Back to Journals » OncoTargets and Therapy » Volume 16
Targeted Treatment of Adults with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL): Tafasitamab in Context
Authors Abdulhaq H , Hwang A, Mahmood O
Received 6 April 2023
Accepted for publication 6 July 2023
Published 20 July 2023 Volume 2023:16 Pages 617—629
DOI https://doi.org/10.2147/OTT.S372783
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Haifaa Abdulhaq, Andrew Hwang, Omar Mahmood
Division of Hematology/Oncology, University of California San Francisco, Fresno, CA, USA
Correspondence: Haifaa Abdulhaq, Email [email protected]
Abstract: The outcomes of Relapsed/Refractory (R/R) Diffuse Large B-cell lymphoma have been historically poor. The recent development of several novel therapies including CD19 directed agents has improved the prognosis of this disease significantly. Chimeric antigen receptor (CAR) T-cell therapy has drastically changed the treatment of R/R DLBCL, but it is still associated with significant barriers and limited access. Tafasitamab (an anti-CD19 engineered monoclonal antibody), in addition to lenalidomide, has shown significant efficacy with exceptionally durable responses in patients with R/R DLBCL who are ineligible for autologous stem cell transplantation (ASCT). Tafasitamab-lenalidomide and certain other therapies (ie, antibody-drug conjugates and bispecific antibodies) are important treatment options for patients who are ineligible for CAR-T due to co-morbidities or lack of access, and patients with rapid progression of disease who are unable to wait for manufacturing of CAR-T. This review will thus discuss currently approved and recently studied targeted treatment options for patients with R/R DLBCL with an emphasis on CAR-T alternative options, particularly Tafasitamab-lenalidomide.
Keywords: DLBCL, tafasitamab, lenalidomide
Introduction
Diffuse Large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL), accounting for 31% of NHL cases in Western countries.1,2 The median age at presentation is 66, and 5-year survival rates decrease by age from 78% for those younger than 55 years to 54% for those over 65 years.3 Patients with DLBCL are typically treated with curative intent, with cure rates following current first-line treatments being around 50–70%. Historically, outcomes were poor for those who had R/R disease and were not candidates for ASCT. This has recently changed, however, with the development of new therapeutic options including CAR-T therapy and other targeted agents. While patients who are not eligible for ASCT may still be candidates for CAR-T, this treatment is currently not readily accessible to many patients in the US and other countries.4 Novel alternative therapies such as tafasitamab with lenalidomide have shown efficacy with durable responses in patients with R/R DLBCL.
CD19, a Biomarker of Development in B Cells
CD19 antigen is a transmembrane glycoprotein present in B cells and is considered a reliable biomarker for B cell identification since it is expressed throughout the various stages of B cell differentiation.5,6
As part of the complement receptor CD21, tetraspanin membrane protein CD81 (TAPA-1), and CD225 complex, CD19 is considered essential in B cell signaling.7 It is involved in B cell receptor (BCR) dependent and independent signal transduction. The latter is mediated by binding of the CD19/CD21 complex to the activated complement fragment C3d which leads to recruitment of several protein kinases5 (Figure 1).
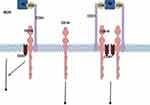 |
Figure 1 CD19 associated signaling complex. Notes: Antigen-C3d complexes can engage the CD19/21 complex in both a B Cell Receptor (BCR)-independent or BCR-dependent fashion. The CD19 complex includes complement receptor CD21, which binds C3d-modified antigen. Reproduced from Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Experimental Hematology & Oncology. 2012;1(1):36.5 |
CD19 also plays a significant role in the body’s immune response given its involvement in B cell antigen-independent development and immunoglobulin-induced activation.
CD19 absence in mice models has been correlated with defects in B cell growth and maturation and dysregulation of the humoral immune response and tolerance induction.8
In B-cell neoplasms, CD19 contributes to lymphomagenesis by chronic activation of BCR to drive aberrant proliferation and survival.9 CD19 engagement has also been shown to amplify Myc expression and activation.9,10
Given that CD19 expression is found in most B-cell malignancies including chronic lymphocytic leukemia (CLL), most acute lymphoblastic leukemia (ALL), and B cell lymphomas, it has been a preferred target for the development of therapeutic drugs.
CD19 Directed Therapies
Tafasitamab Plus Lenalidomide
Anti-CD19 murine immunoconjugates studied in earlier clinical trials have failed due to poor design and the generation of human anti-murine antibodies.11–13 The second generation of anti-CD19 monoclonal antibodies (mAb) was engineered to have specific Fc variant regions in order to further enhance immune effector cell recruitment and complement activation.14,15
Tafasitamab is an engineered, humanized anti-CD19 immunoglobulin G mAb which has two mutations (S239D and I332E) within its Fc region14,15 (Figure 2).
