Back to Journals » Journal of Inflammation Research » Volume 15
Targeting CXCL12/CXCR4 Signaling with AMD3100 Might Selectively Suppress CXCR4+ T-Cell Chemotaxis Leading to the Alleviation of Chronic Prostatitis
Authors Zhang M , Liu Y, Chen J, Chen L, Zhang L, Chen X, Hao Z, Liang C
Received 10 December 2021
Accepted for publication 13 April 2022
Published 21 April 2022 Volume 2022:15 Pages 2551—2566
DOI https://doi.org/10.2147/JIR.S352336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Meng Zhang,1– 3,* Yi Liu,1– 3,* Jing Chen,1– 3,* Lei Chen,1– 3 Li Zhang,1– 3 Xianguo Chen,1– 3 Zongyao Hao,1– 3 Chaozhao Liang1– 3
1Department of Urology, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China; 2Institute of Urology, Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China; 3Anhui Province Key Laboratory of Genitourinary Diseases, Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zongyao Hao; Chaozhao Liang, Department of Urology, The First Affiliated Hospital of Anhui Medical University, Jixi Road 218, Shushan District, Hefei City, 230022, Anhui, People’s Republic of China, Tel/Fax +86 55162923095, Email [email protected]; [email protected]
Background: Chronic nonbacterial prostatitis (CNP) has a high incidence, low cure rate, and unclear pathogenesis. Here, we aimed to systematically identify effective diagnostic and therapeutic targets for CNP.
Methods: Prostate tissues were obtained from established mouse models and negative controls and were used for mRNA array sequencing and immunohistochemistry (IHC) staining. Predominant pathways were identified based on pathway enrichment analysis and pharmaceutical experiments. We also investigated the functional role of CXCL12 on CP, a critical factor belonging to the predominant chemotaxis pathway, and employed IHC staining to explore the influence of the CXCL12/CXCR4 axis on the activation of the NF-κB, AKT, and STAT3 signaling pathways. Serum samples derived from both CNP cases and healthy controls were used to determine the secretion level of CXCL12.
Results: By employing mRNA array sequencing and immunohistochemistry, we found that CXCR4, CXCL12, CD44, and OFLM4 were highly expressed in the infiltrated inflammatory T cells of the prostate tissues generated from CNP mice, while they were rarely expressed on the epithelial cells. Based on the pathway enrichment results, we applied pathway inhibitors to suppress the activity of these classic pathways. We found that targeting the CXCL12/CXCR4 axis with its specific antagonist AMD3100 remarkably alleviated inflammatory infiltration of the prostate in CNP models. Similar results were obtained when we replaced AMD3100 with adenovirus-associated virus (AAV)-shCxcl12. To clarify the potential mechanisms of how the CXCL12/CXCR4 axis influences the pathogenesis of CNP, we tested the classical downstream pathways. The results suggested that p-Akt, p-STAT3, and p-NF-κB were more highly expressed on the inflammatory cells of the prostate derived from the CNP model and were partly suppressed after applying AMD3100 or delivering AAV-shCxcl12, indicating that the CXCL12/CXCR4 axis potentially functioned through AKT/NF-κB and STAT3 signaling to influence the pathogenesis of CNP.
Conclusion: Our study provides potential diagnostic biomarkers and therapeutic targets for CNP.
Keywords: chronic nonbacterial prostatitis, mRNA array sequencing, CXCL12/CXCR4, AMD3100
Background
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a critical health issue that frequently occurs in adulthood in males and is also labeled by the National Institutes of Health (NIH) as “Category III” of chronic prostatitis conditions. Twenty years ago, the NIH published its consensus finding and sub-defined CP/CPPS as chronic bacterial prostatitis (CBP, IIIA) and chronic nonbacterial prostatitis (CNP), depending on whether white blood cells existed in semen and/or expressed prostatic secretions (EPS) or whether post-prostatic massage voided bladder urine (VB3) after prostatic massage.1,2 Generally, the prevalence of CP/CPPS ranges from 5 to 14.2% and is regarded as the most frequent type of prostatitis, accounting for 90% of all prostatitis cases.3 However, little is known concerning the etiology of CP/CPPS, making it difficult to identify novel treatment options.
Since great efforts have been devoted to CP/CPPS research, an inflammatory or autoimmune basis for CP/CPPS has become a very prominent theory.4,5 The self-reactivity of T cells in response to prostatic and seminal plasma proteins derived from CP/CPPS patients has been reported in a set of human surveys.6–9 In addition, studies have found that the expression levels of proinflammatory cytokines are increased in seminal fluid,10–12 and T cells infiltrate intra-acinar tissues even without evidence of infection in CP/CPPS patients.13 However, the detailed mechanisms of how these immune cells infiltrate the prostate and influence the pathogenesis of prostatitis are still unknown. Previously, our group members presented a global expression profile of the differentially expressed microRNAs (miRNAs) between models of chronic prostatitis mice and normal controls and potential miRNA–mRNA regulatory networks; these findings could benefit the identification of novel mechanisms of how miRNAs are involved in the regulation of the immune system and serve as effective therapeutic targets for CP.14
As a supplement and extension to our previous work, we demonstrated the landscape of mRNA, circular RNA (circRNA), and long noncoding RNA (lncRNA) expression profiles between chronic nonbacterial prostatitis (CNP) and normal control groups. Based on these data, we aimed to 1) determine the markers that could more sensitively reflect the inflammatory infiltration status; 2) identify the genes/pathways that play a critical role during inflammation development and reveal the underlying mechanisms; and 3) provide some inspiration for the treatment of CP/CPPS patients.
