Back to Journals » OncoTargets and Therapy » Volume 15
Targeted Therapy for Locally Advanced or Metastatic Urothelial Cancer (mUC): Therapeutic Potential of Sacituzumab Govitecan
Authors Fontes MS, Vargas Pivato de Almeida D, Cavalin C , Tagawa ST
Received 30 July 2022
Accepted for publication 29 November 2022
Published 21 December 2022 Volume 2022:15 Pages 1531—1542
DOI https://doi.org/10.2147/OTT.S339348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Mariane S Fontes,1,2 Daniel Vargas Pivato de Almeida,2,3 Clarissa Cavalin,1 Scott T Tagawa4
1Oncology Department, Oncoclinicas Group, Rio de Janeiro, Brazil; 2LACOG, Latin American Cooperative Oncology Group, Brazil; 3Oncology Department, Oncoclinicas Group, Porto Alegre, Brazil; 4Weill Cornell Medicine, New York, NY, USA
Correspondence: Scott T Tagawa, Email [email protected]
Abstract: Urothelial carcinoma is the second most frequent genitourinary malignancy. Despite the poor prognosis, new treatment options have emerged and have expanded the therapeutic landscape for the disease. Although major improvements have been achieved, many patients experience rapid disease progression and low responses in subsequent lines of therapy. Sacituzumab govitecan is an ADC that targets Trop-2, which is highly expressed in urothelial cancers. Promising results in early clinical trials have led to further drug development which confirmed encouraging efficacy. Sacituzumab govitecan has been given accelerated approval in 2021 for patients with locally advanced and metastatic urothelial cancer who previously received a platinum containing chemotherapy and either a programmed death receptor-1 or programmed death ligand inhibitor. The results are promising, with encouraging efficacy and safety, however responses are not universal. There is a growing comprehension of mechanisms of resistance and predictive biomarkers that are crucial to improving outcomes. In this review, we summarize the current knowledge on antibody–drug conjugates and the clinical findings that led to the approval of Sacituzumab govitecan and discuss the therapeutic potential of new combinations, mechanisms of resistance and predictive biomarkers.
Keywords: antibody–drug conjugate, ADC, bladder cancer, Trop-2, human trophoblast cell surface antigen 2, SN-38
Introduction
Urothelial carcinoma (UC) is among the 10 most common types of cancer, with a steadily rising incidence worldwide, especially in developed and ageing countries. Although advanced disease accounts for the minority of cases, management remains quite challenging and the long-term prognosis is dismal, with a median overall survival (mOS) around 15 months.1,2
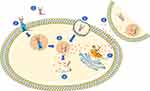 |
Figure 1 Mechanism of Action of Sacituzumab Govitecan. Notes: 1. Tumor penetration, 2. Binding and internalization, 3. Lysosomal activation, 4. Microtubule damage, 5. Alternate route: direct endocytosis, 6. Alternate route: Bystander effect, 7. Direct DNA damage. The figure was partly generated using Servier Medical Art, provided by Servier and licensed under a Creative Commons Attribution 3.0 unported license. (https://creativecommons.org/licenses/by/3.0/. |
Currently, widely accepted first-line regimens consist of cisplatin or carboplatin combined with gemcitabine, or MVAC, followed by maintenance anti-PD-L1 immunotherapy with avelumab.3 In a scenario of chemotherapy-ineligibility or cisplatin-unfit with high-PD-L1 tumor expression, single-agent pembrolizumab4 or atezolizumab5 are reasonable upfront treatment options.6
The therapeutic landscape of metastatic UC (mUC) has been expanded recently with the Food and Drug Administration (FDA) approval of erdafitinib for tumors harboring fibroblast growth factor receptor (FGFR) 2- or FGFR3-activating mutation or fusion7, enfortumab vedotin (EV)8 and sacituzumab govitecan (SG),2 all indicated after progression to platinum-based chemotherapy and either programmed cell death 1 (PD1) or programmed cell death ligand 1 (PD-L1) inhibitor.