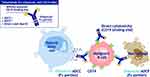 |
Figure 2 Tafasitamab mechanism of action. Notes: Reproduced from Salles G, Długosz-Danecka M, Ghesquières H, Jurczak W. Tafasitamab for the treatment of relapsed or refractory diffuse large B-cell lymphoma. Expert Opin Biol Ther. 2021;21(4):455–463.15 |
In murine xenograft models, tafasitamab has been shown to have highly enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated phagocytosis (ADCP).16
Tafasitamab monotherapy was first studied in patients with R/R CLL in a Phase I study with encouraging results.17
It was subsequently investigated in patients with various subtypes of indolent and aggressive NHL (Non-Hodgkin Lymphoma) including DLBCL in a Phase II study.18,19
The objective response rate (ORR) in DLBCL was 25.7% (9 out of 35) with 7 patients achieving partial response (PR) and 2 complete responses (CR). The median duration of response (DOR) was 20.1 months with 5 DLBCL patients achieving a durable response lasting >12 months18,19 (Figure 3).
 |
Figure 3 Time and duration of response to tafasitamab in non-Hodgkin lymphoma. Abbreviations: DLBCL, diffuse large B-cell lymphoma; NHL, non-Hodgkin’s lymphoma; PR, partial response. Notes: aOne patient with stable disease had PR after 17 months. This patient is not shown in the figure CR, complete response. Reproduced from Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29(5):1266–1272.19 |
The most frequently reported treatment adverse events (AEs) were Infusion-Related Reactions (IRRs) and neutropenia, both occurring in 12% of patients. All IRRs except for one were grade 1 or 2. Eight patients (9%) had grade 3 neutropenia. There were no treatment related deaths.
An exploratory analysis showed longer progression-free survival (PFS) in patients with a peripheral NK-cell count above 100 cells/μL.18,19
Lenalidomide is an immunomodulatory drug which has shown activity across several different subtypes of hematologic malignancies. Its mechanism of action is complex and involves stimulation of the proliferation and activation of CD4/CD8 T cells and NK cells to enhance ADCC and NK cell mediated cytotoxicity. It also affects the tumor microenvironment (TME) via modulating the production of pro-inflammatory T cell cytokines, thus making it less supportive of tumor growth. In addition, it directly acts on malignant B cells via cell cycle arrest and death through inhibition of cyclin-dependent kinases (CDKs) and downregulation or expression of checkpoint inhibitors including Programmed Cell Death Ligand 1 (PD-L1).20,21
In a pooled analysis of NHL-002 and NHL-003 trials which included 134 patients with R/R DLBCL, ORR to lenalidomide was 26% (9% CRs) and the median DOR was 6 months.22 Retrospective analysis of r/r DLBCL patients treated with lenalidomide monotherapy suggests a significantly higher response rate in those with ABC subtype compared to GC subtype per Hans criteria.23
A phase II study by Dr. Wang et al of lenalidomide and rituximab in R/R DLBCL showed an ORR of 33% and median DOR of 10.2 months.24
The rationale for combining tafasitamab and lenalidomide was based on the synergic activation of NK cells noted in the pre-clinical setting. The Fc region modification of tafasitamab further enhances ADCC, ADCP, and NK-cell localization to the malignant B cells25 (Figure 4).
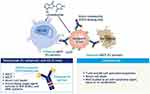 |
Figure 4 Combination mechanism of action of tafasitamab and lenalidomide. Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; DLBCL, diffuse large B-cell lymphoma; iNHL, indolent non-Hodgkin’s lymphoma; mAb, monoclonal antibody; NK, natural killer; R/R, relapsed/refractory. Notes: Reproduced from Düll J, Topp M, Salles G. The use of tafasitamab in diffuse large B-cell lymphoma. Ther Adv Hematol. 2021;12:20406207211027458.11 |
This combination was thought to be promising in treating DLBCL, as low levels of circulating NK-cells have been associated with short event-free survival (EFS) following chemotherapy. Other laboratory observations including gene arrays and cytotoxic assays have also provided supporting evidence for the effect of NK-cells in DLBCL microenvironment.26,27
Tafasitamab plus Lenalidomide in R/R DLBCL: L-MIND, RE-MIND and RE-MIND 2
Tafasitamab-lenalidomide combination was studied in L-MIND, an open-label Phase II single-arm multicenter study which included patients with R/R non-double or triple hit DLBCL and good performance status who had received 1–3 prior regimens and who were ineligible for ASCT. Patients received tafasitamab (12 mg/kg IV) weekly during cycles 1–3 (an additional loading dose was given on day 4 of cycle 1) and every two weeks starting with cycle 4. Lenalidomide was given at 25 mg daily orally on days 1–21 every 28 days up to 12 cycles. Tafasitamab monotherapy was continued until disease progression. The median age was 72 years, and median prior lines of therapy were 2. Half of all patients received one prior therapy (40/81) and 19% had primary refractory disease (15/81). Eleven percent of patients had received prior ASCT.25,28,29
After 35 months of follow-up, ORR was 57.5%, including CR in 40.0%. The median DOR was 43.9 months, the median overall survival (OS) was 33.5 months, and the median progression-free survival (PFS) was 11.6 months. Median DOR and median OS were not reached in patients with CR (Figure 5).
 |
Figure 5 Proportion of patients in remission. Abbreviations: 95% CI, 95% confidence interval; CR, complete response; DoR, duration of response; NE, not evaluable; NR, not reached; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease. Notes: (A–C) Kaplan–Meier plots of duration of response (A), progression-free survival (B) and overall survival (C) after 35 months of follow-up. Reproduced from Duell J, Maddocks KJ, González-Barca E, et al. Long-term outcomes from the Phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417–2426.28 |
In a subgroup analysis, utilizing the tafasitamab-lenalidomide combination was associated with significantly better PFS and OS when used in the second line vs third line or later (23.5 months vs 7.6 months and 45.7 months vs 15.5 months, respectively), thus providing evidence that more benefit is seen when this regimen is utilized earlier.29
Durable responses were seen among several subgroups including those with primary refractory disease; however, median PFS and OS were inferior compared to the overall population (5.3 months and 13.8 months, respectively).