Materials and Methods
Experimental Animals
We purchased male Sprague–Dawley rats from the Experimental Animal Center of Anhui Medical University for prostatic antigen preparation. Healthy male NOD/LtJ (NOD/ShiLtJ, aged four weeks) mice were obtained from the Model Animal Research Center of Nanjing University for the construction of CNP models (Nanjing, China). All mice were acclimated for one week before the start of the experiment. All operations were executed under anesthesia using chloral hydrate, which aimed to minimize suffering.
CNP Induction
CNP induction was conducted as previously described.15–17 Briefly, prostate tissues of SD rats were extracted under aseptic conditions. Then, the tissues were homogenized and centrifuged at four degrees centigrade, and the supernatant was obtained as prostate antigens (PAg). After that, PAg was sufficiently emulsified with an equivalent amount of Freund’s complete adjuvant (Sigma–Aldrich, Inc., St. Louis, USA), and this was intracutaneously injected into NOD mice for CNP induction. The injection was conducted on the 1st day and enhanced on the 28th day. The controls received an equivalent amount of normal saline. The mice were sacrificed at six weeks to verify whether the models were successfully induced.
Sample Collection
Six weeks later, all the mice were sacrificed under anesthesia with 4% chloral hydrate (Aladdin Biotechnology Co. Ltd., Shanghai, China). To remove the adherent blood and seminal fluid, the collected prostate tissues were washed with sterile phosphate-buffered saline (PBS). We divided the prostate samples into two pieces: one was preserved in formalin and used paraffin embedding for hematoxylin and eosin and immunohistochemistry (IHC) staining, which helped evaluate the inflammatory infiltration status of the prostate, and the other section was cryopreserved at −80 °C for RNA sequencing. The inflammation scores were evaluated according to Irani et al’s study.18 Serum samples were collected from CP/CPPS-like symptoms patients and healthy volunteers. The healthy volunteers were confirmed to have no urinary symptoms and had no history of urinary infection. For these CP/CPPS-like symptoms patients, the following requirements should be met: 1) Pelvic pain, or painful ejaculation, and pain domain scores of National Institutes of Health chronic prostate symptom index (NIH-CPSI) ≥ 4; 2) negative white cells in the expressed prostatic secretions (EPS) and urine samples; 3) the course of the disease lasting for 3 months during the past half of year. Enzyme-linked immunosorbent assay (ELISA) was used to determine the secretion difference of CXCL12 between groups (for detailed operations, refer to the manual, Elabscience Biotechnology Inc., Memorial Drive, Texas, USA, Cat.: E-EL-H0052).
RNA Extraction, Quality Control, and RNA Sequencing
Fresh tissue was lapped with liquid nitrogen, and we collected the powder into a 1.5 mL centrifuge tube. Then, 500 µL of TRIzol reagent was added to the tube and vortexed for sufficient mixing. Then, 100 µL chloroform was added to the centrifuge tube, and the tube remained at room temperature for 15 minutes after vortexing for sufficient mixing. Then, the tube was centrifuged at 12,000 rpm for 15 minutes at 4 °C, followed by the top aqueous phase (0.3 mL) being transferred into a new 1.5 mL tube to which a 0.3 mL volume of isopropanol was added, with vortexing for sufficient mixing. Then, the tube was centrifuged at 12,000 rpm for 15 minutes at room temperature. We then added 1 mL 75% ethanol (RNA-free) to wash the pellet and centrifuge it at 3000 rpm for 2 minutes at room temperature. The above procedure was repeated by replacing the 75% ethanol (RNA-free) with absolute ethanol (room temperature, 96–100%). After airing, we added 20 µL of RNA-free water to dissolve the RNA pellet. We quantified the total RNA using a NanoDropnd-2000 (Thermo Fisher Scientific Inc., MA, USA). The integrity of total RNA was evaluated by an Agilent Bioanalyzer 2100 (Agilent Technologies, Inc., Santa Clara, CA, USA). After quality control, the RNA samples were subjected to a standardized procedure from labeling to chip hybridization and elution. First, double-stranded cDNA was synthesized by reverse transcription of total RNA. Next, cDNA was transformed into cRNA labeled with the cyanine-3t test (Cy3). Next, the cRNA was hybridized with the Agilent expression profile chip and then subjected to elution. Finally, the original image was acquired with Agilent Scanner G2505C (Agilent Technologies, Inc., Santa Clara, CA, USA) scanning.
Sequencing Data Analysis
After the standardized data were filtered, the probes were retained for subsequent analyses only when the probe was labeled “detected” for 75% within at least one of the tested groups; the P value of the t test and fold change (FC) were used to evaluate the differentially expressed mRNAs, lncRNAs, and circRNAs, and the differentially expressed genes (DEGs) were considered statistically significant when |log2FC| ≥ 1.0 and P value < 0.05.19,20 Meanwhile, a scatter plot, volcano plot (grouped by biological repetition), and cluster plot were prepared for each group to present the profiles of mRNAs, lncRNAs, and circRNAs. Then, pathway analyses were carried out to evaluate the biological functions or signaling pathways of these genes. Drawing and enrichment analyses were performed by using Python scripts.
