 However, under hypoxic conditions, the activation of hypoxia-inducible factors (HIF-1α) inhibits FTH1 ferritinophagy and OCs ferroptosis, and inhibition of HIF-1α can induce OCs ferroptosis and alleviate OP.112 Thus, hypoxic environments, such as medullary cavities and growth plates, would contribute to the resistance of osteoclasts to ferroptosis, and targeting HIF-1α may be a potential therapeutic direction of OP. In addition, postmenopausal estrogen deficiency leading to reduced inhibition of estrogen on HIF-1α and activation of OCs may be one of the causes of PMOP.113
Compared to OBs and OCs, there have been rare studies on ferroptosis associated with osteocytes in the context of osteoporosis. However, as the most abundant cells in bone, osteocyte ferroptosis undoubtedly plays a role in the pathogenesis of OP. DOP is closely related to abnormal glucolipid homeostasis, and the deposition of glucolipid metabolites was observed in the bone tissue of DOP. Yang et al found that the type 2 diabetes microenvironment promotes the transcription of HO-1 through Nrf2/c-Jun as well as GPX4 reduction and the occurrence of osteocyte ferroptosis, which promotes DOP.114 In addition, inhibition of osteocyte ferroptosis with ferrostatin-1 (Fer-1) or iron chelator desferrioxamine (DFO) can rescue DOP. In addition, Yang et al found that iron overload can induce osteocyte apoptosis and subsequently promote increased secretion of osteocytic RANKL, increasing osteoclast activation and leading to OP.111 In summary, iron overload and ferroptosis significantly promote bone-resorbing and reduce bone-forming. How to reduce osteoblasts and osteocytes ferroptosis or promote osteoclasts ferroptosis should be a focus of future research on osteoporosis (Figure 3).
Ferroptosis and Osteoarthritis
Osteoarthritis (OA) is a common degenerative disease that can cause pain, dysfunction, and deformity. The main mechanisms of OA are cartilage degeneration, cartilage extracellular matrix degradation, synovium inflammation, and subchondral bone sclerosis and hyperplasia.115 The progressive degeneration of cartilage plays an important role in the progression of OA. Chondrocytes are the only cell type in cartilage and can produce extracellular matrix (ECM).116 ECM is mainly composed of water, collagen and proteoglycans, which encapsulates chondrocytes and forms a closed environment.117 This closed environment lacks nerves and blood vessels, so the repair capacity of chondrocytes is poor.
Chondrocyte ferroptosis plays a role in the pathogenesis of OA. Genome-wide RNA-Seq data and several studies have shown a marked reduction of GPX4 in OA cartilage.118–120 The decrease of SLC3A2 in OA cartilage has also been reported.121 Miao et al found that endochondral GPX4 and SLC3A2 were reduced in OA patients, and Fer-1 and DFO can inhibit chondrocyte ferroptosis and OA. Besides, GPX4 inhibition also promoted ECM degradation through the MAPK/NF-κB pathway.118 Yan et al found that Erastin promoted chondrocyte ferroptosis and inhibited the expression of type II collagen (the major collagen protein in the ECM). Fer-1 reversed this process and activated the Nrf2-GPX4 and Nrf2-HO-1 pathways.122 Activation of the Nrf2-GPX4 pathway can ameliorate Erastin-induced ferroptosis in OA chondrocytes.119 In another study, metformin reversed Erastin-induced ferroptosis and p53 pathway activation in OA chondrocytes.123 The AMPK pathway is also involved in the regulation of chondrocyte activity and OA.124 Baicalein promotes the stability of AMPK and nuclear translocation of Nrf2, activates the AMPKα-Nrf2-HO-1 pathway, and alleviates the progression of OA.125 Zhou et al found that in addition to mediating chondrocyte CFDs such as apoptosis and autophagy, HIF-2α inhibited SLC7A11 and GPX4 and promoted lipid peroxidation to mediate chondrocyte ferroptosis.126 D-mannose inhibited HIF-2α and attenuated the above process and OA progression.
Excessive mechanical stimulation is a contributing factor to OA. Piezo1 is an important mechanically-sensitive ion channel in vertebrates and is involved in iron metabolism.127 Wang et al found that excessive mechanical stimulation activated piezo1 channels and increased Ca2+ influx, leading to GSH reduction and GPX4 inhibition, promoting chondrocyte ferroptosis and OA. In addition, FSP1 and CoQ10 can inhibit ferroptosis in parallel with GPX4, indicating that piezo1 induces chondrocyte ferroptosis mainly by inhibiting the SLC7A11-GSH-GPX4 axis.128
Synovium inflammation is also an important factor in the progression of OA. Various inflammatory mediators such as TNF-α, IL-1β, IL-6, cyclooxygenase-2 (COX-2) and NO can promote cartilage injury, and some inflammatory mediators are associated with chondrocytes ferroptosis, such as IL-1β and IL-6.129 Gong et al found that IL-1β played a role in intracellular iron overload, GPX4 inhibition, and ROS production. Cardamonin and DFO could rescue IL-1β-induced chondrocyte ferroptosis and OA through the p53-SLC7A11-GPX4 axis.130 IL-6 can induce chondrocyte ferroptosis by up-regulating hepcidin to inhibit intracellular iron export, increase ROS, and reduce GPX4 activity.131 In intervertebral disc degeneration, miR-10a-5p can inhibit IL-6 and reduce chondrocyte ferroptosis.131
Overall, ferroptosis plays an important role in the pathogenesis of OA, but the specific mechanism still needs to be clarified. Targeting inhibition of chondrocyte ferroptosis, especially the GSH-GPX4 axis, is one of the potential therapeutic directions for OA (Figure 3).
