Back to Journals » Cancer Management and Research » Volume 12
Targeting EGFR Enriches Stem Cell-Like Properties in Salivary Adenoid Cystic Carcinoma by Activating the Notch1 Pathway
Authors Wang Y, Han Y, Xu S, Zhang L , Zhang X, Deng J, Ye W, Liu S
Received 11 March 2020
Accepted for publication 13 July 2020
Published 31 July 2020 Volume 2020:12 Pages 6655—6663
DOI https://doi.org/10.2147/CMAR.S253500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Yang Wang,1– 3,* Yong Han1,3 ,* Shengming Xu1,3 ,* Ling Zhang,1,3 Xiangkai Zhang,1,3 Jiong Deng,4 Weimin Ye,1,3 Shuli Liu1– 3
1Department of Oral and Maxillofacial-Head and Neck Oncology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Laboratory of Oral Microbiota and Systemic Diseases, Shanghai Ninth People’s Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3National Clinical Research Center for Oral Diseases, Shanghai Key Laboratory of Stomatology & Shanghai Research Institute of Stomatology, Shanghai, People’s Republic of China; 4Key Laboratory of Cell Differentiation and Apoptosis of Chinese Minister of Education, Department of Pathophysiology, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuli Liu; Weimin Ye Email [email protected]; [email protected]
Background: Salivary adenoid cystic carcinoma (SACC), a rare cancer arising in the salivary glands, is characterized by high rates of relapse and distant metastasis. Epidermal growth factor receptor (EGFR) has been implicated in SACC carcinogenesis. However, prospective trials of EGFR-targeting therapies in SACC are limited, and the optimum regimen is unclear.
Methods: The effects of erlotinib on cell proliferation, colony formation, ALDH enzymatic activity and tumorsphere formation were investigated in SACC cells. Expression of the cancer stem cell markers Bmi-1 and Oct4 was evaluated using Western blotting.
Results: We found that while it robustly inhibited cell growth, targeting EGFR with erlotinib enriched the ALDH+ cell population and elevated the clonogenicity of SACC cells, suggesting an increase in stem cell-like potential. In addition, we found that suppression of EGFR kinase activity with erlotinib led to the activation of Notch1 signaling, leading to an increase in stem cell-like properties. Moreover, the γ-secretase inhibitor GSI treatment eliminated the erlotinib-induced increase in stem cell-like properties by decreasing Notch activity.
Conclusion: Our results provide an explanation for the worsened survival observed in some studies of erlotinib therapy in SACC and provide potential therapeutic strategies by combined blockade of the EGFR and Notch1 pathways.
Keywords: EGFR, SACC, erlotinib, Notch1, Bmi-1
Introduction
Salivary adenoid cystic carcinoma (SACC), a common subtype of malignant salivary gland cancer in the head and neck, accounts for approximately 30% of all malignant salivary gland tumors. SACCs are characterized by slow and unpredictable growth, high rates of lung metastasis and a poor prognosis with a 5-year survival rate<20% for highly metastatic patients.1 In recent years, complete surgical resection combined with postoperative radiotherapy has been the routine treatment used for SACC.2 However, SACC patients have a poor prognosis and low survival rates because of potential invasiveness and distant metastasis. Therefore, it is important to identify novel approaches to treat SACC. Recent molecular pathology studies found that EGFR, a tyrosine kinase receptor, plays a vital role in controlling tumor invasion, cell proliferation, angiogenesis and cell survival.3 As previously reported, 85% of SACC patients exhibit high EGFR expression, which often leads to EGFR signal activation, making EGFR a potential molecular target for SACC therapy.4 Furthermore, several different EGFR inhibitors that target EGFR and its downstream pathways have been used in clinical trials.5 Although a substantial proportion of SACC tumors showed high EGFR expression, clinical treatment against this target exhibited little objective response. Thus, an increased understanding of the related mechanisms is necessary to improve EGFR-targeted strategies and patient survival.