Although major improvements have been achieved, many patients will experience rapid disease progression and low responses in subsequent lines of therapy. The development of antibody–drug conjugates (ADC) for cancer treatment has brought new insights, with promising ongoing trials. In this review, we aim to depict further ADCs, particularly the role of sacituzumab govitecan in mUC.2
Development of ADC
The development of ADCs can be tracked back to the early 1900s when Paul Ehrlich’s first conceived the Zuberkugel theory - a “magic bullet” that would deliver a toxic drug to target tumor cells while sparing others.9,10 The following decades proved this idea simplistic, and several research technological advancements had to be made until reaching clinically meaningful results, moving from mouse monoclonal antibodies and chimeric murine hybridomas to fully human antibodies linked to drugs 100–1000 times more potent at nano or picomolar concentrations.9–11
Biologic Rationale
Theoretically, the ideal ADC is based on the combination of a monoclonal antibody (mAb) that is highly selective for a tumor-associated antigen, a linker that is stable in blood circulation yet is readily cleavable at the target site and a toxic payload that induces target cell death after tumor cell internalization with payload release.12,13 Each of these steps is intrinsically codependent of each other both in structure and function to perform a more effective therapeutic profile with less systemic toxicity.9,10
The antibody is the main component, and its size accounts for over 90% of the mass of any given ADC. They should possess target specificity, target-binding affinity, good retention, low immunogenicity, low cross-reactivity and appropriate linkage-binding properties. IgG isotype, especially IgG1 subclass, remains the most popular with regard to ADC development due to potent activation of the classical complement pathway and higher binding affinity for IgG-binding Fc-gamma receptors, which are more likely to trigger the desired immune response.14
An ideal tumor target antigen is highly expressed with limited heterogeneity across the tumor and low normal tissue expression (to minimize “on-target, off-tumor toxicity); minimal antigen shedding prevents the antibody binding to its target within the circulation.10,12 A minimum value of tumor-antigen density is a requirement for ADC efficacy, but the desirable cutoff value of antigen expression varies greatly and depends on other target antigen properties such as the internalization rate and binding affinity.10,14,15
Linker chemistry impacts various ADC properties including pharmacokinetics, therapeutic index and efficacy, toxicity, specificity, stability and potency. They can be broadly classified as either cleavable (the payload is able to separate from the mAb at the tumor site) or non-cleavable (payload and mAb remain bound together, mAb is degraded following internalization).9,14 These properties are likely related to a given ADC's ability to target stroma in addition to tumor and to bystander effects.
Another major factor is the number of drug molecules to be loaded onto the antibody: the drug–antibody ratio (DAR). Attaching too few will lead to decreased efficacy, whereas too many may render an unstable ADC with reduced half-life, increased plasma clearance and systemic toxicity.9,13 The optimal DAR is undetermined and highly dependent on other ADC variables; DARs of currently approved ADCs range from 2 to 8.9,10
The cytotoxic payload is the ultimate effector component of an ADC. Its actual concentration in tumor cells is minimal with only 0.1–1% of the administered dose reaching the tumor. Therefore, the optimal chemotherapy drug used should be extremely potent at pico- or nanomolar concentrations and therefore is not suitable for systemic delivery as free drugs in plasma.15,16 The most recent generation of ADCs include both DNA damaging/alkylating agents (duocarmycin, calicheamicin, topoisomerase inhibitors, pyrrolobenzodiazepines) and molecules interfering with microtubule structure (maytansinoids, tubulysins and auristatin derivatives). Though out of scope for this review, it is also possible to deliver radioimmunoconjugates or bispecific agents drawing immune components to tumor cells.9,11,15,16
Mechanism of Action
ADCs have poor oral bioavailability and are therefore generally administered intravenously to prevent degradation by digestive enzymes. They are typically scheduled in a similar way to chemotherapy, with weekly doses.11 In animal models, drug concentrations in cancer cells often peak within 1–2 days following IV administration, reaching levels that can exceed their concentration in nonmalignant tissue by 100-fold.13 After extravasation from capillaries, antibodies reach tumor cells via passive diffusion, often resulting in heterogeneous tissue penetration.13 The antibody–antigen engagement triggers a signal in the tumor cell, which then internalizes the antibody together with the linked cytotoxin, predominantly through receptor-mediated endocytosis.