Ferroptosis and Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a common autoimmune disease. RA is characterized by immune cell infiltration and synovial fibroblast proliferation, leading to the destruction of cartilage and bone, forming aggressive arthritis.132
Ferroptosis is closely related to the pathogenesis of RA. Compared with healthy people, the synovial iron content of RA and OA patients is higher, especially in RA patients.133 Infiltrating immune cells produce various proinflammatory cytokines, such as IL-1β, IL-6 and TNF-α, which improve hepcidin expression and change the glycosylation pattern of transferrin, promote intracellular iron storage and reduce hemoglobin levels.134,135 This may lead to a redistribution of iron in the body, leading to iron deposition in the synovium but iron deficiency anemia in RA patients. Etanercept reversed iron deficiency anemia in RA by down-regulating hepcidin expression.136 In addition, RA patients also showed elevated ROS levels and lipid peroxidation in the synovium.137,138 Importantly, ROS is a key factor to promote the progression of RA. ROS stimulates the continuous production of TNF-α through the NF-κB pathway, forming a ROS/TNF-α positive feedback.138
Fibroblast-like synoviocytes (FLSs) are important cells in RA, which can produce cytokines and promote neovascularization and matrix degradation to promote RA progression.139 It has been mentioned that iron overload and excessive ROS can promote OC differentiation and bone resorption, as well as chondrocyte ferroptosis and type II collagen destruction.140,141 In this case, FLSs ensure normal survival and proliferation through their resistance to ferroptosis. Although ROS was increased in RA FLSs, SLC7A11, GPX4, FTH1 were increased and ACSL4 was decreased, resulting in an increase in free iron and antioxidant/oxidative capacity.142 Wu et al found that in collagen-induced arthritis (CIA), TNF promotes SLC7A11 expression and GSH biosynthesis in FLSs, increasing ferroptosis resistance.143 In addition, macrophages showed a protective effect against ferroptosis to FLSs when treated with RSL3.143 Cheng et al found that increased Semaphorin 5A (SEMA5A) produced by synovial CD68+ macrophages in RA patients promoted GPX4 and SCD1 expression through the PI3K-Akt-mTOR pathway and inhibited lipid peroxidation and ferroptosis of FLSs.144 Due to the resistance of FLS to ferroptosis and the redistribution of iron, iron supplementation in RA patients with iron deficiency anemia may lead to exacerbation of RA.145
Targeting ferroptosis has already shown positive effects in RA. Icariin can promote the SLC7A11-GSH-GPX4 axis to alleviate ferroptosis and lipopolysaccharide-induced synovitis.146 Several studies anticipated that activation of the FSP1-CoQ10H2 axis might help to suppress ROS/TNF-α positive feedback and attenuate RA.138,147 Galectin-1 derived peptide 3 (G1dP3) showed an inhibitory effect on FLS cell line MH7A cells. G1dP3 promotes ferroptosis of MH7A cells by activating the p53-SLC7A11 axis, and the knockout of p53 abolished the inhibitory effect of G1dP3 on MH7A cells.148 Xiang et al identified SLC2A3 as a possible marker of RA. RSL3 could cause SLC2A3, SLC7A11, GPX4, and FTH1 inhibition in RA FLSs and promote ferroptosis.149 The combination of TNF blocker etanercept and ferroptosis inducer imidazole ketone erastin can promote FLS ferroptosis and alleviate CIA.143 Glycine, an important component of GSH, also promotes the expression of S-adenosylmethionine (SAM). SAM promoted the GPX4 promoter methylation and inhibited GPX4 expression. A study showed that both glycine and SAM treatment can promote ferroptosis of FLS cells and improve CIA.142 Glycine also inhibited FTH1 expression and increased intracellular free iron in FLSs. Transient receptor potential melastatin 7 (TRPM7) is highly permeable to Ca2+ and Mg2+, and TRPM7 channel activation promotes intracellular Ca2+ and ROS accumulation.150,151 Zhou et al found that TRPM7 channel is elevated in RA patients and adjuvant arthritis (AA) and promotes chondrocyte ferroptosis via the PKCα-NOX4 axis to promote RA and AA.152 Although studies on osteoblastic ferroptosis in RA are still lacking, there is no doubt that FLS, OCs and chondrocytes have different sensitivity to ferroptosis in RA. A large number of studies are still needed to explore the unique mechanisms of ferroptosis in different cells in arthritis and provide guidance for more specific targeted therapies for RA (Figure 3).
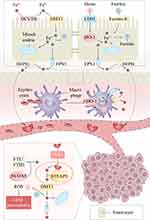
Ferroptosis and Osteosarcoma
Osteosarcoma (OS), a familiar primary malignant bone tumor, originated from primitive mesenchymal cells and is aggressive and prone to lung metastasis.153 At present, the treatment of OS includes surgery, radiotherapy, chemotherapy, neoadjuvant chemotherapy, etc. However, the treatment effect of metastatic, recurrent and drug-resistant OS is not satisfactory.154 Inducing tumor cell death and inhibiting tumor cell proliferation is an important part of cancer treatment.155 Targeted induction of ferroptosis in tumor cells has brought new opportunities for the treatment of OS.
Studies showed that Sulfasalazine, Tirapazamine and miRNA-1297-5p can promote OS cell ferroptosis by inhibiting SLC7A11-GSH-GPX4 axis.156–158 KDM4A knockdown inhibited H3K9me3 demethylation in SLC7A11 promoter region and reduced SLC7A11 expression in OS cells.159 Baicalin can bind to Nrf2 and promote Nrf2 degradation, inducing MG63 and 143B ferroptosis through the Nrf2-SLC7A11-GPX4 axis.160 RNA sequencing analysis revealed that zoledronic acid upregulated P450 oxidoreductase (POR), leading to excessive ROS and lipid peroxidation in OS cells.161 Lv et al found that β-Phenethyl isothiocyanate (PEITC) can promote multiple CFDs in OS cells, such as apoptosis, autophagy and ferroptosis.162 PEITC can promote the increase of intracellular free iron and ROS and the decrease of GSH, which may be related to the activation of mitogen-activated protein kinase (MAPK) signaling.163 EF24, an antitumor compound, has been shown to induce tumor cell death in osteosarcoma cell line U2os and Saos-2.164 This process is inhibited by Fer-1 but not by inhibitors of apoptosis, autophagy, or necroptosis. Further study found EF24 promotes HMOX1 and HO-1 expression and inhibits GPX4 expression, improving ROS accumulation and lipid peroxidation.