Histological Analyses
To confirm the presence of prostate inflammation, we fixed the prostate tissues in formalin, dehydrated them, and then embedded them in paraffin. Tissues were sliced into 4 μm thick sections for HE and IHC staining. Tissue was considered positive for prostatitis if inflammatory cells infiltrated the prostatic stroma.15,21 Irani et al18 graded the inflammatory infiltration of the prostate as 0 (lack of inflammatory cells), 1 (no lymphoid nodule within the stroma but with scattered inflammatory cell infiltration), 2 (nonconfluent lymphoid nodules) and 3 (large inflammatory areas with confluence of infiltrate). The inflammatory scores of each mouse were determined according to this criterion. The histological analysis was performed blindly by a veterinary pathologist. To further evaluate the inflammatory status and cytokines within the prostate, IHC staining of COX-2 and IL-1β was performed. In addition, IHC staining was also performed to validate the novel markers identified by mRNA array analyses. Detailed information on the antibodies used in the current study is listed in Supplementary Table S1.
RNA Extraction and Real-Time Polymerase Chain Reaction Quantification (qRT–PCR)
Total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific Inc., MA, USA) and reverse transcribed to cDNA by using a FastQuant RT kit (KR10; Tiangen Biotech Co., Ltd., Beijing, China). Then, qPCR of DEGs, lncRNAs, and circRNAs was performed using SuperReal PreMix Plus (SYBR Green; FP215, Tiangen Biotech Co., Ltd.) as determined by an Applied Biosystems 7500 PCR System (Thermo Fisher Scientific Inc., MA, USA). The specific primers were purchased from Sangon Biotech (Sangon Biotech Co., Ltd. Shanghai, China) and are listed in Supplementary Tables S2 and 3.
Administration of Pathway Inhibitors
After the acquisition of enriched pathways related to CNP by employing KEGG pathway enrichment analysis, we selected the three top classic enriched pathways and treated CNP mice with pathway inhibitors to evaluate the clinical significance of these selected pathways. These inhibitors were as follows: C29 (1.314 mM/g, intraperitoneal injection), R406 (10 mg/kg, orally), and plerixafor (AMD3100) (6 mg/kg, intraperitoneal injection), which reacted on the toll-like receptor (TLR) signaling pathway, B-cell receptor signaling pathway, and chemokine signaling pathway, respectively. After two weeks of treatment, the mice were sacrificed, and prostate samples were collected for HE and IHC staining.
shRNA Delivery
To further prove the significance of CXCL12, we purchased adeno-associated virus (AAV)-vector/shCxcl12 virus from Sangon Biotech (Sangon Biotech Co., Ltd. Shanghai, China) to validate whether silencing the expression of Cxcl12 could influence the pathogenesis of chronic prostatitis. Sixteen healthy NOD/LtJ (NOD/ShiLtJ, aged four weeks) mice were assigned to the control-vector, control-shCxcl12, CNP-vector, and CNP-shCxcl12 groups. The virus was delivered to the mice via tail vein injection two times (one week before CNP model induction and strengthened before the second induction). Subsequently, HE assays were performed to verify the real effects of CXCL12.
Results
HE Staining of Human and CNP Mouse Prostate Tissues
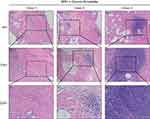
We obtained histological sections from patients who were diagnosed with benign prostatic hypertrophy (BPH) after surgical treatment and histologically confirmed to have inflammatory cell infiltration and CP/CPPS-like symptoms (Supplementary Table S4). The infiltrated inflammatory cells were mainly lymphocytes and neutrophils (Figure 1), a result consistent with previous findings.9,22,23 After establishing the CNP model, we found that many lymphocytes and neutrophils infiltrated the interstitial space of the CNP mice, while little inflammatory cell infiltration was observed in the negative control group (Supplementary Figure S1). Since COX-2 and IL-1β are recognized markers of chronic prostatitis, we applied IHC to detect their expression; nevertheless, weak differences (universal expression of these markers on epithelial, stromal, and inflammatory cells) were uncovered between the CNP models and negative controls (data not shown). Therefore, identifying new and prominent markers for the diagnosis of CNP is warranted. Taking Figure 1 and Supplementary Figure S1 in combination, we established a stable CNP model and revealed some similarities between the clinical patients and CNP models.
Differential Expressed Gene Analysis
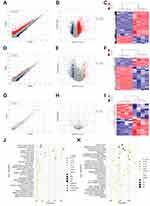
Comparing the tissues derived from CNP models (n = 4) with normal controls (n = 4) using DEGseq224 software at the mRNA, lncRNA, and circRNA levels, we identified 2855 mRNAs, 768 lncRNAs, and 38 circRNAs as DEGs, with a |log2FC < 1| and P value < 0.05 (Figure 2A–I, Supplementary Tables S5 and 6). The top 10 upregulated and downregulated genes are listed in Table 1, along with their annotations. Most of these genes have been reported to be related to the function of the immune process (Supplementary Figure S2). Taken together, these results suggest that aberrant expression of critical immune-related genes is a risk factor for chronic prostatitis pathogenesis.
 |
Table 1 Representative Differentially Expression Genes Between Chronic Non-Bacterial Prostatitis (CNP) Models and Normal Controls |
GO and KEGG Enrichment Analysis
GO and KEGG pathway enrichment analyses were performed to clarify the potential function of these differentially expressed genes. The top 20 enriched GO and KEGG pathways are presented in Figure 2J and K, Supplementary Figures S3, and S4.