The most common AEs were neutropenia (51%) and anemia (37%). Twenty-one patients (52.5%) had treatment interruption during extended tafasitamab monotherapy, mostly due to neutropenia and respiratory tract infections. Both hematologic and non-hematologic adverse events became less common during tafasitamab monotherapy, with a tolerability profile mirroring that of previous single-agent tafasitamab studies. The most common cause of treatment discontinuation was disease progression (32/50 patients; 64%). It is important to note that prior treatment with tafasitamab-lenalidomide did not preclude patients from receiving subsequent ASCT or CAR-T cell therapy.
Overall, tafasitamab plus lenalidomide showed highly durable response rates and was generally well tolerated with notably no cytokine release syndrome events and low rates of infusion reactions. This regimen is thus an attractive and effective option for frail patients who may prioritize treatments that may not severely impact their quality of life. Longer follow-up is needed to determine whether this combination could be a curative option for some patients.
Tafasitamab plus lenalidomide regimen was granted FDA (Food and Drug Administration) accelerated approval for patients with R/R DLBCL not eligible for ASCT based on this study’s high ORR and prolonged DOR.
To delineate the contribution of tafasitamab to the combination, the RE-MIND study (an observational retrospective cohort of lenalidomide monotherapy) was conducted to generate a historic control for the L-MIND study.30
In this study, a pool of 140 patients with R/R DLBCL who received lenalidomide monotherapy were eligible for matching to the L-MIND cohort. After balancing nine prespecified prognostic baseline covariates, 76 patients were identified, and outcomes were compared. The combination was associated with higher ORR than lenalidomide monotherapy (67.1% versus 34.2%) (p < 0.0001) and higher CR (39.5% vs 13.2%). Median DOR was 20.5 months in the combination cohort versus 6.6 months for lenalidomide monotherapy. Survival endpoints favored combination therapy as well.30
Given the lack of high-quality trial data comparing tafasitamab-lenalidomide with other established treatments for R/R DLBCL, another retrospective study (RE-MIND2) was conducted to compare the efficacy of tafasitamab-lenalidomide as studied in L-MIND with matched patient populations treated with other commonly used therapies, including CAR-T, lenalidomide-rituximab (R2), and polatuzumab vedotin + bendamustine-rituximab (Pola-BR).31
In this study, 3454 patients with DLBCL and at least 2 prior systemic therapies (including at least 1 anti-CD20 therapy) were enrolled. Matched patient pairs were created for the comparative analysis using 1:1 nearest neighbor matching. The matched pairs consisted of tafasitamab-lenalidomide versus CAR-T (n = 37 pairs), versus Pola-BR (n = 24 pairs), and versus R2 (n = 33 pairs). Tafasitamab-lenalidomide was associated with a significantly higher OS benefit compared to Pola-BR (HR 0.44, p = 0.038) and R2 (HR 0.44, p = 0.014) with no significant difference compared to CAR-T (HR 0.95, p = 0.891).
While RE-MIND and RE-MIND2 are not equivalent to Phase III randomized trials, combining real-world data (RWD) with clinical trial data in these studies provides a means of comparison for the effectiveness of these different treatment regimens in lieu of expensive and time-consuming head-to-head trials.
Other combinations with tafasitamab are also currently being studied. The B-MIND study is a randomized phase II/III trial of bendamustine + either tafasitamab or rituximab in patients with R/R DLBCL who are not eligible for ASCT.32
Combinations of tafasitamab plus lenalidomide with other novel therapies such as bispecific antibodies are also being considered in future clinical trials.
To summarize, data from the L-MIND study support the use of tafasitamab-lenalidomide as an effective second-line option in patients with R/R DLBCL who are not eligible for ASCT. However, the availability of other treatment options besides tafasitamab that target CD19 (ie, CAR-T and the antibody–drug conjugate Loncastuximab Tesirine) raises the question of how these therapies are best sequenced.
CAR-T
CAR-T cell therapy utilizes autologous genetically modified T lymphocytes to target specific surface antigens expressed on cancer cells. It has emerged as an effective treatment option for R/R DLBCL, especially for patients who are unable to achieve complete response (CR) after first-line therapy or who have early relapse (<12 months). In the US, FDA approval of CAR-T for DLBCL was first granted in 2017 for treatment after 2 prior lines of therapy. There are presently 3 FDA approved CAR-T products for use in the relapsed/refractory setting, all of which target CD19.