Cancer stem cells (CSCs), which often lead to clinical treatment failure, have emerged as a vital factor in cancer chemoresistance, tumor recurrence and cancer metastasis. CSCs can promote tumor initiation and maintenance by undergoing self-renewal and differentiation. Previous studies revealed the presence of SACC CSCs expressing CSC-related factors (OCT4, NANOG, SOX2 and Bmi-1) and some surface markers (CD44, ABCG2, CD133).6–8 Bmi-1 had been reported to involve in self renewal of neuronal, hematopoietic and mammary stem cells, and has been reported in the tumorigenesis of various cancers.9–12 As a cancer stem cell marker and an important epigenetic regulator, Bmi-1 controls stem cell self-renewal through the modification of histones and chromatin. In SACC PDX models, the population of ALDH+ cells exhibited strong tumorigenic ability, and Aldefluor was used as the sole marker to isolate this population of CSCs based on ALDH functional activities.13 As CSCs often drive both tumorigenesis and cancer metastasis, elimination of these cells is required for the successful treatment of patients, and the development of new therapeutic approaches targeting this population is needed. Targeting the signaling pathways critical for self-renewal and differentiation is an important strategy. Importantly, several candidate pathways were identified, including the Wnt, Hedgehog and Notch pathways. Notch proteins which play a vital role in cell fate decisions are a family of transmembrane receptors. As reported, four Notch receptors (Notch 1–4) and five ligands (Jagged-1 and −2, Delta-like-1, −3, and −4) are found in mammalian cells.14 However, CSCs usually lie dormant to avoid being killed by chemotherapeutic drugs.15,16 Therefore, understanding how to promote the recovery of CSCs out of this dormant stage is quite important.
In our current study, we demonstrated that while targeting EGFR with erlotinib suppressed tumor cell growth, it also contributed to the increase of stem cell-like properties in a Notch1-dependent manner. Our study provides a plausible explanation for EGFR treatment failure and represents a novel strategy for the treatment of SACC.
Materials and Methods
Cell Cultures and Reagents
The paired SACC-83 and SACC-LM cell lines were established by Peking University School of Stomatology. The SACC-83 cell line originates from a patient’s sublingual gland; SACC-LM cells with enhanced lung metastatic behavior were isolated in vivo following injection of SACC-83 cells into the tail vein of immunodeficient mice. Cells were cultured at 37°C in the humidified atmosphere of 95% air with 5% CO2. All cells were maintained in RPMI-1640 (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Gibco). Antibodies against GAPDH, p-EGFR, EGFR, cyclin D1, PCNA, Oct4, Bmi-1, Notch1, HES1 and HRP-conjugated secondary antibodies were bought from Cell Signaling Technology. (Beverley, MA,). The Notch signal inhibitor DAPT (GSI-IX, Cat. No:S2215) was purchased from Selleckchem.
Western Blotting
Cells grown to 80% confluence were lysed in ice-cold RIPA lysis buffer (Cell Signaling Technology) containing 125 mM Tris–HCl (pH 6.8), 5% w/v SDS, and 24.75% glycerol, and subjected to total protein extraction according to standard procedure. Total 30 ug protein per lane of each sample was loaded and electrophoretically separated by 10% SDS–PAGE and then transferred onto polyvinylidene difluoride membranes using a wet transfer system (Invitrogen). The membranes were blocked with 5% non-fat milk powder in TBS for 1 h and probed with different antibodies at 4°C overnight. After thorough washing, the blot was incubated with secondary antibody and coupled to horseradish peroxidase-conjugated anti-rabbit (1:2000) or antimouse IgG (1:2000) for 1 h at room temperature. The blots were developed, and the immunoreactive bands were scanned and analyzed using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA samples were extracted with TriPure Isolation Reagent (Roche, Switzerland) and cDNA prepared from 1 microg of total RNA using the SuperScript III System (Invitrogen Life Technologies). The mRNAs expression levels were determined via RT-PCR, using the following primers: Notch1 (F: 5ʹ- GCCGCCTTTGTGCTTCTGTTC-3ʹ and R: 5ʹ-CCGGTGGTCTGTCTGGTCGTC-3ʹ); Notch2 (F:5ʹ- GTGAACCCTGTAAGAATGGA-3ʹand R:5ʹ- TCAGTGCACTCATTGATGTT-3ʹ); Notch3 F:5ʹ-AGACGCTCGTCAGTTCTTAG-3ʹ R: 5ʹ- TGGAAAGAGAAGAGGATGAA-3ʹ); Notch4 (F:5ʹ-AGTCCAGGCCTTGCCAGAACG-3ʹand R: 5ʹ- GTAGAAGGCATTGGCCAGAGAG-3ʹ); HES1 (F:5ʹ- AAAATGCCAGCTGATATAATGGAG-3ʹand R: 5ʹ- GGTCTGTGCTCAGCGCAGCCGTCA-3ʹ); GAPDH (F: 5ʹ-TCCACCACCCTGTTGCTGTA −3ʹ and R: 5ʹ-ACCACAGTCCATGCCATCAC-3ʹ); Bmi-1 (F: 5ʹ-TGCTGGAGAACTGGAAAGTG-3ʹ and R: 5ʹ-GATGAGGAGACTGCACTGGA-3ʹ);
Cell Viability Assay
Cell Counting Kit-8 (DOJINDO, Kumamoto, Japan) were used to examine the cell proliferation ability according to the manufacturer’s guidance. SACC-83 cells or SACC-LM cells (2000 per well) were seeded in 96-well plates with three replicates and cultured overnight. Then cells were treated with different erlotinib concentrations. After treatment, ten microliters of CCK-8 solution was added with a mixture of 100 μL fresh medium. Cells were then incubated for another 4 h at 37 °C in a humidified incubator. Finally, the number of viable cells was measured by a microplate reader (Varioskan Flash, Thermo Scientific) at optical density (OD) 450 nm. Measurements were performed in triplicate.