Once inside lysosomes or endosomes, acidic, proteolytic or redox conditions cause the ADC payloads to be released from their antibody carriers, so they can diffuse into the cytosol and throughout the cell to act on their target substrates, ultimately resulting in cell death.13 Antibody-dependent cellular cytotoxicity also contributes to cell death through complement-dependent cytotoxicity and/or antibody-dependent cellular phagocytosis.9,13
Hydrophobic payloads can also diffuse through cell membranes, and some ADCs with weaker linkers may release payload in tumor microenvironment which can result in cytotoxic activity against neighboring cells irrespective of their expression of the target antigen. This “bystander effect” might be an important contributor to the efficacy of ADCs, especially in tumors with heterogeneous expression of the antibody target.13 (Figure 1)
Use of ADCs in UC
Although clinical experience with ADCs was initially centered in the treatment of hematological malignancies and HER2-positive metastatic breast cancer,17,18 the high expression of selected cellular antigens in the surface of tumor cells and their relationship with tumor growth, invasion, metastatic progression, and treatment resistance has emerged UC as an interesting model for the clinical development of ADCs. Potential surface markers targeted under investigation in clinical trials are Nectin-4, human epithelial growth factor 2 (HER2), epidermal growth factor receptor (EGFR), trophoblast cell surface antigen 2 (Trop 2), epithelial cell adhesion molecule (EpCAM), SLIT and NTRK-like protein 6 (SLITRK6), Integrin β6, B7 homologue 3 (B7-H3), and CD25 (Table 1).
 |
Table 1 Antibody–Drug Conjugates Under Clinical Investigation in Urothelial Carcinoma |
EV is a fully humanized monoclonal antibody constructed with a protease-cleavable linker to a cytotoxic payload of monomethyl auristatin E (MMAE) directed against Nectin-4, a surface protein highly expressed in UC.20 Following a Phase 1 study demonstrating safety and preliminary efficacy,21 Cohort 1 of the EV-201 trial in patients with previous exposure to platinum-based chemotherapy and anti-PD-L1 immune checkpoint inhibitor demonstrated EV’s promising antitumoral activity in UC with objective response rate (ORR) 44% (12% CR and 32% PR), leading to accelerated US FDA approval.22 In the confirmatory Phase III trial, EV improved OS (mOS, 12.88 vs 8.97 months; hazard ratio (HR) for death, 0.70; 95% confidence interval (CI), 0.56 to 0.89; P = 0.001) and PFS (mPFS, 5.55 vs 3.71 months; HR for progression or death, 0.62; 95% CI, 0.51 to 0.75; P < 0.001) in comparison to chemotherapy (docetaxel, paclitaxel, or vinflunine), resulting in its full regulatory approval by the FDA.23 There is an interesting preliminary efficacy of this drug in combination with pembrolizumab, and it is being studied in additional combinations for metastatic disease and as single-agent and in combination in the neoadjuvant setting (NCT 03288545, NCT04223856, NCT03924895).
The HER2 protein is encoded by the HER2/neu oncogene and contributes to cell proliferation through downstream activation of mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K/Akt) pathways.24 The prevalence of HER2/neu amplification occurs in up to 8% of urothelial tumors, while overexpression of HER2 protein can be present in 52% of muscle-invasive urothelial cancers.25 At least four ADCs targeting HER2 – disitamab vedotin, trastuzumab duocarmazine, trastuzumab emtansine and trastuzumab deruxtecan – are under clinical investigation for the treatment of UC, either in monotherapy or combined with other drugs.26 Disitamab vedotin (RC-48) has the most information to date, with preliminary data leading to the US FDA breakthrough designation.27 The Phase 2 trial of patients with HER2+ (IHC status 3+ or 2+) locally advanced or mUC who previously failed at least one line of systemic chemotherapy showed an ORR of 51.2%, a median PFS and OS of 6.9 months (95% CI, 5.6–8.9) and 13.9 months (95% CI, 9.1-NE), respectively.27 Recently, the RC48-C011 trial assessed the efficacy and safety of disitamab vedotin in HER2-low tumors, demonstrating that the ORR was 26.3% (95% CI 9.1% to 51.2%), median PFS and OS were 5.5 months (95% CI 3.9 to 6.8) and 16.4 months (95% CI 7.1 to 21.7), respectively.28 Combinations with anti-PD1 are also being explored with promising activity and manageable safety profiles.29