We summarized the top 20 most enriched Gene Ontology (GO) terms for biological processes (BPs), cellular components (CCs), and molecular functions (MFs) in Figure 2J, Supplementary Figure S3A–C, and Supplementary Figure S4A–C. These DEGs appeared to be mostly enriched for BPs, such as the immune system process, inflammatory response, chemotaxis, neutrophil chemotaxis, and cell adhesion. Meanwhile, the most enriched CCs were membrane, plasma membrane, extracellular region, cell surface, extracellular space, etc., and the most enriched MFs were protein binding, chemokine activity, carbohydrate-binding, integrin binding, cytokine activity, etc. (Figure 2J). In addition, we also performed pathway enrichment analyses for the differentially expressed circRNAs and lncRNAs, and the results were partly similar to the enrichment results obtained at the mRNA expression level (Supplementary Figures S3A–C and S4A–C).
When comparing CNP models with negative controls, these KEGG pathways were tightly linked to hematopoietic cell lineage, leishmaniasis, Staphylococcus aureus infection, tuberculosis, etc., whose pathogenesis is closely associated with the functional aberration of immune systems (Figure 2K). Similarly, KEGG analyses were also conducted for these differentially expressed lncRNAs and circRNAs (Supplementary Figures S3D and S4D), and the top five enriched KEGG pathways for lncRNAs were influenza A, Epstein–Barr virus infection, measles, pertussis, tuberculosis, etc., while the top enriched KEGG pathways for circRNAs were TCA cycle, SNARE interactions in vesicular transport, platelet activation, etc. Taken together, these results indicate that aberrant expression of critical genes potentially leads to the activation or repression of the related signaling transductions participating in the inflammatory and immune responses.
Identification of Diagnostic Markers for Chronic Prostatitis
As mentioned above, although COX-2 and IL-1β could discriminate inflammatory tissues from normal controls, they were universally expressed on epithelial cells and infiltrated inflammatory cells. Thus, identifying novel markers that are specifically expressed on inflammatory cells instead of epithelial cells would help us better evaluate the inflammation status.
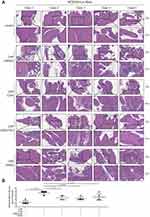
Based on the correlations between these top DEGs and immune processes, we selected eight of them to perform IHC validation (CLU, CXCR4, HSP70, TLR2, MMP12, CYP2B19, OLFM4, and CD44). Notably, we found that CXCR4, CD44, and OLFM4 were expressed only on the inflammatory cells of the CNP models (Figure 3, and Supplementary Figure S5), and the epithelial cells of both the CNP models and negative controls rarely expressed these proteins, highlighting their roles in serving as potential therapeutic targets. Taken together, these results highlight the diagnostic roles of CD44, OLFM4, and CXCR4 in discriminating CNP models from normal controls, which also hold therapeutic potential.
Targeting the CXCL12/CXCR4 Axis Successfully Alleviated Chronic Prostatitis
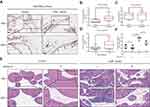
Based on the pathway enrichment results obtained above, we tried to apply pathway inhibitors to identify critical signaling pathways during the pathogenesis of chronic prostatitis. The CNP mice were randomly assigned to four groups (one control and three inhibitor treatment groups). Three inhibitors targeting the toll-like receptor pathway (C29), CXCR4 (AMD3100), and SYK pathway (R406, targeting B-cell signaling) were applied to the CNP models. We found that C29 alleviated more than half of the CNP cases in the CNP-C29 group, and R406 had less capacity to deal with prostatic inflammation. Notably, the CXCR4 inhibitor AMD3100 showed the best treatment effects, which alleviated most of the prostatitis cases in the CNP-AMD3100 group (Figure 4 and Supplementary Figure S6). Since Th1 cells are proven to play a critical role during the pathogenesis of chronic prostatitis, we tested the proportion of variations in Th1 cells with or without AMD3100 treatment via immunofluorescence. The results suggested that after treating CNP mice with AMD3100, the proportion of Th1 cells was significantly reduced, a result consistent with the overall phenotype (Supplementary Figure S7).