Axicabtagen Ciloleucel (Axi-Cel)
Axi-cel was first clinically evaluated in the Phase 1/2 ZUMA-1 trial which included heavily pre-treated patients (the majority of whom had received at least 3 prior lines of treatment). CR was achieved in 54% of patients with an 18-month OS of 52%. At 2-year follow-up, ORR was 83% and CR was 58%. The median PFS was 5.9 months, and the median OS was not reached. Based on these results, Axi-cel became the first FDA-approved CAR-T product for DLBCL in 2017.33,34
The ZUMA-7 Phase 3 trial subsequently compared Axi-cel to second-line chemoimmunotherapy followed by high-dose chemotherapy/ASCT in responding patients (standard of care [SoC]) in R/R DLBCL within 12 months of 1st line treatment. An RR of 83% was seen with Axi-cel vs 50% with SoC. Two-year event-free survival (EFS) was 41% with Axi-cel and 16% with SoC (P < 0.001). Two-year estimated OS was 61% with Axi-cel vs 52% with SoC. Despite higher rates of grade 3 or higher AEs (91% vs 83%), only 1 patient death was attributed to Axi-cel (HBV reactivation) vs 2 deaths with SoC. In the CAR-T arm, 11 patients (6%) had grade 3 or higher CRS and 36 patients (21%) had grade 3 or higher neurotoxicity. Axi-Cel was thus granted FDA approval for use in the second-line setting in 2022.35
Lisocabtagene Maraleucel (Liso-Cel)
Liso-cel received FDA approval for third-line treatment of DLBCL in 2021 following the results of the single arm multi-center TRANSCEND trial; patients were heavily pre-treated with median 3 prior lines of therapy and 33% having received prior ASCT. ORR was 73% with 54% CR; median PFS was 6.8 months and median OS was 27.3 months.36 Liso-Cel subsequently received approval for second-line treatment in 2022 based on the phase 3 TRANSFORM trial in which it was compared to SoC. Median EFS was 10.1 months vs 2.3 months. Grade 3 or higher AEs were comparable in both arms (92% Liso-Cel vs 87% SoC) with the Liso-Cel arm having only 1 patient (1%) with grade 3 CRS and 4 patients (4%) with grade 3 neurotoxicity.37
Tisagenlecleucel (Tisa-Cel)
Tisa-cel was FDA approved in 2018 for use as a third line option in R/R DLBCL based on the Phase 2 JULIET trial which reported ORR and CR rates of 52% and 40%, respectively, in heavily pretreated patients who either were ineligible or progressed after chemotherapy/ASCT. Twenty-four patients (22%) had grade 3 or higher CRS; 13 patients (12%) had grade 3 or higher neurotoxicity.38 However, the phase 3 BELINDA trial evaluating second-line use in early R/R patients did not show superiority of Tisa-cel compared to SoC.39
With regard to safety, the most notable adverse effects seen in all three CAR-T products are CRS and immune effector cell-associated neurotoxicity syndrome (ICANS). Although there is no direct comparison, trial data suggest that these complications may be higher in patients treated with Axi-cel compared to Liso-cel or Tisa-cel. Of note, CAR-T clinical trials have included older patients (ie, 65 or older) and those with comorbidities; response rates and adverse events were consistent with younger and healthier participants. Thus, patients who otherwise would be considered unfit for autologous HCT may still be candidates for CAR-T.
Given their robust response rates and otherwise dismal outcomes with salvage chemotherapy, CAR-T therapy with either Axi-cel or Liso-cel should be considered as standard of care for eligible patients with early relapsed/refractory DLBCL. It remains to be seen if CAR-T is superior to salvage treatment/autologous transplant in patients who relapse after 12 months and otherwise have chemo-sensitive disease.
Despite the promising efficacy of CAR-T, however, there are several important limitations which need to be considered. CAR-T therapy is only available at select certified institutions given its complex manufacturing and storage process as well as the need for specialized care during administration to monitor for potentially life-threatening complications. Accessibility, therefore, is a significant challenge for patients who live in more remote areas without nearby access to larger tertiary care facilities. Additionally, other factors such as ethnicity, socioeconomic stratum, and insurance coverage have been shown to be contributors to the disparities seen in CAR-T accessibility and treatment. Review of the Vizient Clinical Database (CDB) showed that African American (AA) patients were less likely to receive CAR-T therapy compared to other ethnicities. CAR-T trials have also been limited by a lack of adequate representation/inclusion of non-Caucasian ethnic minorities.40
Furthermore, patients with clinically aggressive disease may not have the luxury of time to wait for CAR-T approval and/or manufacturing. The role of bridging therapy (defined in studies as treatment given between T-cell collection and CAR-T infusion) adds more complexity to the interpretation of the CAR-T trials and is still an area requiring further investigation. The ZUMA-7 and TRANSFORM trials did not allow for bridging therapy with standard salvage regimens. Several real-world retrospective studies as well as the JULIET and TRANSCEND trials included patients who received bridging therapy; outcomes and toxicity were notably worse in bridged patients compared to non-bridged patients. It is unknown whether these findings are due to bridged patients having more aggressive disease or if bridging therapy itself adversely affects response to CAR-T treatment. The use of other novel targeted therapeutic agents mentioned in this review and radiation for bridging therapy may be interesting areas for further study.