As an important tumor suppressor, p53 regulates multiple CFDs. p53 is frequently inactivated in osteosarcoma, leading to resistance of OS cells to apoptosis and ferroptosis.165 Studies have shown that p53 regulates typical and atypical ferroptosis, and p53-SLC7A11 binding is the primary mechanism. Mutation or inactivation of p53 can inhibit p53-SLC7A11 binding and ferroptosis in OS cells.166 p53 also regulates cellular ROS, iron and lipids. Flavonoids bavachin can inhibit signal transducer and activator of transcription 3 (STAT3) phosphorylation, promote p53 and inhibit SLC7A11 expression through STAT3-p53-SLC7A11 axis. In addition, bavachin increased intracellular free iron and inhibited the proliferation of OS cell lines MG63 and HOS. DFO and Fer-1 reversed bavachin-induced ferroptosis.44 STAT3 phosphorylation inhibition also impaired Nrf2-GPX4 axis to promote OS cell ferroptosis and increase the sensitivity to cisplatin.167 Fanconi anemia complementation group D2 (FANCD2) can inhibit JAK2-STAT3 pathway and ferroptosis and promote temozolomide resistance in OS cells.168 Hypoxia is a characteristic of solid tumors such as osteosarcoma and is closely related to drug resistance.169 An ultrasound-activatable DOX-Fe(VI)@HMS-HE-PEG (DFHHP) nanomedicine developed based on reoxygenation and ferroptosis has been confirmed to induce apoptosis and ferroptosis in hypoxic Saos-2 cells and promote doxorubicin chemotherapy efficacy by inhibiting HIF-1α, MDR1, P-pg, GSH, GPX4 and promoting Fenton reaction.170
Overall, ferroptosis as an emerging CFD provides a strong direction for the treatment of OS, but more studies are still needed to explore the specific role of ferroptosis in OS cells and how to reduce the side effects on normal cells. Some studies have identified several genes associated with OS ferroptosis by bioinformatics analysis, which may provide research directions for this purpose (Figure 3).171–175
Conclusion
As a novel CFD, ferroptosis is tightly regulated at different levels and is involved in the pathogenesis of many diseases. Since its discovery in 2012, ferroptosis has been increasingly investigated in bone-related diseases. In this manuscript, we summarize the main regulatory mechanisms of ferroptosis and its role in bone-related diseases. In general, ferroptosis plays an important role in a variety of bone-related diseases such as OP, OA, RA and OS. The different responses of different cells to ferroptosis, such as OBs, OCs, BMSCs, chondrocytes, FLSs and osteosarcoma cells, lead to the pathological progression of various bone-related diseases. Interfering with the ferroptosis pathway in these cells shows great clinical potential in the treatment of bone-related diseases. However, current studies still remain many gaps. Some progress has been made in the application of ferroptosis inhibitors or inducers, ferroptosis inhibitors such as FSP-1, Fer-1, and DFO have shown therapeutic potential in bone-related diseases, but most of these studies remain in animal or in vitro experiments, and relevant clinical studies are still lacking.118,138,176 Because different cells (OBs, OCs, BMSCs, chondrocytes, FLSs, OS cells) have different tolerance to ferroptosis, ferroptosis is a double-edged sword in bone-related diseases. Clinical studies are needed to explore how to use ferroptosis inducers or inhibitors in specific bone-related diseases. In addition, ferroptosis and other CFDs such as apoptosis and autophagy constitute cell fate regulatory networks, more studies are needed to explore their interactions in bone-related diseases. Finally, iron is also an essential element for normal cells. How to specifically target ferroptosis in bone-related diseases while reducing the damage to normal cells and organs is also a challenge for subsequent research.
In conclusion, ferroptosis is closely related to bone-related diseases such as osteoporosis, osteoarthritis, rheumatoid arthritis, and osteosarcoma. An in-depth study of ferroptosis-related regulatory mechanisms will provide new targets for the diagnosis and treatment of bone-related diseases.
Abbreviations
ACSL4, acyl-CoA synthetase long-chain family member 4; ALOX15, arachidonate lipoxygenase 15; AMPK, AMP-activated protein kinase; Akt, protein kinase B; BH4, tetrahydrobiopterin; BMSC, bone marrow-derived mesenchymal stem cell; CoQ10, coenzyme Q10; Cys, cysteine; Cys2, cysteine; DCYTB, duodenal cytochrome B; DDP4, dipeptidyl peptidase-4; DMT1, divalent metal transporter 1; ECAD, E-cadherin; ECM, extracellular matrix; 4EBP, eukaryotic initiation factor 4E-binding proteins; Fer-1, ferrostatin-1; FLS, fibroblast-like synoviocyte; FPN1, ferroportin 1; FSP1, ferroptosis suppressor protein 1; FTH1, ferritin heavy chain 1; FTL, ferritin light chain; GCH1, GTP cyclohydrolase-1; GLS2, glutaminase 2; Glu, glutamate; GPX4, glutathione peroxidase 4; GSH, glutathione; GSSG, glutathione disulfide; HEPH, hephaestin; HIF-1α, hypoxia-inducible factors; HO-1, heme oxygenase-1; Keap1, Kelch-like ECH-associated protein 1; LPCAT3, lysophosphatidylcholine acyltransferase 3; mTOR, mammalian target of rapamycin; MUFA, monounsaturated fatty acyl; NCOA4, nuclear receptor coactivator 4; NF2, neurofibromatosis 2; NOX, NADPH oxidase; Nrf2, nuclear factor E2-related factor 2; OB, osteoblast; OC, osteoclast; PI3K, phosphatidylinositol-3 kinase; PL, phospholipid; PLOO•, phospholipid peroxyl radical; PUFA, polyunsaturated fatty acyl; RANKL, receptor activator of the nuclear factor-κB ligand; ROS, reactive oxygen species; Runx2, Runt-related transcription factor 2; SAT1, spermidine/spermine N1-acetyltransferase 1; SCD1, stearoyl-CoA desaturase-1; SEMA5A, semaphorin 5A; SLC7A11, subunit solute carrier family 7 member 11; STAT3, signal transducer and activator of transcription 3; STEAP3, six transmembrane epithelial antigen of the prostate 3; TAZ, WW domain-containing transcription regulator protein 1; TF, transferrin; TFR1, transferrin receptor 1; YAP, yes-associated protein 1.
Funding
The work was founded by Project of Chengdu Municipal Health Commission (2022573).