CXCR4 is commonly expressed on immunocytes and cells in the central nervous system25,26 and mediates leukocyte and hematopoietic progenitor migration in response to its ligand, SDF-1, also termed CXCL12.27–30 In our recent single-cell RNA sequencing study,31 we found that more than 90% of human CD4+ T cells express CXCR4 protein (Supplementary Figure S8). To clarify how CXCR4 exerts its function, we tested the expression of CXCL12, the ligand of CXCR4, in CNP models and found that CXCL12 was expressed in prostate stromal cells and infiltrated inflammatory cells in the CNP model, while epithelial cells in both CNP models and normal controls were weakly expressed (Figure 5A). We tested the expression of CXCL12 among CP/CPPS patients compared with healthy controls and found that the expression of CXCL12 was elevated in the serum samples derived from CP/CPPS patients (Figure 5B and C, and Supplementary Table S7) compared with healthy controls. In addition, CXCL12 was also more highly expressed in elderly people than in younger patients (Figure 5D). These results indicated that increased secretion of CXCL12 would enhance its interaction with CXCR4+ T cells, thus promoting the chemotaxis of immune cells to the local prostate.
To further validate this hypothesis, we used AAV-packaged shCxcl12 and delivered it to mice through tail vein injection (normal control-vector, normal control-shCxcl12, CNP-vector, and CNP-shCxcl12). Six weeks later, we sacrificed the mice and extracted the prostate tissues to determine whether the inflammatory infiltration was relieved. HE staining results showed that after knocking down Cxcl12 expression, the immunocytes that infiltrated the prostate were reduced compared with the vector control (Figure 5E and F). Taken together, our results suggest that targeting the CXCL12/CXCR4 axis serves as a novel therapeutic option for chronic prostatitis.
Mechanism Dissection
To further reveal the mechanisms of how the CXCL12/CXCR4 axis influences the pathogenesis of chronic prostatitis, we tested the activation status of its classical downstream Akt, STAT3, and NF-κB pathways, which are reported to participate in the movement of immunocytes from peripheral blood to the inflammatory site. Our results found that the phosphorylation levels of Akt, STAT3, and NF-κB were increased in the prostate tissues derived from CNP models compared with negative controls, while after AMD3100 treatment, the p-Akt-, p-STAT3-, or p-NF-κB-positive inflammatory cells were decreased (Figure 6). These results suggested that the activation of the Akt, STAT3, and NF-kB pathways might be involved in CXCL12/CXCR4-mediated T-cell chemotaxis regulation. Taken together with the results from Figures 1–6, targeting the CXCL12/CXCR4 axis decreases the chemotaxis of CXCR4+ Th1 cells to the prostate, leading to better suppression of chronic prostatitis. We summarized the potential mechanisms with an illustration in Figure 7.
Discussion
CP/CPPS is recognized as a complex and frustrating syndrome caused by undiscovered pathological/immune mechanisms and a lack of effective therapy. Massive efforts have been made to identify crucial pathogenic factors that mediate the initiation and progression of CP/CPPS. Since increasing evidence indicates that one-third of biopsies have inflammation, the critical role of inflammation in inducing the symptoms of CP/CPPS, such as chronic pelvic pain, should not be ignored.32–34 Recently, investigations have indicated that the coexistence of inflammation and immune processes could be observed in diverse diseases.35 Correspondingly, immune mechanisms have been revealed to participate in the etiology, pathogenesis, and symptom development of CP/CPPS.34 Nevertheless, a comprehensive understanding of the underlying molecular mechanisms, particularly for the immune regulatory networks that take part in CP/CPPS, remains unknown.
In our work, we performed microarray sequencing on prostate tissues derived from CNP models and normal controls and found a set of differentially expressed mRNAs/lncRNAs/circRNAs. Based on their connection with inflammation, we selected eight proteins to execute IHC validation. We revealed that CXCR4, CD44, and OLFM4 were expressed on infiltrated inflammatory T cells, instead of epithelial cells, thereby serving as effective diagnostic markers. In addition, pathway analyses found that all these significantly expressed DEGs were enriched in immune process-related pathways. Referring to the pathway enrichment results, we applied pathway inhibitors to decrease pathway activity to determine which pathway plays the most critical role during the pathogenesis of CNP, and the results suggested that the CXCL12/CXCR4 axis inhibitor AMD3100 showed the best treatment effect.
The chemokine CXC motif ligand 12 (CXCL12), also termed stromal cell-derived factor 1 (SDF-1), has attracted increasing attention. CXCL12 is commonly expressed on stromal cells derived from diverse organs.36 CXCR4 is the predominant receptor of CXCL12. Targeting the CXCL12/CXCR4 axis using nucleoside reverse transcriptase inhibitors mediates neuropathic pain in patients with human immunodeficiency virus (HIV).37 As indicated in a previous study,38 upregulation of CXCL12 in the dorsal root ganglia (DRG) activates ERK signaling and leads to the development and maintenance of neuropathic pain, while intrathecal injection of AMD3100 reduces the allodynia and phosphorylation of ERK following SNI10. Notably, Saha et al39 found that prostate stromal cells express a high level of CXCL12, and the increased expression of CXCL12 in HMVP2 cells contributes to the rapid activation of STAT3, NF-κB, and MAPK signaling, which are subsequently suppressed by knocking down CXCR4 expression or using an AMD3100 inhibitor.