The prevalence of EGFR genomic alterations (base substitutions, small insertions and deletions, amplifications, or rearrangements) in UC can reach 4.1%, with gene amplification being the most frequent alteration (64% of the altered cases).30 Serclutamab talirine (ABBV-321) is an antibody with high affinity to EGFR and a potent pyrrolobenzodiazepine dimer toxin currently under clinical investigation in UC.31,32
High expression of the transmembrane glycoprotein Trop 2 has been described in diverse epithelial tumors, with higher Trop 2 expression being linked to poor prognosis.33,34 SG and SKB264 represent ADCs directed to Trop 2 in clinical development.2,35
EpCAM, a transmembrane glycoprotein associated with E-cadherin dependent cell adhesion, has been demonstrated to be expressed in up to 54% of UC, including non-muscle invasive (NMI) tumors (Tis, Ta, and T1).36 Oportuzumab monatox targets EpCAM and has demonstrated clinical activity in Phase 1 and 2 studies with a complete response at 3 months in subjects with carcinoma in situ (CIS) of 29% and 40%, respectively.37 The Phase III VISTA trial in patients with BCG-unresponsive NMI UC showed a 42% CR rate at 3-months in subjects with CIS recurring within 12 months of the last BCG and a 68% recurrence-free rate at 3-months in recurrent papillary-only NMI bladder cancer.38 Additional clinical and statistical data were requested by the FDA to evaluate the potential approval of the agent.
Sirtratumab vedotin is composed by a SLITRK6 antibody linked to an MMAE payload via a protease-cleavable linker. Expression of SLITRK6 in bladder cancer can occur in up to 100% of the metastatic tumors, while only weak and diffuse expression is seen in other normal tissues.39 Initial results of clinical experience with sirtatumab vedotin in mUC demonstrated an overall response rate of 33% and a median progression-free survival of 16 weeks showing a promising antitumor activity and tolerability.40
Other ADCs being investigated in early phase clinical trials in UC are SGN-B6A, DS-7300 and camidanlumab tesirine targeting integrin β6, B7-H3 transmembrane glycoprotein and anti-CD25.41–43
Role of Human Trophoblast Cell Surface Antigen 2
Initially described in trophoblasts and fetal tissues, the expression of Trop 2 was actually explored in the 1990s after its recognition as a unique marker of trophoblasts and neoplastic cells.44 Trop 2 is a 36-kDa transmembrane calcium signal transducing glycoprotein encoded by the TACSTD2 gene mapped on chromosome 1p32 and modified post-translationally by N-linked glycosylation to form a type-1 transmembrane protein.45,46 Through the calcium signaling regulation, Trop 2 activates ERK1/2 (extracellular signal regulated kinase)-MAPK pathways, promoting cell cycle progression and deregulation of stem cell functions via Notch, Hedgehog and Wnt pathways.45 Of interest, the Trop 2 overexpression does not result from gene amplification or inactivating mutation as seen in other oncogenes.46 A strong correlation was demonstrated between the Trop 2 expression and cyclin D1, cyclin E, p27, p16, ER⍺ and HER2, known markers of proliferation and differentiation.46 The overexpression of Trop 2 was found to be a predictive marker of poorer OS (pooled HR: 2.77, 95% CI: 1.45–2.35), disease-free survival (pooled HR: 2.77, 95% CI: 1.73–2.42), and PFS (pooled HR: 1.71, 95% CI: 1.25–2.35) in a meta-analysis including 16 types of primary tumors.47
Mechanism of Action of SG
SG represents a third-generation ADC developed by site-specific conjugation of SN-38 (govitecan), an irinotecan active metabolite covalently linked via a hydrolysable CL2A linker to a humanized mAb directed against Trop 2 (hRS7).48 This conjugation results in an average DAR of 7.6:1. SN-38 is a semi-synthetic camptothecin with cytotoxic effect through topoisomerase I inhibition, resulting in double-stranded DNA break during S-phase of the cell cycle.49 The IC50 of SN-38 is approximately 1.0–6.0 nM when tested in vitro in different cell lines.50,51 Notwithstanding, beyond the cytotoxic effects resulting from the internalization of SN-38, it is advocated that SG also acts through a bystander effect via the release of SN-38 in the tumor microenvironment.50
Pharmacokinetics