Disclosure
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
References
1. Chen H, Han Z, Luo Q, et al. Radiotherapy modulates tumor cell fate decisions: a review. Radiat Oncol. 2022;17(1):196. doi:10.1186/s13014-022-02171-7
2. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
3. Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi:10.1038/s41419-020-2298-2
4. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–125. doi:10.1038/s41422-020-00441-1
5. Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381–396. doi:10.1038/s41568-022-00459-0
6. Wang K, Chen XZ, Wang YH, et al. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death Discov. 2022;8(1):394. doi:10.1038/s41420-022-01183-2
7. Wang J, Liu Y, Wang Y, Sun L. The cross-link between ferroptosis and kidney diseases. Oxid Med Cell Longev. 2021;2021:6654887. doi:10.1155/2021/6654887
8. Zhang Y, Huang X, Qi B, et al. Ferroptosis and musculoskeletal diseases: “Iron maiden” cell death may be a promising therapeutic target. Front Immunol. 2022;13:972753. doi:10.3389/fimmu.2022.972753
9. Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2014;307(4):G397–G409. doi:10.1152/ajpgi.00348.2013
10. Huang L, Bian M, Zhang J, Jiang L. Iron metabolism and ferroptosis in peripheral nerve injury. Oxid Med Cell Longev. 2022;2022:5918218. doi:10.1155/2022/5918218
11. Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106(Suppl 6):1559S–1566S. doi:10.3945/ajcn.117.155804
12. Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23(3):95–104. doi:10.1016/j.blre.2008.08.001
13. Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta. 2012;1823(9):1426–1433. doi:10.1016/j.bbamcr.2012.03.004
14. Kawabata H. Transferrin and transferrin receptors update. Free Radic Biol Med. 2019;133:46–54. doi:10.1016/j.freeradbiomed.2018.06.037
15. Capelletti MM, Manceau H, Puy H, Peoc’h K. Ferroptosis in liver diseases: an overview. Int J Mol Sci. 2020;21(14):4908. doi:10.3390/ijms21144908
16. DeGregorio-Rocasolano N, Marti-Sistac O, Ponce J, et al. Iron-loaded transferrin (tf) is detrimental whereas iron-free tf confers protection against brain ischemia by modifying blood tf saturation and subsequent neuronal damage. Redox Biol. 2018;15:143–158. doi:10.1016/j.redox.2017.11.026
17. Lei P, Bai T, Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. doi:10.3389/fphys.2019.00139
18. Morales M, Xue X. Targeting iron metabolism in cancer therapy. Theranostics. 2021;11(17):8412–8429. doi:10.7150/thno.59092
19. Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13(5):342–355. doi:10.1038/nrc3495
20. Santana-Codina N, Gikandi A, Mancias JD. The role of ncoa4-mediated ferritinophagy in ferroptosis. Adv Exp Med Biol. 2021;1301:41–57.
21. Billesbolle CB, Azumaya CM, Kretsch RC, et al. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature. 2020;586(7831):807–811. doi:10.1038/s41586-020-2668-z
22. Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol. 2018;19(5):281–296. doi:10.1038/nrm.2017.138
23. Lei G, Mao C, Yan Y, Zhuang L, Gan B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell. 2021;12(11):836–857. doi:10.1007/s13238-021-00841-y
24. Doll S, Proneth B, Tyurina YY, et al. Acsl4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi:10.1038/nchembio.2239
25. Reed A, Ichu TA, Milosevich N, et al. Lpcat3 inhibitors remodel the polyunsaturated phospholipid content of human cells and protect from ferroptosis. ACS Chem Biol. 2022;17(6):1607–1618. doi:10.1021/acschembio.2c00317
26. Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054–2081. doi:10.1080/15548627.2020.1810918
27. Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82(12):2215–2227. doi:10.1016/j.molcel.2022.03.022
28. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–282. doi:10.1038/s41580-020-00324-8
29. Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966–E4975. doi:10.1073/pnas.1603244113
30. Maiorino M, Conrad M, Ursini F. Gpx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid Redox Signal. 2018;29(1):61–74. doi:10.1089/ars.2017.7115
31. Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter slc7a11/xct at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018;38(1):12. doi:10.1186/s40880-018-0288-x
32. Parker JL, Deme JC, Kolokouris D, et al. Molecular basis for redox control by the human cystine/glutamate antiporter system xc(). Nat Commun. 2021;12(1):7147. doi:10.1038/s41467-021-27414-1
33. Koppula P, Zhuang L, Gan B. Cystine transporter slc7a11/xct in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620. doi:10.1007/s13238-020-00789-5
34. Liu X, Olszewski K, Zhang Y, et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat Cell Biol. 2020;22(4):476–486. doi:10.1038/s41556-020-0496-x
35. Wang Y, Hekimi S. Understanding ubiquinone. Trends Cell Biol. 2016;26(5):367–378. doi:10.1016/j.tcb.2015.12.007
36. Elguindy MM, Nakamaru-Ogiso E. Apoptosis-inducing factor (AIF) and its family member protein, AMID, are rotenone-sensitive NADH: ubiquinone oxidoreductases (NDH-2). J Biol Chem. 2015;290(34):20815–20826. doi:10.1074/jbc.M115.641498
37. Bersuker K, Hendricks JM, Li Z, et al. The coq oxidoreductase fsp1 acts parallel to gpx4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi:10.1038/s41586-019-1705-2
38. Doll S, Freitas FP, Shah R, et al. Fsp1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi:10.1038/s41586-019-1707-0
39. Kraft VAN, Bezjian CT, Pfeiffer S, et al. Gtp cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6(1):41–53. doi:10.1021/acscentsci.9b01063
40. Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. doi:10.1042/bj3470001
41. Soula M, Weber RA, Zilka O, et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol. 2020;16(12):1351–1360. doi:10.1038/s41589-020-0613-y
42. Hu Q, Wei W, Wu D, et al. Blockade of gch1/bh4 axis activates ferritinophagy to mitigate the resistance of colorectal cancer to erastin-induced ferroptosis. Front Cell Dev Biol. 2022;10:810327. doi:10.3389/fcell.2022.810327
43. Wei X, Yi X, Zhu XH, Jiang DS, Lloret A. Posttranslational modifications in ferroptosis. Oxid Med Cell Longev. 2020;2020:8832043. doi:10.1155/2020/8832043
44. Luo Y, Gao X, Zou L, Lei M, Feng J, Hu Z. Bavachin induces ferroptosis through the stat3/p53/slc7a11 axis in osteosarcoma cells. Oxid Med Cell Longev. 2021;2021:1783485. doi:10.1155/2021/1783485
45. Zeng C, Lin J, Zhang K, et al. Sharpin promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/slc7a11/gpx4 signaling. Cancer Sci. 2022;113(11):3766–3775. doi:10.1111/cas.15531
46. Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi:10.1038/nature14344
47. Wang SJ, Li D, Ou Y, et al. Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Rep. 2016;17(2):366–373. doi:10.1016/j.celrep.2016.09.022