As summarized in García-Cuesta et al’s40 work, they found that the CXCL12/CXCR4 axis is able to regulate the migration and polarization of regulatory T cells, lymphocytes, or macrophages and mediate angiogenesis during the pathogenesis of inflammatory diseases. In a skin inflammation model established in VEGF-A transgenic mice, inhibition of CXCR4 alleviated skin inflammation; in addition, angiogenesis and inflammatory cell accumulation were compromised.41 Evidence suggests that during autoimmune diseases that occur in the central nervous system (CNS), the intraparenchymal migration of mononuclear cells is limited by CXCL12. In addition, P2G-CXCL12, a molecule derived from CXCL12, alleviated the progression of experimental autoimmune encephalomyelitis (EAE).42 Studies have also found that CXCL12 and CXCR4 are overexpressed in inflammatory bowel disease (IBD) and are involved in the recruitment of T cells, particularly memory Th1 cells.43–45 In a colitis model induced by dextran sulfate sodium, delivering a CXCR4 antagonist compromised colonic inflammation; in addition, the production of TNF-α and IFN-γ by mesenteric lymph node cells was decreased by the CXCR4 antagonist. As indicated in one recent study,46 the authors identified that in patients with primary biliary cholangitis, mucosal-associated invariant T cells were attracted to the liver tissue via the CXCL12/CXCR4-mediated chemotaxis pathway, and this kind of accumulation would lead to the production of proinflammatory cytokines, initiating portal inflammation. The upregulation of CXCR4 and CXCL12 expression was identified in our CNP mouse model. We hypothesized that the upregulation of CXCL12 contributed to CXCR4+ T-cell chemotaxis in the prostate and resulted in prostatitis. Therefore, we first intraperitoneally injected AMD3100 into the CNP mouse model to block the interaction between CXCL12 and CXCR4, and HE assays revealed that the chemotaxis of T cells in the prostate was reduced after treatment, which supported our hypothesis. Consistently, similar results were obtained after knocking down CXCL12 expression in the CNP mouse model, and the results suggested that silencing CXCL12 expression decreased CXCR4+ T-cell chemotaxis to the prostate. We conducted HE and IHC on human prostate samples and found that some of the infiltrating T cells were CXCR4+ Th1 cells, which was consistent with the cell type revealed in the animal model. In addition, ELISA was conducted to determine the expression level of CXCL12 in human blood serum samples and found that the expression levels of CXCL12 were higher in the blood serum samples of CP/CPPS patients than in those of healthy controls. In addition, as age increased, the serum CXCL12 level increased. Combining our results and previous publications, we predicted that the secretion of CXCL12 by prostate stromal cells would increase with age and that the increased secretion would enhance the chemotaxis of CXCR4+ T cells to the prostate, accentuating chronic prostatitis.
As previous studies indicated, COX-2, IL-17A and TNFA were more highly expressed in prostatitis tissues than in normal controls and can be used as diagnostic markers for prostatitis.47–51 However, these established biomarkers are mostly found in other inflammatory/immune-related diseases and extend to chronic prostatitis. Alternatively, they could be used to predict a set of inflammatory/immune-related diseases and lack specificity. Second, many of these markers were generally expressed on epithelial cells, stromal cells, inflammatory diseases, etc. Although targeting these biomarkers might alleviate inflammation, these cells, which positively express these markers, might be damaged. Meanwhile, we identified four novel markers, CD44, CXCR4, OFLM4, and CXCL12, which were expressed only on the infiltrated inflammatory cells instead of epithelial cells in the prostate. With these markers, we can easily discriminate the inflammatory cells that infiltrated the prostate tissues. In addition, based on their function related to the inflammatory process, they also hold the possibility to be therapeutic targets for chronic prostatitis patients.
To conclude, we first demonstrated the mRNA, lncRNA, and circRNA expression differences between CNP models and negative controls with microarray sequencing technology and identified CXCR4, CXCL12, CD44, and OLFM4 as diagnostic markers. The CXCL12/CXCR4 axis potentially functions through AKT/NF-κB and STAT3 signaling to influence disease pathogenesis. Together, these results suggest that targeting this newly identified CXCL12/CXCR4 axis with the small-molecule AMD3100 or AAV-shCxcl12 may help develop novel therapies to better suppress chronic prostatitis. However, since CP/CPPS is a complex disease, the current model cannot fully represent the characteristics of human disease, and more research should be executed to investigate the similarities and differences between this model and human CP/CPPS and improve the former.
Ethical Approval
Animal Care and Use Committee of Anhui Medical University and the Ethics Committee of the First Affiliated Hospital of Anhui Medical University have reviewed and approved our study, in accordance with the Chinese Guideline of Welfare and Ethics for Laboratory Animals and Declaration of Helsinki, respectively (for animal sources: LLSC20211051, for human sources: PJ2021-08-25). In addition, written informed consent were obtained from each patient and healthy individual.
Acknowledgment
We thank OE Biotech Company (Shanghai, China) for performing microarray sequencing and analyses.
Funding
The presented research was financially supported by the National Natural Science Foundation of China (31430028, 81970597, 81630019, and 81870519), the National Science Foundation for Young Scientists (82000719), the Scientific Research Foundation of the Institute for Translational Medicine of Anhui Province (2017ZHYX02), and Natural Science Research Project Funding of Higher Education Institutions of Anhui Province (No. KJ2019A0279).
Disclosure
All authors declare no competing interests in this work.