Loncastuximab
Loncastuximab is an anti-CD19 humanized monoclonal antibody conjugated to a cytotoxic, cross-linking agent pyrrolobenzodiazepine (PBD) dimer.41 The LOTIS-2 phase 3 trial, which led to the FDA approval of loncastuximab in patients with R/R DLBCL after two or more lines of treatment, included 145 patients who received loncastuximab every 3 weeks for up to one year. Patients who had clinical response had the option of continuing treatment. ORR was 48% with 24% CR. Median DOR was 10 months. However, patients who achieved CR had a longer median DOR of 13 months. Notably, 5 patients with double-hit/triple-hit lymphoma had a CR. Median PFS and OS were 5 and 10 months, respectively. ORR was not significantly different based on the number of prior lines of treatment, de novo vs transformed disease, or germinal center vs activated B-cell type. Lower ORR was seen in those who had disease refractory to most recent prior treatment vs not (37% vs 67%.) Interestingly, 6 of 13 patients who received prior CAR-T therapy had responses with 2 patients achieving CR. Most common grade 3 or higher AEs were neutropenia (26%), thrombocytopenia (18%), and increased γ-glutamyltransferase (17%). Fluid retention including edema or effusion was seen in 31% of patients.
Loncastuximab is currently being evaluated in several combinations including a phase 3 trial comparing loncastuximab-rituximab vs gemcitabine-oxaliplatin-rituximab in patients with R/R DLBCL.42
An important aspect when considering sequencing therapy for R/R DLBCL patients is the reduction in CD19 expression following CAR-T therapy. This has been thought of as a tumor escape mechanism resulting in relapse and may prevent efficacy of subsequent CD19-targeted therapy.43
More promising data were reported for other CD19-targeted therapies. In a study of 14 patients with DLBCL who received loncastuximab in phase 1 and 2 studies, CD19 was still positive by immunohistochemistry after treatment, and CD19-directed CAR-T therapy, given after a median 120 days from loncastuximab failure, resulted in ORR of 50% (43% CR and 7% PR).44
In vitro studies of CAR-T19 cells incubated with tafasitamab showed that these cells continued to exhibit strong functionality despite having competition for CD19 binding.45
Recent limited clinical data from the L-MIND study also support the persistence of CD19 expression following tafasitamab treatment, with one report of a patient achieving CR from CAR-T after being a participant in L-MIND.46 Newer methods, however, may be needed to evaluate conclusively for CD19 persistence and rule out masking of the receptor after CD19 directed therapy.
Non CD19 Directed Therapies
Bendamustine/Rituximab Plus Polatuzumab (BR-Pola)
Polatuzumab is an antibody–drug conjugate which consists of a humanized anti-CD79b monoclonal antibody covalently attached to monomethyl auristatin E (MMAE).
The combination of polatuzumab vedotin, bendamustine and rituximab (pola-BR) was FDA approved for patients with R/R DLBCL after at least two prior therapies based on phase 1b/2 study which compared pola-BR with BR in 80 patients with transplantation-ineligible R/R DLBCL. Patients with transformed follicular lymphoma (FL) or DHL were excluded. The medium number of prior therapies was two. Patients received six 21-day cycles.
There were higher CR rates with pola-BR (40% vs 17.5%, p = 0.026) as well as higher PFS (9.5 vs 3.7 months, p = 0.026) and higher OS (12.4 vs 4.7 months, p = 0.002). Post hoc subgroup analysis showed similar survival regardless of the number of prior lines of therapy, DLBCL subtype and prior ASCT, but statistical significance could not be established due to small sample size. Peripheral neuropathy was seen more frequently with pola-BR (44% vs 3%), but it was mild (grade 1–2) and reversible in most patients. Although there were higher rates of hematological AEs with pola-BR, there was no increase in risk of febrile neutropenia (10% vs 13%).47,48 The combination of polatuzumab with rituximab, gemcitabine and oxaliplatin (Pola-R Gem-Ox) is currently compared to R Gem-Ox in the phase 3 trial.
Selinexor
Selinexor is an oral agent that belongs to the class of selective inhibitors of nuclear export (SINE). Currently, this drug is approved in R/R DLBCL after at least 2 lines of systemic therapy. The accelerated FDA approval in June 2020 was based on the Phase 2 SADAL trial results.
In this trial, 127 patients received 60 mg of selinexor on day 1 and day 3 of each week till disease progression. ORR was 28% (12% CR and 16% PR). Safety profile noted for Nausea (58%) and fatigue (47%). Hematologic toxicities were noted for thrombocytopenia and anemia (43%). Selinexor is a strong emetogenic agent, and patients are recommended to receive two prophylactic antiemetics while receiving treatment.49
Bispecific Antibodies
Bispecific T-Cell Engagers (BiTEs) are dual-targeted antibodies engaging the effector T-cells with the target tumor cells, offering a bypass to the MCH-restricted antigen presentation to cytotoxic T-cells and eliminating the issue of rejection of transplanted effector T-cells (Table 1). CRS and ICANS are the most observed adverse events.