Analysis of Semisolid Medium Anchorage-Independent Colony Formation
Both SACC-83 and SACC-LM cells were treated with or without erlotinib and suspended in 0.2 mL of Matrigel (Sigma). The matrigel was diluted 1: 1 (vol/vol) with 10% FBS in growth medium. Cell suspensions were then placed in each well of a 24-well plate on the top of a previously cast semisolid layer. Colonies were cultured over 2 weeks at 37°C in a humidified incubator. Colony numbers were then counted and photographed in four different microscopic fields under an inverted microscope. More than 50 aggregated cells were defined to be colonies. We calculated the means and standard error of the number of colonies in four different microscopic fields. Three independent experiments were performed.
Tumorsphere
The sphere formation assay was performed as described before.16 Briefly, both SACC-83 and SACC-LM cells were cultured in serum free Dulbecco’s modified Eagle’s medium (DMEM; Gibco), with or without treatment of erlotinib in a low adhesion plate. After 14 days, all the spheres were flowed through a 70 μm-filter membrane. The spheres that more than 70 μm was counted. The efficiency of sphere formation was calculated.
Aldefluor Assay
We used the Aldeflour kit (Stem Cell Technologies, Durham) to isolate the high ALDH activity population in accordance with the manufacturer’s guidance. SACC cells were suspended in ALDH substrate buffer and incubated at 37 °C water bath for 50 mins. Cells treated with 50 mmol/l diethylaminobenzaldehyde (DEAB) of each samples was used as negative control. Following incubation, centrifuge all samples for 5 minutes at 250 x g and remove supernatant. Resuspend cell pellets in 0.5 mL of Aldeflour Assay Buffer and store the cells on ice. Then flow cytometry was performed to all samples.
Statistical Analysis
SPSS (Statistic Package for Social Sciences) 13.0 was used for data analysis. Unpaired Student’s t-tests and U-Mann Whitney tests were used for statistical analysis. P values <0.05 was considered significant.
Results
Targeting EGFR with Erlotinib Suppressed the Proliferation of SACC Cells in vitro
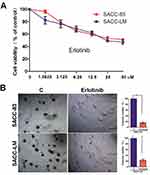
Our previous study demonstrated that EGFR activation is required for the lung metastasis of SACC cells, and inhibition of EGFR by erlotinib could largely decrease the migration and invasion abilities of these cells.17 To further explore the biological role of erlotinib in SACC cells, we first examined the effects of erlotinib on cell proliferation and anchorage-independent growth. We identified the dose-response curves in two SACC cancer cell lines, SACC-83 and SACC-LM cells. As shown in Figure 1A, exposure of SACC-83 and SACC-LM cells to erlotinib inhibited cell viability in a dose-dependent manner. When the erlotinib concentration was 2 uM, the killing effects for both the two cells was about 20%. Thus, to further explore the effect of erlotinib on ACC cells in addition to growth inhibition, we selected 2 uM concentration to stimulate the cells. Moreover, the colony formation abilities of both SACC-83 and SACC-LM cells were significantly suppressed upon treatment with erlotinib (Figure 1B).