48. Ma XH, Liu JH, Liu CY, et al. Alox15-launched PUFA-phospholipids peroxidation increases the susceptibility of ferroptosis in ischemia-induced myocardial damage. Signal Transduct Target Ther. 2022;7(1):288. doi:10.1038/s41392-022-01090-z
49. Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–168. doi:10.1016/j.freeradbiomed.2018.05.074
50. Suzuki S, Venkatesh D, Kanda H, et al. Gls2 is a tumor suppressor and a regulator of ferroptosis in hepatocellular carcinoma. Cancer Res. 2022;82(18):3209–3222. doi:10.1158/0008-5472.CAN-21-3914
51. Liu J, Zhang C, Wang J, Hu W, Feng Z. The regulation of ferroptosis by tumor suppressor p53 and its pathway. Int J Mol Sci. 2020;21(21):8387. doi:10.3390/ijms21218387
52. Tarangelo A, Magtanong L, Bieging-Rolett KT, et al. P53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 2018;22(3):569–575. doi:10.1016/j.celrep.2017.12.077
53. Xie Y, Zhu S, Song X, et al. The tumor suppressor p53 limits ferroptosis by blocking dpp4 activity. Cell Rep. 2017;20(7):1692–1704. doi:10.1016/j.celrep.2017.07.055
54. Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD. Modulating nrf2 in disease: timing is everything. Annu Rev Pharmacol Toxicol. 2019;59(1):555–575. doi:10.1146/annurev-pharmtox-010818-021856
55. Sun X, Ou Z, Chen R, et al. Activation of the p62-keap1-nrf2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi:10.1002/hep.28251
56. Cheng H, Wang P, Wang N, et al. Neuroprotection of nrf2 against ferroptosis after traumatic brain injury in mice. Antioxidants. 2023;12(3):731. doi:10.3390/antiox12030731
57. Dong H, Xia Y, Jin S, et al. Nrf2 attenuates ferroptosis-mediated iir-ali by modulating tert and slc7a11. Cell Death Dis. 2021;12(11):1027. doi:10.1038/s41419-021-04307-1
58. Dang R, Wang M, Li X, et al. Edaravone ameliorates depressive and anxiety-like behaviors via sirt1/nrf2/ho-1/gpx4 pathway. J Neuroinflammation. 2022;19(1):41. doi:10.1186/s12974-022-02400-6
59. Cano M, Datta S, Wang L, et al. Nrf2 deficiency decreases NADPH from impaired IDH shuttle and pentose phosphate pathway in retinal pigmented epithelial cells to magnify oxidative stress-induced mitochondrial dysfunction. Aging Cell. 2021;20(8):e13444. doi:10.1111/acel.13444
60. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of nrf2/ho-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–3247. doi:10.1007/s00018-016-2223-0
61. Rochette L, Zeller M, Cottin Y, Vergely C. Redox functions of heme oxygenase-1 and biliverdin reductase in diabetes. Trends Endocrinol Metab. 2018;29(2):74–85. doi:10.1016/j.tem.2017.11.005
62. Tang Z, Ju Y, Dai X, et al. Ho-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biol. 2021;43:101971. doi:10.1016/j.redox.2021.101971
63. Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313. doi:10.1016/j.cmet.2017.10.009
64. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi:10.1038/nrm.2017.95
65. Song X, Zhu S, Chen P, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system X(c)(-) activity. Curr Biol. 2018;28(15):2388–2399 e5. doi:10.1016/j.cub.2018.05.094
66. Liu MY, Li HM, Wang XY, et al. TIGAR drives colorectal cancer ferroptosis resistance through ROS/AMPK/SCD1 pathway. Free Radic Biol Med. 2022;182:219–231. doi:10.1016/j.freeradbiomed.2022.03.002
67. Zhao Y, Li M, Yao X, et al. Hcar1/mct1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020;33(10):108487. doi:10.1016/j.celrep.2020.108487
68. Wang X, Chen X, Zhou W, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022;12(2):708–722. doi:10.1016/j.apsb.2021.10.005
69. Lu Q, Yang L, Xiao JJ, et al. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic Biol Med. 2023;195:89–102. doi:10.1016/j.freeradbiomed.2022.12.088
70. Lee H, Zandkarimi F, Zhang Y, et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22(2):225–234. doi:10.1038/s41556-020-0461-8
71. Hunkeler M, Hagmann A, Stuttfeld E, et al. Structural basis for regulation of human acetyl-coa carboxylase. Nature. 2018;558(7710):470–474. doi:10.1038/s41586-018-0201-4
72. Wu J, Minikes AM, Gao M, et al. Intercellular interaction dictates cancer cell ferroptosis via nf2-yap signalling. Nature. 2019;572(7769):402–406. doi:10.1038/s41586-019-1426-6
73. Gao R, Kalathur RKR, Coto-Llerena M, et al. Yap/taz and atf4 drive resistance to sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13(12):e14351. doi:10.15252/emmm.202114351
74. Magesh S, Cai D. Roles of yap/taz in ferroptosis. Trends Cell Biol. 2022;32(9):729–732. doi:10.1016/j.tcb.2022.05.005
75. Ma S, Meng Z, Chen R, Guan KL. The hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88(1):577–604. doi:10.1146/annurev-biochem-013118-111829
76. Piccolo S, Dupont S, Cordenonsi M. The biology of yap/taz: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. doi:10.1152/physrev.00005.2014
77. Sun T, Chi JT. Regulation of ferroptosis in cancer cells by yap/taz and hippo pathways: the therapeutic implications. Genes Dis. 2021;8(3):241–249. doi:10.1016/j.gendis.2020.05.004
78. Yang WH, Chi JT. Hippo pathway effectors yap/taz as novel determinants of ferroptosis. Mol Cell Oncol. 2020;7(1):1699375. doi:10.1080/23723556.2019.1699375
79. Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A taz-angptl4-nox2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol Cancer Res. 2020;18(1):79–90. doi:10.1158/1541-7786.MCR-19-0691
80. Rozycki M, Bialik JF, Speight P, et al. Myocardin-related transcription factor regulates nox4 protein expression: linking cytoskeletal organization to redox state. J Biol Chem. 2016;291(1):227–243. doi:10.1074/jbc.M115.674606
81. Yang WH, Ding CC, Sun T, et al. The hippo pathway effector taz regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28(10):2501–2508 e4. doi:10.1016/j.celrep.2019.07.107
82. Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mtor for cancer therapy. J Hematol Oncol. 2019;12(1):71. doi:10.1186/s13045-019-0754-1
83. Alzahrani AS. Pi3k/akt/mtor inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi:10.1016/j.semcancer.2019.07.009
84. Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of pi3k-akt-mtor signaling suppresses ferroptosis via srebp-mediated lipogenesis. Proc Natl Acad Sci U S A. 2020;117(49):31189–31197. doi:10.1073/pnas.2017152117
85. Chen H, Qi Q, Wu N, et al. Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutant colorectal cancer. Redox Biol. 2022;55:102426. doi:10.1016/j.redox.2022.102426
86. Han D, Jiang L, Gu X, et al. SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels. J Cell Physiol. 2020;235(11):8839–8851. doi:10.1002/jcp.29727
87. Zhang Y, Swanda RV, Nie L, et al. Mtorc1 couples cyst(e)ine availability with gpx4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12(1):1589. doi:10.1038/s41467-021-21841-w