 |
Table 1 Bi-Specific T-Cell Engagers (BiTE) in the Setting of Relapsed Refractory DLBCL |
Blinatumomab
Blinatumomab was the first BiTE used in lymphoma. Bargou et al reported Blinatumomab’s efficacy in this setting.50,51 This signal was eventually confirmed with further subsequent studies. Goebeler et al reported the results of phase 1 study that included 11 DLBCL patients with ORR 55% (36% CR). Neurologic AE was the most common dose-limiting side effect.52 A phase 2 trial for R/R DLBCL patients with a median of 3 prior treatments showed ORR 43% (19% CR) with neurologic AE > Grade 3 reported in 22% leading to treatment discontinuation.53 The suboptimal pharmacokinetics requiring a long duration for infusion, as well as the significant neurotoxic side effect profile of Blinatumomab, led to the discontinuation of further trials in this drug in DLBCL and the development of several subsequent BiTEs.
Mosunetuzumab
In phase 1b trial, Schuster et al reported disease response in 119 patients with aggressive NHL (including transformed NHL) who were heavily pre-treated with a median of 3 lines of treatment, including a subset of patients who received CAR-T.54 ORR reported as 34.7% (18.6% CR) and the adverse event profile was more favorable than Blinatumomab. CRS Grade 3 occurred in 1.1% with no Grade 4 CRS. ICANS was reported in 1.1%.
Glofitamab
In a phase 1 trial of glofitamab for R/R B-cell Lymphomas that included 73 DLBCL patients,55 ORR for the DLBCL cohort was reported at 41.4% (CR 28.8%) and the median DOR was reported as 5.5 months in the aggressive lymphoma arm. PFS for the aggressive lymphoma subset was 2.9 months; however, those who achieved CR continue to be responding at 12 months. In the phase 2 study, CR was 32% including patients who received prior CAR-T. The majority of CRs were ongoing at 12 months. AE grade 3 or higher occurred in 62% of the patients. Grade 3 or higher CRS and neurological events were uncommon and occurred in 4% and 3% of patients, respectively.56
Epcoritamab
Hutchings et al reported the results of their phase I/II trial including 46 patients with DLBCL. ORR in patients with R/R DLBCL was 68%. At 48 mg, the ORR was 88% and CR was 38%. CRS was reported on 59% of the cases, all were grade 1–2. Neurologic AEs were reported in 14% at different dose levels (7% were grade 3).57
Odronextumab
In an early phase I dose escalation trial, odronextumab was evaluated in 78 R/R DLBCL patients. ORR for those without prior CAR-T was 53% (all were CR), compared to 33% for those with prior CAR-T (27% were CR). The median DOR was not reached in both groups with the longest duration reported at 32.4 months (no prior CAR-T) and 20.5 months (with prior CAR-T). In terms of safety, CRS of any grade was seen in 61% (7% were grade 3 or higher). Twelve percent ICANS-like symptoms were seen (3% were grade 3 or higher).58
Plamotamab
In an early phase I dose escalation trial, plamotamab demonstrated efficacy in heavily pretreated R/R DLBCL with a median of 4 prior treatments achieving ORR 47.4% (26.3% were CR).59 The majority of DLBCL patients had prior CAR T-cell treatment (13/19) and the ORR in that subset was 46.2% (30.8% were in CR). In terms of safety profile, CRS was reported in 72.2% but no grade 3 or higher was seen. CRS was mostly confined to cycle 1.
Imvotamab
Budde et al reported the results of their phase I trial including patients with R/R DLBCL who achieved ORR of 31% (25% were in CR).60 The safety profile was favorable with CR reported in 19% (4% were grade 3), no ICANS-like events, and Grade 1 neutropenia seen in 1 patient only.
BiTEs have come a long way since the first use of Blinatumomab in the setting of R/R DLBCL in 2008. Many advances have been made with more stable pharmacodynamics and, more recently, subcutaneous formulations (epcoritamab) and more novel BiTEs are developed as IgM-based rather than the usual IgG-based Ab with promising results and favorable safety profile. The potential efficacy of bispecific antibodies in the setting of CAR-T failure results in an increasing interest in BiTEs and supporting the T-cell effector approach in treating DLBCL.
Several clinical trials are evaluating the combinations of BiTEs with other novel therapies including tafasitamab.
Other Therapies
Bruton kinase inhibitors (ie, ibrutinib), BCL2 inhibitors (ie, venetoclax), immune checkpoint inhibitors, and epigenetic modifiers are being studied in different combinations in clinical trials.
Conclusion
There are currently several effective therapies in patients with R/R DLBCL. Tafasitamab plus lenalidomide is an efficacious regimen with highly durable responses. It is an attractive second-line treatment option for patients non-eligible for ASCT or CAR-T, and it can be used as a later-line treatment option. Early limited clinical data show maintenance of CD19 expression after tafasitamab and support the use of tafasitamab prior to CAR-T. Using this regimen as a bridge to CAR-T is worth exploring.
Combinations of tafasitamab–lenalidomide with bispecific anti-CD20 antibodies, or antibody-drug conjugates, are also worth being considered to improve response to treatment and help prevent relapse.