References
1. Krieger JN, Nyberg L
2. Krieger JN, Ross SO, Deutsch L, Riley DE. The NIH Consensus concept of chronic prostatitis/chronic pelvic pain syndrome compared with traditional concepts of nonbacterial prostatitis and prostatodynia. Curr Urol Rep. 2002;3(4):301–306. doi:10.1007/s11934-002-0054-z
3. Schaeffer AJ. Clinical practice. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med. 2006;355(16):1690–1698. doi:10.1056/NEJMcp060423
4. Motrich RD, Maccioni M, Riera CM, Rivero VE. Autoimmune prostatitis: state of the art. Scand J Immunol. 2007;66(2–3):217–227. doi:10.1111/j.1365-3083.2007.01971.x
5. Rivero VE, Iribarren P, Riera CM. Mast cells in accessory glands of experimentally induced prostatitis in male Wistar rats. Clin Immunol Immunopathol. 1995;74(3):236–242. doi:10.1006/clin.1995.1035
6. Alexander RB, Brady F, Ponniah S. Autoimmune prostatitis: evidence of T cell reactivity with normal prostatic proteins. Urology. 1997;50(6):893–899. doi:10.1016/S0090-4295(97)00456-1
7. Batstone GR, Doble A, Gaston JS. Autoimmune T cell responses to seminal plasma in chronic pelvic pain syndrome (CPPS). Clin Exp Immunol. 2002;128(2):302–307. doi:10.1046/j.1365-2249.2002.01853.x
8. Kouiavskaia DV, Southwood S, Berard CA, Klyushnenkova EN, Alexander RB. T-cell recognition of prostatic peptides in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2009;182(5):2483–2489. doi:10.1016/j.juro.2009.07.067
9. Motrich RD, Maccioni M, Molina R, et al. Presence of INFgamma-secreting lymphocytes specific to prostate antigens in a group of chronic prostatitis patients. Clin Immunol. 2005;116(2):149–157. doi:10.1016/j.clim.2005.03.011
10. Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998;52(5):744–749. doi:10.1016/S0090-4295(98)00390-2
11. Motrich RD, Maccioni M, Molina R, et al. Reduced semen quality in chronic prostatitis patients that have cellular autoimmune response to prostate antigens. Hum Reprod. 2005;20(9):2567–2572. doi:10.1093/humrep/dei073
12. Nadler RB, Koch AE, Calhoun EA, et al. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000;164(1):214–218. doi:10.1016/S0022-5347(05)67497-6
13. John H, John H, Barghorn A, et al. Noninflammatory chronic pelvic pain syndrome: immunological study in blood, ejaculate and prostate tissue. Eur Urol. 2001;39(1):72–78. doi:10.1159/000052415
14. Zhang L, Liu Y, Chen X-G, et al. MicroRNA expression profile in chronic nonbacterial prostatitis revealed by next-generation small RNA sequencing. Asian J Androl. 2019;21(4):351–359. doi:10.4103/aja.aja_97_18
15. Breser ML, Motrich RD, Sanchez LR, Rivero VE. Chronic pelvic pain development and prostate inflammation in strains of mice with different susceptibility to experimental autoimmune prostatitis. Prostate. 2017;77(1):94–104. doi:10.1002/pros.23252
16. Du H, Chen X, Zhang L, et al. Experimental autoimmune prostatitis induces learning-memory impairment and structural neuroplastic changes in mice. Cell Mol Neurobiol. 2020;40(1):99–111. doi:10.1007/s10571-019-00723-2
17. Chen J, Zhan C, Zhang L, et al. The hypermethylation of foxp3 promoter impairs the function of treg cells in EAP. Inflammation. 2019;42(5):1705–1718. doi:10.1007/s10753-019-01030-0
18. Irani J, Levillain P, Goujon JM, Bon D, Doré B, Aubert J. Inflammation in benign prostatic hyperplasia: correlation with prostate specific antigen value. J Urol. 1997;157(4):1301–1303. doi:10.1016/S0022-5347(01)64957-7
19. Trapnell C, Williams BA, Pertea G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–515. doi:10.1038/nbt.1621
20. Zhang SJ, Chen X, Li CP, et al. Identification and characterization of circular rnas as a new class of putative biomarkers in diabetes retinopathy. Invest Ophthalmol Vis Sci. 2017;58(14):6500–6509. doi:10.1167/iovs.17-22698
21. Elkahwaji JE, Hauke RJ, Brawner CM. Chronic bacterial inflammation induces prostatic intraepithelial neoplasia in mouse prostate. Br J Cancer. 2009;101(10):1740–1748. doi:10.1038/sj.bjc.6605370
22. Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51(5):1202–1216. doi:10.1016/j.eururo.2006.12.011
23. Nickel JC, True LD, Krieger JN, Berger RE, Boag AH, Young ID. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001;87(9):797–805. doi:10.1046/j.1464-410x.2001.02193.x
24. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi:10.1186/s13059-014-0550-8
25. Moepps B, Frodl R, Rodewald HR, Baggiolini M, Gierschik P. Two murine homologues of the human chemokine receptor CXCR4 mediating stromal cell-derived factor 1alpha activation of Gi2 are differentially expressed in vivo. Eur J Immunol. 1997;27(8):2102–2112. doi:10.1002/eji.1830270839
26. Jazin EE, Soderstrom S, Ebendal T, Larhammar D. Embryonic expression of the mRNA for the rat homologue of the fusin/CXCR-4 HIV-1 co-receptor. J Neuroimmunol. 1997;79(2):148–154.
27. Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–835. doi:10.1038/382833a0
28. Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382(6594):829–833. doi:10.1038/382829a0
29. Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185(1):111–120. doi:10.1084/jem.185.1.111
30. Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996;184(3):1101–1109. doi:10.1084/jem.184.3.1101
31. Zhang M, Liu Y, Chen J, et al. Single-cell multi-omics analysis presents the landscape of peripheral blood T-cell subsets in human chronic prostatitis/chronic pelvic pain syndrome. J Cell Mol Med. 2020;24(23):14099–14109. doi:10.1111/jcmm.16021
32. Watanabe T, Inoue M, Sasaki K, et al. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011;108(2):248–251. doi:10.1111/j.1464-410X.2010.09716.x
33. Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. Examination of the relationship between symptoms of prostatitis and histological inflammation: baseline data from the REDUCE chemoprevention trial. J Urol. 2007;178(3Pt 1):
34. Breser ML, Salazar FC, Rivero VE, Motrich RD. Immunological mechanisms underlying chronic pelvic pain and prostate inflammation in chronic pelvic pain syndrome. Front Immunol. 2017;8:898. doi:10.3389/fimmu.2017.00898
35. Marques RE, Marques PE, Guabiraba R, Teixeira MM. Exploring the homeostatic and sensory roles of the immune system. Front Immunol. 2016;7:125. doi:10.3389/fimmu.2016.00125
36. Sanchez-Martin L, Estecha A, Samaniego R, Sanchez-Ramon S, Vega MA, Sanchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117(1):88–97. doi:10.1182/blood-2009-12-258186
37. Bhangoo SK, Ren D, Miller RJ, et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007;21(5):581–591. doi:10.1016/j.bbi.2006.12.003
38. Bai L, Wang X, Li Z, et al. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull. 2016;32(1):27–40. doi:10.1007/s12264-015-0007-4
39. Saha A, Ahn S, Blando J, Su F, Kolonin MG, DiGiovanni J. Proinflammatory CXCL12-CXCR4/CXCR7 signaling axis drives myc-induced prostate cancer in obese mice. Cancer Res. 2017;77(18):5158–5168. doi:10.1158/0008-5472.CAN-17-0284
40. García-Cuesta EM, Santiago CA, Vallejo-Díaz J, Juarranz Y, Rodríguez-Frade JM, Mellado M. The role of the CXCL12/CXCR4/ACKR3 axis in autoimmune diseases. Front Endocrinol (Lausanne). 2019;10:585. doi:10.3389/fendo.2019.00585
41. Zgraggen S, Huggenberger R, Kerl K, Detmar M. An important role of the SDF-1/CXCR4 axis in chronic skin inflammation. PLoS One. 2014;9(4):e93665. doi:10.1371/journal.pone.0093665
42. Kohler RE, Comerford I, Townley S, Haylock-Jacobs S, Clark-Lewis I, McColl SR. Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis. Brain Pathol. 2008;18(4):504–516. doi:10.1111/j.1750-3639.2008.00154.x
43. Agace WW, Amara A, Roberts AI, et al. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr Biol. 2000;10(6):325–328. doi:10.1016/S0960-9822(00)00380-8
44. Dotan I, Werner L, Vigodman S, et al. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16(4):583–592. doi:10.1002/ibd.21106
45. Werner L, Elad H, Brazowski E, et al. Reciprocal regulation of CXCR4 and CXCR7 in intestinal mucosal homeostasis and inflammatory bowel disease. J Leukoc Biol. 2011;90(3):583–590. doi:10.1189/jlb.0111101
46. Chen Z, Liu S, He C, et al. CXCL12-CXCR4-mediated chemotaxis supports accumulation of mucosal-associated invariant T cells into the liver of patients with PBC. Front Immunol. 2021;12:578548. doi:10.3389/fimmu.2021.578548
47. Jia YL, Liu X, Yan JY, et al. The alteration of inflammatory markers and apoptosis on chronic prostatitis induced by estrogen and androgen. Int Urol Nephrol. 2015;47(1):39–46. doi:10.1007/s11255-014-0845-4
48. Jeon SH, Zhu GQ, Kwon EB, et al. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFkappaB pathway in a prostatitis rat model. Prostate. 2019;79(13):1498–1504. doi:10.1002/pros.23880
49. Murphy SF, Schaeffer AJ, Done J, et al. IL17 mediates pelvic pain in experimental autoimmune prostatitis (EAP). PLoS One. 2015;10(5):e0125623. doi:10.1371/journal.pone.0125623
50. Altuntas CZ, Daneshgari F, Veizi E, et al. A novel murine model of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) induced by immunization with a spermine binding protein (p25) peptide. Am J Physiol Regul Integr Comp Physiol. 2013;304(6):R415–422. doi:10.1152/ajpregu.00147.2012
51. Lu JC, Shen JM, Hu XC, Peng LP, Hong ZW, Yao B. Identification and preliminary study of immunogens involved in autoimmune prostatitis in human males. Prostate. 2018;78:1092–1102. doi:10.1002/pros.23684
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.