Primary data from the first-in-human phase 1 trial of multiple diseases and dose cohorts established the dose of 10mg/kg of SG for future development.52 At this dose, there is a proportional increase in the antibody’s concentration with continued treatment, the half-lives of SG and free SN38 are 16 and 18 h, respectively. The clearance of systemic SG is around 11 to 14 h, whereas the naked antibody is cleared over approximately 103 to 114 h. SN38 has minimal renal excretion, but there are no safety data for patients with creatinine clearance below 30 mL/min and dosing in patients with hepatic impairment remains unexplored.48 The metabolism of SN-38 is mediated by UGT1A1, and the glucuronide metabolite SN-38G can be detectable in serum. Patients with UGT1A1*28 allele homozygosis may have an increased risk of neutropenia when treated with SG.53
Current Approvals of SG
The safety and efficacy of SG was evaluated in the phase I/II basket trial IMMU-132-01 (NCT01631552) in adult patients with advanced epithelial cancers presenting with disease progression after at least one line of standard systemic treatment.54 Among the 108 patients with metastatic triple-negative breast cancer who received SG as a third- or higher-line of therapy, 66% presented with visceral metastasis, 42% had liver metastasis, and 2% had brain metastasis. The median number of prior systemic treatments was 3 (range: 2–10). The overall response rate as assessed by the investigators was 33.3% (95% IC: 24.6–43.1), including three complete responses. The median duration of response was 7.7 months (95% IC: 4.9–10.8). Among the responders, 55.6% and 16.7% presented response duration of at least 6 and 12 months, respectively.55 Based on those results, SG received an accelerated approval by the FDA in February 2020.56
In the confirmatory multicentric, randomized, phase III ASCENT trial (NCT02574455), SG was compared to physician’s choice of chemotherapy (eribulin, vinorelbine, capecitabine, or gemcitabine) in 529 patients with metastatic triple-negative breast cancer previously treated with at least two lines of standard systemic therapy. Of note, the enrollment of patients with brain metastasis was pre-defined to a maximum of 15% of the patients. The primary objective of the study was PFS in patients without brain metastasis as analyzed by independent central review. The mPFS analyzed for the primary outcome was 5.6 months (95% CI: 4.3–6.3) with SG and 1.7 months (95% CI: 1.5–2.6) with chemotherapy (HR: 0.41, 95% CI: 0.32–0.52, P < 0.001). The mOS of the population without brain metastasis, a secondary endpoint, was 12.1 months (95% CI: 10.7–14.0) and 6.7 months (95% CI: 5.8–7.7) with SG and chemotherapy, respectively (HR: 0.48, 95% IC: 0.38–0.59, P < 0.001). When all the randomized patients were analyzed, the benefit towards treatment with SG was maintained both in PFS (HR: 0.43, 95% IC: 0.35–0.54, P < 0.0001) and OS (HR: 0.51, 95% CI: 0.41–0.62, P < 0.0001). The objective response rates were 31% with SG and 4% with chemotherapy, and the median duration of response with the ADC was 6.3 months (95% CI: 5.5–9.0).55 Following those results, the regular approval was granted by the FDA for the use of SG in previously treated metastatic triple-negative breast cancer in April 2021.57 More recently, the results of the phase III TROPiCS-02 demonstrated the benefit of SG over chemotherapy in patients with ER positive and HER2 negative metastatic breast cancer with improved PFS.58
The favorable efficacy results of SG for the treatment of advanced UC were initially seen in the IMMU 132-01 trial and later in the TROPHY trial (NCT03547973). In the initial portion of the phase 1 trial, better than expected activity in patients with advanced UC was observed.59 In an expansion cohort of the phase 1/2 basket study, 45 UC patients received SG 10 mg/kg with a median of 2 prior lines of therapies. The ORR was 31%; median duration of response was 12.9 months, the mPFS was 7.3 months and mOS was 16.3 months.60 The encouraging results led to further investigation in a dedicated phase 2 trial, the TROPHY trial.2 Cohort 1 of the single-arm Phase II TROPHY trial included 113 patients of whom 96% had metastatic disease, 66% had visceral metastasis, 34% had liver metastasis, and 50% had previously received at least three prior lines of systemic therapy. The overall response rate as determined by blinded independent central review was 27.4% (95% CI: 19.5–36.6), including six complete responses. The clinical benefit rate (including complete responses, partial responses, and stable disease lasting at least 6 months) was 37.2% (95% CI: 28.3–46.8). The response rate in patients presenting with liver metastasis was 31.6% (95% CI: 17.5–48.7) and in those with prior treatment with EV was 30%. Any reduction in target lesions was achieved by 77% of patients. The median duration of response was 7.2 months (95% CI: 4.7–8.6).2 The results led to the accelerated approval of SG for the treatment of patients with locally advanced or mUC previously treated with a platinum-based chemotherapeutic regimen and either a PD-1 or a programmed death ligand 1 (PD-L1) inhibitor in April 2021 by the FDA.56 The confirmatory phase III TROPiCS-04 trial is ongoing, seeking full regulatory approval of SG for the treatment of advanced urothelial carcinoma.61