Disclosure
Dr Haifaa Abdulhaq reports grants, personal fees from Morphosys, personal fees from Novartis, personal fees from BMS, grants from Genentech, personal fees from Amgen, personal fees from AbbVie, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Martelli M, Ferreri AJM, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146–171. doi:10.1016/j.critrevonc.2012.12.009
2. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748. doi:10.1038/s41375-022-01620-2
3. Howlader NNA, Krapcho M, Miller D, et al. SEER cancer statistics review, 1975–2017; 2020.
4. Kuhln A, Kirkwood A, Roddie C, et al. CAR T in patients with large B-cell lymphoma not fit for autologous transplant [epub ahead of print]. Br J Haematol. 2023. doi:10.1111/bjh.18810
5. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. doi:10.1186/2162-3619-1-36
6. Del Nagro CJ, Otero DC, Anzelon AN, Omori SA, Kolla RV, Rickert RC. CD19 function in central and peripheral B-cell development. Immunol Res. 2005;31(2):119–131. doi:10.1385/ir:31:2:119
7. Tedder TF, Inaoki M, Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6(2):107–118. doi:10.1016/s1074-7613(00)80418-5
8. Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3(1):39–50. doi:10.1016/1074-7613(95)90157-4
9. Hojer C, Frankenberger S, Strobl LJ, et al. B-cell expansion and lymphomagenesis induced by chronic CD40 signaling is strictly dependent on CD19. Cancer Res. 2014;74(16):4318–4328. doi:10.1158/0008-5472.Can-13-3274
10. Chung EY, Psathas JN, Yu D, Li Y, Weiss MJ, Thomas-Tikhonenko A. CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis. J Clin Invest. 2012;122(6):2257–2266. doi:10.1172/jci45851
11. Düll J, Topp M, Salles G. The use of tafasitamab in diffuse large B-cell lymphoma. Ther Adv Hematol. 2021;12:20406207211027458. doi:10.1177/20406207211027458
12. Makita S, Tobinai K. Antibody therapy targeting CD19 for B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29(5):1086–1089. doi:10.1093/annonc/mdy092
13. Katz BZ, Herishanu Y. Therapeutic targeting of CD19 in hematological malignancies: past, present, future and beyond. Leuk Lymphoma. 2014;55(5):999–1006. doi:10.3109/10428194.2013.828354
14. Lazar GA, Dang W, Karki S, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103(11):4005–4010. doi:10.1073/pnas.0508123103
15. Salles G, Długosz-Danecka M, Ghesquières H, Jurczak W. Tafasitamab for the treatment of relapsed or refractory diffuse large B-cell lymphoma. Expert Opin Biol Ther. 2021;21(4):455–463. doi:10.1080/14712598.2021.1884677
16. Horton HM, Bernett MJ, Pong E, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68(19):8049–8057. doi:10.1158/0008-5472.Can-08-2268
17. Woyach JA, Awan F, Flinn IW, et al. A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood. 2014;124(24):3553–3560. doi:10.1182/blood-2014-08-593269
18. Jurczak W, Zinzani PL, Hess G, et al. A Phase IIa, open-label, multicenter study of single-agent tafasitamab (MOR208), an Fc-optimized anti-CD19 antibody, in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma: long-term follow-up, final analysis. Blood. 2019;134:4078. doi:10.1182/blood-2019-124297
19. Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29(5):1266–1272. doi:10.1093/annonc/mdy056
20. Hernandez-Ilizaliturri FJ, Batoo SA. The emerging role of lenalidomide in the management of lymphoid malignancies. Ther Adv Hematol. 2011;2(1):45–53. doi:10.1177/2040620710390547
21. Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J Clin Oncol. 2015;33(25):2803–2811. doi:10.1200/jco.2014.59.5363
22. Witzig TE, Nowakowski GS, Habermann TM, et al. A comprehensive review of lenalidomide therapy for B-cell non-Hodgkin lymphoma. Ann Oncol. 2015;26(8):1667–1677. doi:10.1093/annonc/mdv102
23. Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer. 2011;117(22):5058–5066. doi:10.1002/cncr.26135
24. Wang M, Fowler N, Wagner-Bartak N, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia. 2013;27(9):1902–1909. doi:10.1038/leu.2013.95
25. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. doi:10.1016/S1470-2045(20)30225-4
26. Danielou-Lazareth A, Henry G, Geromin D, et al. At diagnosis, diffuse large B-cell lymphoma patients show impaired rituximab-mediated NK-cell cytotoxicity. Eur J Immunol. 2013;43(5):1383–1388. doi:10.1002/eji.201242733
27. Sarkar S, Sabhachandani P, Ravi D, et al. Dynamic analysis of human natural killer cell response at single-cell resolution in B-cell non-Hodgkin lymphoma. Front Immunol. 2017;8:1736. doi:10.3389/fimmu.2017.01736
28. Duell J, Maddocks KJ, González-Barca E, et al. Long-term outcomes from the Phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417–2426. doi:10.3324/haematol.2020.275958
29. Maddocks KJ, Duell J, González-Barca E, et al. Long-term subgroup analyses from L-mind, a Phase II study of tafasitamab (MOR208) combined with lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Blood. 2020;136(Supplement 1):19–21. doi:10.1182/blood-2020-140314
30. Zinzani PL, Rodgers T, Marino D, et al. RE-MIND: Comparing Tafasitamab + Lenalidomide (L-MIND) with a real-world lenalidomide monotherapy cohort in relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2021;27(22):6124–6134. doi:10.1158/1078-0432.Ccr-21-1471
31. Nowakowski GS, Yoon DH, Peters A, et al. Improved efficacy of tafasitamab plus lenalidomide versus systemic therapies for relapsed/refractory DLBCL: RE-MIND2, an observational retrospective matched cohort study. Clin Cancer Res. 2022;28(18):4003–4017. doi:10.1158/1078-0432.Ccr-21-3648
32. A trial to evaluate the efficacy and safety of tafasitamab with Bendamustine (BEN) Versus Rituximab (RTX) With BEN in adult patients with relapsed or refractory Diffuse Large B-cell Lymphoma (DLBCL) - full text view - ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT02763319.
33. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi:10.1056/NEJMoa1707447
34. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi:10.1016/s1470-2045(18)30864-7
35. Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2021;386(7):640–654. doi:10.1056/NEJMoa2116133
36. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi:10.1016/S0140-6736(20)31366-0
37. Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi:10.1016/S0140-6736(22)00662-6
38. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2018;380(1):45–56. doi:10.1056/NEJMoa1804980
39. Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2021;386(7):629–639. doi:10.1056/NEJMoa2116596
40. Ahmed N, Shahzad M, Shippey E, et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transplant Cell Ther. 2022;28(7):358–364. doi:10.1016/j.jtct.2022.04.008
41. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790–800. doi:10.1016/s1470-2045(21)00139-x
42. Hamadani M, Linhares Y, Gandhi M, et al. Phase 3 randomized study of loncastuximab tesirine in combination with rituximab (Lonca-R) versus immunochemotherapy in patients with R/R DLBCL (LOTIS-5). J Clin Oncol. 2022;40(16_suppl):TPS7591–TPS7591. doi:10.1200/JCO.2022.40.16_suppl.TPS7591
43. Shalabi H, Kraft IL, Wang HW, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103(5):e215–e218. doi:10.3324/haematol.2017.183459
44. Thapa B, Caimi PF, Ardeshna KM, et al. CD19 antibody-drug conjugate therapy in DLBCL does not preclude subsequent responses to CD19-directed CAR T-cell therapy. Blood Adv. 2020;4(16):3850–3852. doi:10.1182/bloodadvances.2020002587
45. Horvei P, Sakemura R, Cox MJ, et al. Targeting of CD19 by tafasitamab does not impair CD19 directed chimeric antigen receptor T cell activity in vitro. Biol Blood Marrow Transplant. 2020;26(3):S223–S224. doi:10.1016/j.bbmt.2019.12.201
46. Duell J, Obr A, Augustin M, et al. CD19 expression is maintained in DLBCL patients after treatment with tafasitamab plus lenalidomide in the L-MIND study. Leuk Lymphoma. 2022;63(2):468–472. doi:10.1080/10428194.2021.1986219
47. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155–165. doi:10.1200/jco.19.00172
48. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533–543. doi:10.1182/bloodadvances.2021005794
49. Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7(7):e511–e522. doi:10.1016/S2352-3026(20)30120-4
50. Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891). doi:10.1126/science.1158545
51. Dufner V, Sayehli CM, Chatterjee M, et al. Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv. 2019;3(16):2491–2498. doi:10.1182/bloodadvances.2019000025
52. Goebeler ME, Knop S, Viardot A, et al. Bispecific T-Cell Engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a Phase I study. J Clin Oncol. 2016;34(10). doi:10.1200/JCO.2014.59.1586
53. Viardot A, Goebeler ME, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127(11):1410–1416. doi:10.1182/blood-2015-06-651380
54. Schuster SJ, Bartlett NL, Assouline S, et al. Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after Chimeric Antigen Receptor T-Cell (CAR-T) therapies, and is active in treatment through multiple lines. Blood. 2019;134:6. doi:10.1182/blood-2019-123742
55. Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a Phase I trial. J Clin Oncol. 2021;39(18):1959–1970. doi:10.1200/jco.20.03175
56. Dickinson MJ, Carlo-Stella C, Morschhauser F, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022;387(24):2220–2231. doi:10.1056/NEJMoa2206913
57. Hutchings M, Mous R, Clausen MR, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet. 2021;398(10306):1157–1169. doi:10.1016/s0140-6736(21)00889-8
58. Bannerji R, Arnason JE, Advani RH, et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9(5):e327–e339. doi:10.1016/S2352-3026(22)00072-2
59. Patel K. A Phase 1 Study of Plamotamab, an Anti-CD20 x Anti-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma: Recommended Dose Safety/Efficacy Update and Escalation Exposure-Response Analysis. ASH; 2022.
60. Budde E, Gopal AK, Kim WS, et al. A phase 1 dose escalation study of Igm-2323, a novel anti-CD20 x anti-CD3 IgM T Cell Engager (TCE) in patients with advanced B-cell malignancies. Blood. 2021;138:132. doi:10.1182/blood-2021-153355
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