88. Buckwalter JA, Cooper RR. Bone structure and function. Instr Course Lect. 1987;36:27–48.
89. Delgado-Calle J, Bellido T. The osteocyte as a signaling cell. Physiol Rev. 2022;102(1):379–410. doi:10.1152/physrev.00043.2020
90. Cui J, Shibata Y, Zhu T, Zhou J, Zhang J. Osteocytes in bone aging: advances, challenges, and future perspectives. Ageing Res Rev. 2022;77:101608. doi:10.1016/j.arr.2022.101608
91. Ponzetti M, Rucci N. Osteoblast differentiation and signaling: established concepts and emerging topics. Int J Mol Sci. 2021;22(13). doi:10.3390/ijms22136651
92. Sommerfeldt DW, Rubin CT. Biology of bone and how it orchestrates the form and function of the skeleton. Eur Spine J. 2001;10(Suppl 2):S86–S95. doi:10.1007/s005860100283
93. Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92(10):860–867. doi:10.1177/0022034513500306
94. Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-osteoclast communication and bone homeostasis. Cells. 2020;9(9):2073. doi:10.3390/cells9092073
95. Armas LA, Recker RR. Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am. 2012;41(3):475–486. doi:10.1016/j.ecl.2012.04.006
96. Gao Z, Chen Z, Xiong Z, Liu X. Ferroptosis - a new target of osteoporosis. Exp Gerontol. 2022;165:111836. doi:10.1016/j.exger.2022.111836
97. Guggenbuhl P, Filmon R, Mabilleau G, Basle MF, Chappard D. Iron inhibits hydroxyapatite crystal growth in vitro. Metabolism. 2008;57(7):903–910. doi:10.1016/j.metabol.2008.02.004
98. Li Z, Li D, Chen R, Gao S, Xu Z, Li N. Cell death regulation: a new way for natural products to treat osteoporosis. Pharmacol Res. 2023;187:106635. doi:10.1016/j.phrs.2022.106635
99. Lin Z, He H, Wang M, Liang J. microRNA-130a controls bone marrow mesenchymal stem cell differentiation towards the osteoblastic and adipogenic fate. Cell Prolif. 2019;52(6):e12688. doi:10.1111/cpr.12688
100. Komori T. Whole aspect of runx2 functions in skeletal development. Int J Mol Sci. 2022;23(10):5776. doi:10.3390/ijms23105776
101. Balogh E, Tolnai E, Nagy B, et al. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta. 2016;1862(9):1640–1649. doi:10.1016/j.bbadis.2016.06.003
102. Lan D, Qi S, Yao C, et al. Quercetin protects rat bmscs from oxidative stress via ferroptosis. J Mol Endocrinol. 2022;69(3):401–413. doi:10.1530/JME-22-0086
103. Messer JG, Kilbarger AK, Erikson KM, Kipp DE. Iron overload alters iron-regulatory genes and proteins, down-regulates osteoblastic phenotype, and is associated with apoptosis in fetal rat calvaria cultures. Bone. 2009;45(5):972–979. doi:10.1016/j.bone.2009.07.073
104. Jiang Z, Wang H, Qi G, Jiang C, Chen K, Yan Z. Iron overload-induced ferroptosis of osteoblasts inhibits osteogenesis and promotes osteoporosis: an in vitro and in vivo study. IUBMB Life. 2022;74(11):1052–1069. doi:10.1002/iub.2656
105. Ma H, Wang X, Zhang W, et al. Melatonin suppresses ferroptosis induced by high glucose via activation of the nrf2/ho-1 signaling pathway in type 2 diabetic osteoporosis. Oxid Med Cell Longev. 2020;2020:9067610. doi:10.1155/2020/9067610
106. Xu P, Lin B, Deng X, Huang K, Zhang Y, Wang N. Vdr activation attenuates osteoblastic ferroptosis and senescence by stimulating the nrf2/gpx4 pathway in age-related osteoporosis. Free Radic Biol Med. 2022;193(Pt 2):720–735. doi:10.1016/j.freeradbiomed.2022.11.013
107. Jin C, Tan K, Yao Z, et al. A novel anti-osteoporosis mechanism of VK2: interfering with ferroptosis via AMPK/SIRT1 pathway in type 2 diabetic osteoporosis. J Agric Food Chem. 2023;71(6):2745–2761. doi:10.1021/acs.jafc.2c05632
108. Tian Q, Wu S, Dai Z, et al. Iron overload induced death of osteoblasts in vitro: involvement of the mitochondrial apoptotic pathway. PeerJ. 2016;4:e2611. doi:10.7717/peerj.2611
109. Wang B, Zhan Y, Yan L, Hao D. How zoledronic acid improves osteoporosis by acting on osteoclasts. Front Pharmacol. 2022;13:961941. doi:10.3389/fphar.2022.961941
110. Ma J, Wang A, Zhang H, et al. Iron overload induced osteocytes apoptosis and led to bone loss in hepcidin(-/-) mice through increasing sclerostin and rankl/opg. Bone. 2022;164:116511. doi:10.1016/j.bone.2022.116511
111. Yang J, Dong D, Luo X, Zhou J, Shang P, Zhang H. Iron overload-induced osteocyte apoptosis stimulates osteoclast differentiation through increasing osteocytic rankl production in vitro. Calcif Tissue Int. 2020;107(5):499–509. doi:10.1007/s00223-020-00735-x
112. Ni S, Yuan Y, Qian Z, et al. Hypoxia inhibits rankl-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radic Biol Med. 2021;169:271–282. doi:10.1016/j.freeradbiomed.2021.04.027
113. Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080–1092. doi:10.1016/S0140-6736(21)02646-5
114. Yang Y, Lin Y, Wang M, et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022;10(1):26. doi:10.1038/s41413-022-00198-w
115. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi:10.1016/S0140-6736(14)60802-3
116. Charlier E, Deroyer C, Ciregia F, et al. Chondrocyte dedifferentiation and osteoarthritis (oa). Biochem Pharmacol. 2019;165:49–65. doi:10.1016/j.bcp.2019.02.036
117. Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71–72:40–50. doi:10.1016/j.matbio.2018.05.008
118. Miao Y, Chen Y, Xue F, et al. Contribution of ferroptosis and gpx4’s dual functions to osteoarthritis progression. EBioMedicine. 2022;76:103847. doi:10.1016/j.ebiom.2022.103847
119. Xu C, Ni S, Xu N, et al. Theaflavin-3,3’-digallate inhibits erastin-induced chondrocytes ferroptosis via the nrf2/gpx4 signaling pathway in osteoarthritis. Oxid Med Cell Longev. 2022;2022:3531995. doi:10.1155/2022/3531995