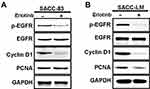
As shown in Figure 2, consistent with the inhibitory effects of erlotinib on proliferation, expression of p-EGFR, cyclin D1 and PCNA detected by Western blotting was significantly suppressed compared with that in untreated cells. Taken together, these results suggest that targeting EGFR with erlotinib inhibited the growth of SACC cells.
EGFR Targeting with Erlotinib-Induced Stem Cell-Like Properties in SACC Cells
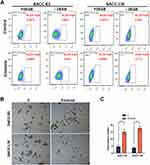
To examine the possible relationship between EGFR inhibition and the induction of stem cell-like properties in SACC cells, we examined the level of ALDH, a detoxifying enzyme that plays an important role in stem cell early differentiation and is responsible for the oxidation of retinol to retinoic acid. Increased ALDH activity is correlated with several types of stem/progenitor cells in both mice and humans. As shown in Figure 3A, erlotinib treatment significantly increased the ALDH level in both SACC-83 and SACC-LM cells. To further confirm these findings, we also performed a tumorsphere-formation assay in both SAC-83 and SACC-LM cells. To our surprise, erlotinib treatment increased tumorsphere formation by approximately 3-fold and 4-fold in SACC-83 and SACC-LM cells, respectively (Figure 3B and C). Together, these results indicate that targeting EGFR with erlotinib conferred stem cell-like properties to SACC cells with enhanced tumorsphere formation and ALDH activity.
Erlotinib Treatment Upregulated Expression of the CSC Markers Oct4 and Bmi-1 in SACC Cells
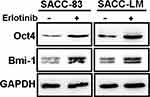
Our results showed that targeting EGFR with erlotinib conferred stem cell-like properties to SACC cells with enhanced tumorsphere formation and ALDH activity. To further explore the impact of erlotinib on the expression of CSC markers in SACC cells, we treated SACC-83 and SACC-LM cells with erlotinib and detected the expression of Oct4 and Bmi-1 via Western blot analysis. We found that erlotinib treatment significantly enhanced the expression of these stem cell markers (Oct4 and Bmi-1) (Figure 4) compared with that in the corresponding control cells. This result suggested that erlotinib treatment could effectively increase the self-renewal of SACC cells in vitro.
Targeting of EGFR by Erlotinib Activated Notch-1 Signaling and Notch-1 Target Genes
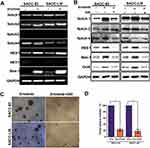
Activation of Notch signaling regulates a variety of cellular processes, including cellular differentiation and the maintenance stem cell characteristics.18 A previous study found that inhibition of EGFR kinase activity induced Notch activation and enriched the population of ALDH high+ cells.19 To further detect whether the Notch signaling pathway was also activated in erlotinib-treated SACC cells, we first examined the expression of Notch1–4 receptors and the Notch target genes HES1 and HEY1 at the mRNA level using RT-PCR. Interestingly, SACC-83 and SACC-LM cells exhibited increased expression of the Notch target gene HES1 and the CSC marker Bmi-1 at the mRNA level. However, the expression of the other Notch receptors was not significantly different (Figure 5A), and no Notch3 or HEY1 was detected (data not shown). To further investigate this finding, we detected the expression of these markers by Western blotting. As shown in Figure 5B, Notch1 was significantly increased in both SACC-83 and SACC-LM cells treated with erlotinib. Furthermore, the expression of HES1 and Bmi-1, which are target genes downstream of Notch1, was upregulated in cells treated with erlotinib (Figure 5B). These findings clearly indicate that erlotinib treatment could activate the Notch1 signaling pathway in SACC cells.
GSI Treatment Decreased Notch Activity and Eliminated Erlotinib-Induced Stem Cell-Like Properties
GSIs, the major Notch pathway inhibitors examined in clinical trials, can prevent intracellular domain release into the cytoplasm and subsequent translocation to the nucleus by inhibiting cleavage of the Notch receptor.20 In this study, we found that the EGFR inhibitor erlotinib activated Notch activity and increased stem cell-like properties. We sought to determine whether these erlotinib-induced effects could be inhibited by treatment with GSI, a novel γ-secretase inhibitor that inhibits Aβ production (GSI-IX, DAPT). As shown in Figure 5B, erlotinib treatment increased the expression of HES1, Bmi-1 and Oct4 in both SACC-83 and SACC-LM cells, but this effect was sensitive to GSI treatment. Moreover, combined treatment with a GSI and erlotinib reduced the number of tumorspheres formed by both SACC-83 and SACC-LM cells compared to that upon treatment with erlotinib alone (Figure 5C and D). These results support the hypothesis that targeting EGFR with erlotinib enriches CSC properties through the activation of Notch signaling. More importantly, our research also provides a potential clinical strategy to overcome this effect in the form of combined Notch and EGFR inhibition.