Patient selection for treatment with SG is based on clinical factors, locally advanced or mUC previously treated with a platinum-based chemotherapeutic regimen and either a PD-1 or a PD-L1 inhibitor. Selection based on a specific biomarker is not recommended, and the benefit seen in the second and third line occurs despite antigen expression and is due to be confirmed in the Phase 3 trial. Currently, SG is most commonly used in later lines of therapy (third or fourth line), after EV, but trials are exploring the role of SG monotherapy and in combination earlier in the treatment of UC.
Toxicity and Safety
Sacituzumab govitecan is safe and has a predictable and manageable toxicity profile. The most common adverse events (AE) are nausea (66%), diarrhea (65%), fatigue (62%), neutropenia (61%), alopecia (45%), anemia (42%), vomiting (39%), constipation (37%), decreased appetite (34%), rash (32%) and abdominal pain (28%).2,52,54,55
Neutropenia and diarrhea are the most common AEs, leading to drug interruptions or dose reductions. Although grade 3/4 neutropenia occurred in 46% of patients with advanced UC in cohort 1 of the TROPHY-U-01 study, 10% of febrile neutropenia was observed. Sixty-six percent and 10% of patients had any grade and grade 3/4 diarrhea, respectively. Neutropenic colitis and intestinal perforation have been reported but are rare events. The incidence of any grade and grade 3/4 nausea was of 60% and 4% and that of any grade and grade 3/4 vomiting was of 30% and 1%, respectively. For adequate control of symptoms preventive measures with steroids and 5HT3 receptor antagonists or NK1 receptor antagonists may be necessary.2,52,54,55
Uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1) is responsible for metabolizing SN38. The activity of UGT1A1 can be reduced in some populations due to the presence of allele gene variants, UGT1A1*28; therefore, the genotype status has been evaluated as a predictor of AE.62 Some studies have shown that the pattern and incidence of AE are similar in patients heterozygous for the UGT1A1*28 allele and wild type, though in TROPHY U-01, neutropenia appeared to be worse in heterozygotes. The incidence of neutropenia, diarrhea and anemia is numerically higher in patients homozygous for UGT1A1*28 allele than in heterozygous or wild-type patients with neutropenia specifically identified as worse in homozygotes.48,54 Nevertheless, the percentage of patients who permanently discontinued treatment is similar regardless of the UGT1A1 genotype status.52,54 Currently, there is no formal recommendation for the adoption of a preemptive UGT1A1 genotyping to increase safety in clinical practice, and additional validation is needed prior to the use of genetic testing for clinical decision-making.
Biomarkers of Response
The target antigen expression is heterogeneous and dynamic for many solid tumors; therefore, its measurements can aid patient selection for ADC treatment. Although many ADCs have been approved regardless of the levels of antigen expression, there are signals suggesting that the level of antigen expression correlates with therapy response. In the trial that led to the accelerated approval of SG in UC, 19% of patients had progressive disease and 34% had stable disease as best response suggesting that de novo resistance mechanism to this agent is common emphasizing the need for predictive biomarkers for better patient selection.
Genomic and transcriptomic analysis of tumor tissue from patients with metastatic triple negative breast cancer treated with SG showed that patients with progressive disease as best response had undetectable TROP2 RNA expression63 these findings are supported by data demonstrating that absence of TROP2 expression is commonly associated with primary resistance to SG.55 These findings highlight that TROP2 expression can potentially be used in patient selection for SG.