120. Zhang S, Xu J, Si H, Wu Y, Zhou S, Shen B. The role played by ferroptosis in osteoarthritis: evidence based on iron dyshomeostasis and lipid peroxidation. Antioxidants. 2022;11(9):1668. doi:10.3390/antiox11091668
121. Liu H, Deng Z, Yu B, et al. Identification of slc3a2 as a potential therapeutic target of osteoarthritis involved in ferroptosis by integrating bioinformatics, clinical factors and experiments. Cells. 2022;11(21):3430. doi:10.3390/cells11213430
122. Yao X, Sun K, Yu S, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Translat. 2021;27:33–43. doi:10.1016/j.jot.2020.09.006
123. Yan J, Feng G, Ma L, Chen Z, Jin Q. Metformin alleviates osteoarthritis in mice by inhibiting chondrocyte ferroptosis and improving subchondral osteosclerosis and angiogenesis. J Orthop Surg Res. 2022;17(1):333. doi:10.1186/s13018-022-03225-y
124. Yi D, Yu H, Lu K, et al. AMPK signaling in energy control, cartilage biology, and osteoarthritis. Front Cell Dev Biol. 2021;9:696602. doi:10.3389/fcell.2021.696602
125. Wan Y, Shen K, Yu H, Fan W. Baicalein limits osteoarthritis development by inhibiting chondrocyte ferroptosis. Free Radic Biol Med. 2023;196:108–120. doi:10.1016/j.freeradbiomed.2023.01.006
126. Zhou X, Zheng Y, Sun W, et al. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a hif-2alpha-dependent manner. Cell Prolif. 2021;54(11):e13134. doi:10.1111/cpr.13134
127. Ma S, Dubin AE, Zhang Y, et al. A role of piezo1 in iron metabolism in mice and humans. Cell. 2021;184(4):969–982 e13. doi:10.1016/j.cell.2021.01.024
128. Wang S, Li W, Zhang P, et al. Mechanical overloading induces gpx4-regulated chondrocyte ferroptosis in osteoarthritis via piezo1 channel facilitated calcium influx. J Adv Res. 2022;41:63–75. doi:10.1016/j.jare.2022.01.004
129. Tong L, Yu H, Huang X, et al. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022;10(1):60. doi:10.1038/s41413-022-00226-9
130. Gong Z, Wang Y, Li L, Li X, Qiu B, Hu Y. Cardamonin alleviates chondrocytes inflammation and cartilage degradation of osteoarthritis by inhibiting ferroptosis via p53 pathway. Food Chem Toxicol. 2023;174:113644. doi:10.1016/j.fct.2023.113644
131. Bin S, Xin L, Lin Z, Jinhua Z, Rui G, Xiang Z. Targeting mir-10a-5p/il-6r axis for reducing il-6-induced cartilage cell ferroptosis. Exp Mol Pathol. 2021;118:104570. doi:10.1016/j.yexmp.2020.104570
132. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi:10.1016/S0140-6736(16)30173-8
133. Ogilvie-Harris DJ, Fornaiser VL. Synovial iron deposition in osteoarthritis and rheumatoid arthritis. J Rheumatol. 1980;7(1):30–36.
134. Chen Y, Xu W, Yang H, et al. Serum levels of hepcidin in rheumatoid arthritis and its correlation with disease activity and anemia: a meta-analysis. Immunol Invest. 2021;50(2–3):243–258. doi:10.1080/08820139.2020.1742731
135. Cylwik B, Gruszewska E, Gindzienska-Sieskiewicz E, Kowal-Bielecka O, Chrostek L. Serum profile of transferrin isoforms in rheumatoid arthritis treated with biological drugs. Clin Biochem. 2019;74:31–35. doi:10.1016/j.clinbiochem.2019.10.005
136. Elsheemy MS, Hasanin AH, Mansour A, Mehrez SI, Abdel-Bary M. Etanercept improved anemia and decreased hepcidin gene expression in a rat model of rheumatoid arthritis. Biomed Pharmacother. 2019;112:108740. doi:10.1016/j.biopha.2019.108740
137. Fan XX, Xu MZ, Leung EL, Jun C, Yuan Z, Liu L. Ros-responsive berberine polymeric micelles effectively suppressed the inflammation of rheumatoid arthritis by targeting mitochondria. Nanomicro Lett. 2020;12(1):76. doi:10.1007/s40820-020-0410-x
138. Xie Z, Hou H, Luo D, An R, Zhao Y, Qiu C. Ros-dependent lipid peroxidation and reliant antioxidant ferroptosis-suppressor-protein 1 in rheumatoid arthritis: a covert clue for potential therapy. Inflammation. 2021;44(1):35–47. doi:10.1007/s10753-020-01338-2
139. Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):110. doi:10.1186/s13075-017-1303-3
140. Chang S, Tang M, Zhang B, Xiang D, Li F. Ferroptosis in inflammatory arthritis: a promising future. Front Immunol. 2022;13:955069. doi:10.3389/fimmu.2022.955069
141. Liu P, Wang W, Li Z, et al. Ferroptosis: a new regulatory mechanism in osteoporosis. Oxid Med Cell Longev. 2022;2022:2634431. doi:10.1155/2022/2634431
142. Ling H, Li M, Yang C, et al. Glycine increased ferroptosis via sam-mediated gpx4 promoter methylation in rheumatoid arthritis. Rheumatology. 2022;61(11):4521–4534. doi:10.1093/rheumatology/keac069
143. Wu J, Feng Z, Chen L, et al. Tnf antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat Commun. 2022;13(1):676. doi:10.1038/s41467-021-27948-4
144. Cheng Q, Chen M, Liu M, et al. Semaphorin 5a suppresses ferroptosis through activation of pi3k-akt-mtor signaling in rheumatoid arthritis. Cell Death Dis. 2022;13(7):608. doi:10.1038/s41419-022-05065-4
145. Ooi M, Hibbs S, Chen FE. The safety of modern intravenous iron infusions in patients with rheumatoid arthritis - a review of the literature. Hematology. 2020;25(1):108–111. doi:10.1080/16078454.2020.1730557
146. Luo H, Zhang R. Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the xc-/gpx4 axis. Exp Ther Med. 2021;21(1):72. doi:10.3892/etm.2020.9504
147. Abdollahzad H, Aghdashi MA, Asghari Jafarabadi M, Alipour B. Effects of coenzyme q10 supplementation on inflammatory cytokines (tnf-alpha, il-6) and oxidative stress in rheumatoid arthritis patients: a randomized controlled trial. Arch Med Res. 2015;46(7):527–533. doi:10.1016/j.arcmed.2015.08.006
148. Hu J, Zhang R, Chang Q, et al. P53: a regulator of ferroptosis induced by galectin-1 derived peptide 3 in mh7a cells. Front Genet. 2022;13:920273. doi:10.3389/fgene.2022.920273