Discussion
In this study, we show that while EGFR targeting in SACC significantly inhibited tumor cell growth, it also extensively enriched stem cell-like properties by inducing Notch signaling. As a receptor tyrosine kinase, EGFR plays an important role in controlling tumor cell proliferation, invasion, motility, adhesion and angiogenesis.3 In total, 85% of patients with SACC present with EGFR overexpression.4 Our previous study reported that EREG promotes SACC lung metastasis by inducing Snail and Slug expression via the activation of EGFR signaling.17 For metastatic SACC patients with EGFR activation without a genetic mutation, EGFR-targeted therapy may represent a novel treatment strategy. However, clinical studies on EGFR inhibitors did not report any major objective responses.2 Thus, uncovering the specific molecular details underlying the progression of SACC may contribute to the discovery of effective and new strategies for EGFR-targeted therapy.
CSCs are a distinct tumor cell population that plays an important role in tumor recurrence and metastatic spread.7 Previous studies identified SACC CSCs expressing CD44, ABCG2, and CD133, which are surface markers, and the other CSC-related factors SOX2, OCT4, NANOG and Bmi-1.8 Upregulation of these stemness regulators is crucial to maintain self-renewal ability and support the pluripotent state by controlling cellular reprogramming in both somatic and malignantly transformed normal cells.20,21 Therefore, the identification of CSCs might pave the new way for therapeutic strategies for SACC. To date, the effect of EGFR inhibitors on CSC characteristics in SACC remains unclear. In this study, we show that treatment of SACC cells with the EGFR inhibitor erlotinib enhanced CSC-like properties. We found a relationship between EGFR inhibition and the induction of stem cell-like properties in SACC cells. Targeting EGFR with erlotinib increased the level of ALDH activity and tumorsphere formation in SACC cell lines. Additionally, expression of the CSC markers Oct4 and Bmi-1 was elevated. These results suggest an important cross-regulatory mechanism between EGFR and CSCs in SACC. Furthermore, our study is consistent with a recent study that also suggested that the EGFR inhibitors erlotinib and gefitinib enhanced CSC-like features in lung cancer cells.19
Activation of Notch signaling regulates a variety of cellular processes, including cellular differentiation, cell proliferation, apoptosis, and the maintenance of stem cell properties.18 Cross regulation between EGFR and Notch signaling has been well documented, and these pathways can be either synergistic or antagonistic, depending on the tissue and developmental context.22 EGFR signaling could negatively regulate Notch1 gene expression through downregulating transcription of the p53 gene. Moreover, inhibition of EGFR activity also caused Notch signal activation and activated Notch transcriptional target genes in a γ-secretase inhibitor-sensitive manner.23 Our study revealed that Notch1 and its downstream target genes HES1 and Bmi-1 were significantly upregulated in both SACC-83 and SACC-LM cells treated with erlotinib. Combined treatment with a GSI and erlotinib inhibited the expression of HES1, Bmi-1 and Oct4 and reduced the number of formed tumorspheres in both SACC-83 and SACC-LM cells, indicating a potential clinical strategy to overcome EGFR inhibitor treatment failure through combined EGFR and Notch inhibition.
Taken together, these results show that inhibition of EGFR induces stem cell-like properties in SACC cells by potentially inducing Notch1 expression. Therefore, the use of Notch1 inhibitors may be beneficial for SACC with EGFR inhibitor treatment failure.