149. Xiang J, Chen H, Lin Z, Chen J, Luo L. Identification and experimental validation of ferroptosis-related gene slc2a3 is involved in rheumatoid arthritis. Eur J Pharmacol. 2023;943:175568. doi:10.1016/j.ejphar.2023.175568
150. Sonou T, Ohya M, Yashiro M, et al. Magnesium prevents phosphate-induced vascular calcification via trpm7 and pit-1 in an aortic tissue culture model. Hypertens Res. 2017;40(6):562–567. doi:10.1038/hr.2016.188
151. Schappe MS, Szteyn K, Stremska ME, et al. Chanzyme trpm7 mediates the ca(2+) influx essential for lipopolysaccharide-induced toll-like receptor 4 endocytosis and macrophage activation. Immunity. 2018;48(1):59–74 e5. doi:10.1016/j.immuni.2017.11.026
152. Zhou R, Chen Y, Li S, et al. Trpm7 channel inhibition attenuates rheumatoid arthritis articular chondrocyte ferroptosis by suppression of the pkcalpha-nox4 axis. Redox Biol. 2022;55:102411. doi:10.1016/j.redox.2022.102411
153. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–vii325. doi:10.1093/annonc/mdq276
154. Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma: fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1–10. doi:10.1016/j.canlet.2020.12.024
155. Liu J, Hong M, Li Y, Chen D, Wu Y, Hu Y. Programmed cell death tunes tumor immunity. Front Immunol. 2022;13:847345. doi:10.3389/fimmu.2022.847345
156. Shi Y, Gong M, Deng Z, et al. Tirapazamine suppress osteosarcoma cells in part through slc7a11 mediated ferroptosis. Biochem Biophys Res Commun. 2021;567:118–124. doi:10.1016/j.bbrc.2021.06.036
157. Liu J, Lou C, Zhen C, Wang Y, Shang P, Lv H. Iron plays a role in sulfasalazine-induced ferroptosis with autophagic flux blockage in k7m2 osteosarcoma cells. Metallomics. 2022;14(5). doi:10.1093/mtomcs/mfac027
158. Xu Z, Chen L, Wang C, Zhang L, Xu W. microRNA-1287-5p promotes ferroptosis of osteosarcoma cells through inhibiting GPX4. Free Radic Res. 2021;55(11–12):1119–1129. doi:10.1080/10715762.2021.2024816
159. Chen M, Jiang Y, Sun Y. Kdm4a-mediated histone demethylation of slc7a11 inhibits cell ferroptosis in osteosarcoma. Biochem Biophys Res Commun. 2021;550:77–83. doi:10.1016/j.bbrc.2021.02.137
160. Wen RJ, Dong X, Zhuang HW, et al. Baicalin induces ferroptosis in osteosarcomas through a novel nrf2/xct/gpx4 regulatory axis. Phytomedicine. 2023;116:154881. doi:10.1016/j.phymed.2023.154881
161. Jiacong H, Qirui Y, Haonan L, Yichang S, Yan C, Keng C. Zoledronic acid induces ferroptosis by upregulating por in osteosarcoma. Med Oncol. 2023;40(5):141. doi:10.1007/s12032-023-01988-w
162. Lv H, Zhen C, Liu J, Shang P. Beta-phenethyl isothiocyanate induces cell death in human osteosarcoma through altering iron metabolism, disturbing the redox balance, and activating the MAPK signaling pathway. Oxid Med Cell Longev. 2020;2020:5021983. doi:10.1155/2020/5021983
163. Lv HH, Zhen CX, Liu JY, Shang P. Peitc triggers multiple forms of cell death by gsh-iron-ros regulation in k7m2 murine osteosarcoma cells. Acta Pharmacol Sin. 2020;41(8):1119–1132. doi:10.1038/s41401-020-0376-8
164. Lin H, Chen X, Zhang C, et al. Ef24 induces ferroptosis in osteosarcoma cells through hmox1. Biomed Pharmacother. 2021;136:111202. doi:10.1016/j.biopha.2020.111202
165. Velletri T, Xie N, Wang Y, et al. P53 functional abnormality in mesenchymal stem cells promotes osteosarcoma development. Cell Death Dis. 2016;7(1):e2015. doi:10.1038/cddis.2015.367
166. Wang L, Pan S. The regulatory effects of p53 on the typical and atypical ferroptosis in the pathogenesis of osteosarcoma: a systematic review. Front Genet. 2023;14:1154299. doi:10.3389/fgene.2023.1154299
167. Liu Q, Wang K. The induction of ferroptosis by impairing stat3/nrf2/gpx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int. 2019;43(11):1245–1256. doi:10.1002/cbin.11121
168. Li X, Liu J. Fancd2 inhibits ferroptosis by regulating the jak2/stat3 pathway in osteosarcoma. BMC Cancer. 2023;23(1):179. doi:10.1186/s12885-023-10626-7
169. Zhang W, Lyu P, Andreev D, Jia Y, Zhang F, Bozec A. Hypoxia-immune-related microenvironment prognostic signature for osteosarcoma. Front Cell Dev Biol. 2022;10:974851. doi:10.3389/fcell.2022.974851
170. Fu J, Li T, Yang Y, et al. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials. 2021;268:120537. doi:10.1016/j.biomaterials.2020.120537
171. Jiang R, He S, Gong H, et al. Identification of atg7 as a regulator of proferroptosis and oxidative stress in osteosarcoma. Oxid Med Cell Longev. 2022;2022:8441676. doi:10.1155/2022/8441676
172. Yang L, Liu J, Liu S. Clinical significance and immune landscape of a novel ferroptosis-related prognosis signature in osteosarcoma. BMC Cancer. 2023;23(1):229. doi:10.1186/s12885-023-10688-7
173. Jiang M, Jike Y, Gan F, et al. Verification of ferroptosis subcluster-associated genes related to osteosarcoma and exploration of immune targeted therapy. Oxid Med Cell Longev. 2022;2022:9942014. doi:10.1155/2022/9942014
174. Wang X, Xia G, Xiao S, et al. A ferroptosis-related gene signature associated with immune landscape and therapeutic response in osteosarcoma. Front Oncol. 2022;12:1024915. doi:10.3389/fonc.2022.1024915
175. Liu X, Du S, Wang S, Ye K. Ferroptosis in osteosarcoma: a promising future. Front Oncol. 2022;12:1031779. doi:10.3389/fonc.2022.1031779
176. Zhang F, Yan Y, Cai Y, et al. Current insights into the functional roles of ferroptosis in musculoskeletal diseases and therapeutic implications. Front Cell Dev Biol. 2023;11:1112751. doi:10.3389/fcell.2023.1112751
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.