Ethical Approval
This study was approved by the ethics committee of shanghai ninth people’s hospital.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (81802696 to S.L Liu), the Shanghai Natural Science Foundation of China 17ZR1416300 (to Y Wang).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42(8):759–769. doi:10.1016/j.oraloncology.2006.01.001
2. Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011;12(8):815–824. doi:10.1016/S1470-2045(10)70245-X
3. Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999;82(2–3):241–250. doi:10.1016/S0163-7258(98)00045-X
4. Vered M, Braunstein E, Buchner A. Immunohistochemical study of epidermal growth factor receptor in adenoid cystic carcinoma of salivary gland origin. Head Neck. 2002;24(7):632–636. doi:10.1002/hed.10104
5. Chae YK, Chung SY, Davis AA, et al. Adenoid cystic carcinoma: current therapy and potential therapeutic advances based on genomic profiling. Oncotarget. 2015;6(35):37117–37134. doi:10.18632/oncotarget.5076
6. Destro Rodrigues MF, Sedassari BT, Esteves CM, et al. Nunes, Embryonic stem cells markers Oct4 and Nanog correlate with perineural invasion in human salivary gland mucoepidermoid carcinoma. J Oral Pathol Med. 2017;46(2):112–120. doi:10.1111/jop.12449
7. Dai W, Tan X, Sun C, Zhou Q. High expression of SOX2 is associated with poor prognosis in patients with salivary gland adenoid cystic carcinoma. Int J Mol Sci. 2014;15(5):8393–8406. doi:10.3390/ijms15058393
8. Chang B, Li S, He Q, et al. Deregulation of Bmi-1 is associated with enhanced migration, invasion and poor prognosis in salivary adenoid cystic carcinoma. Biochim Biophys Acta. 2014;1840(12):3285–3291. doi:10.1016/j.bbagen.2014.08.005
9. Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–305. doi:10.1038/nature01587
10. Wang Y, Guan Y, Wang F, Huang A, Wang S, Zhang YA. Bmi-1 regulates self-renewal, proliferation and senescence of human fetal neural stem cells in vitro. Neurosci Lett. 2010;476(2):74–78. doi:10.1016/j.neulet.2010.04.006
11. Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi:10.1158/0008-5472.CAN-06-0054
12. Shen Q, Liu S, Hu J, et al. The differential expression pattern of the BMI-1, SALL4 and ABCA3 genes in myeloid leukemia. Cancer Cell Int. 2012;12(1):42. doi:10.1186/1475-2867-12-42
13. Sun S, Wang ZS. ALDH high adenoid cystic carcinoma cells display cancer stem cell properties and are responsible for mediating metastasis. Biochem Biophys Res Commun. 2010;396(4):843–848. doi:10.1016/j.bbrc.2010.04.170
14. Bell D, Hanna EY, Miele L, Roberts D, Weber RS, El-Naggar AK. El-Naggar, Expression and significance of notch signaling pathway in salivary adenoid cystic carcinoma. Ann Diagn Pathol. 2014;18(1):10–13. doi:10.1016/j.anndiagpath.2013.10.001
15. Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10(3):201–209. doi:10.1038/nri2726
16. Price JV, Savenye ED, Lum D, Breitkreutz A. Dominant enhancers of Egfr in Drosophila melanogaster: genetic links between the Notch and Egfr signaling pathways. Genetics. 1997;147(3):1139–1153.
17. Liu S, Ye D, Xu D, et al. Autocrine epiregulin activates EGFR pathway for lung metastasis via EMT in salivary adenoid cystic carcinoma. Oncotarget. 2016;7(18):25251–25263. doi:10.18632/oncotarget.7940
18. Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11(1):41–52. doi:10.1007/s10911-006-9011-7
19. Arasada RR, Amann JM, Rahman MA, Huppert SS, Carbone DP. EGFR blockade enriches for lung cancer stem-like cells through Notch3-dependent signaling. Cancer Res. 2014;74(19):5572–5584. doi:10.1158/0008-5472.CAN-13-3724
20. Chien CS, Wang ML, Chu PY, et al. Lin28B/Let-7 regulates expression of oct4 and sox2 and reprograms oral squamous cell carcinoma cells to a stem-like state. Cancer Res. 2015;75(12):2553–2565. doi:10.1158/0008-5472.CAN-14-2215
21. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi:10.1016/j.cell.2007.11.019
22. Doroquez DB, Rebay I. Signal integration during development: mechanisms of EGFR and Notch pathway function and cross-talk. Crit Rev Biochem Mol Biol. 2006;41(6):339–385. doi:10.1080/10409230600914344
23. Kolev V, Mandinova A, Guinea-Viniegra J, et al. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10(8):902–911. doi:10.1038/ncb1750
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.