Mechanisms of Resistance
The mechanisms of resistance for ADCs are not completely understood, but initial evidence suggests that acquired resistance mechanisms are complex and multifactorial involving antibody-antigen engagement due to antigen downregulation or loss, alteration in ADC internalization and payload tolerance or efflux.
Antigen downregulation or loss impacts antibody binding and release of payload. The altered expression within the tumor may be a result of gene mutations and by selection of non-antigen expressing cells within a highly heterogeneous cell population. The relationship between Trop-2 expression in triple negative breast cancer and response to SG has been previously described, and patients with high and moderate expression of Trop-2 benefit more from treatment with SG than patients with low or no Trop2 expression.55,63 In an analysis of the expression of TROP2 and NECTIN4 in published datasets, cell lines, and patient tumor samples, both were highly expressed in most samples.64 TROP2 was more highly expressed in human patient samples and cell lines. Most subtypes of urothelial carcinoma maintained TROP2 expression except the neuroendocrine subtype. In preclinical studies, expression levels were associated with ADC sensitivity. When examining EV resistant cells, NECTIN4 expression was low, but TROP2 expression was maintained and these cells remained sensitive to SG, consistent with the hypothesis that SG is not cross resistant to EV. An exploratory analysis of TROP2 expression and relationship to outcome with SG in cohorts 1 and 2 of the TROPHY-U-01 study demonstrated that the vast majority (95%) had intermediate to high expression and responses occurred across expression levels though the utility of SG in those with low TROP2 expression is understudied.65 Studies are needed to explore ways to overcome this mechanism of resistance, such as targeting two tumor antigens with a bispecific antibody.
The cytotoxic payload is susceptible to the same multidrug resistance mechanisms as conventional chemotherapy drugs. Increased elimination of the payload via ATP-binding cassette transporters (MDR1, MRP1 and BCRP) may contribute to resistance to ADC. Resistance mutations to the molecular target of the payload may arise ensuing decreased susceptibility to treatment, such as resistance mutations in Topoisomerase-1.55,63 In addition, there is evidence that parallel genomic alteration of antigen and payload targets may occur63 Identifying how these mutations temporally emerge under selective pressure from ADCs, will enable the development of sequential strategies to improve outcomes of patients treated with these agents.
Clonal expansion of resistant cells due to treatment pressure and intratumoral heterogeneity representing populations of cells that are primarily resistant within the same tumor are likely playing a role in resistance mechanisms to ADCs. In addition, acquired resistance may share common mechanisms involved in resistance to chemotherapy, such as activating mutations in PIK3CA.
A more accurate understanding of these mechanisms of resistance may provide insights regarding the drug intrinsic mechanism of action and can help shape strategies to overcome resistance. Some potential strategies involve ADC-like approaches which abandon the traditional antibody backbone entirely in favor of “miniaturized” small-molecule drug conjugates that utilize targeting moieties such as peptide fragments, single-chain variable fragments or diabodies to deliver their toxic payloads to antigen-expressing cells. These efforts are largely motivated by hopes that smaller drug conjugates will have improved tumor tissue penetration and thus payload delivery can be limited by rapid plasma clearance. Reduction of drug resistance and efficacy can perhaps be achieved by combination therapies, and potentially molecular imaging may be used to study the biodistribution and pharmacodynamics of ADCs to determine target expression in the tumor and to detect interlesional heterogeneity in order to predict response.66
Combinations with SG
Combinations with SG are being studied in multiple cohorts in the TROPHY U-01 trial. The interim efficacy results of cohort 3 of SG in combination with pembrolizumab as a second-line therapy in patients who progressed after platinum-based chemotherapy were recently presented and showed an ORR of 34% by investigator assessment and short follow-up, a clinical benefit rate of 44% and a 6 months PFS rate of 47%. (61) The most common treatment-related AE was diarrhea (76%), nausea (59%), anemia (56%), neutropenia (44%), and asthenia (41%).67 Cohort 4 assessing the combination of SG with cisplatin followed by maintenance SG and avelumab is ongoing with additional cohorts planned.19
Other combinations are being explored on ongoing clinical trials with SG and other ADCs in the metastatic setting such as SG and atezolizumab; SG, ipilimumab and nivolumab; SG and EV; EV and cisplatin/carboplatin; EV and gemcitabine; and EV, platinum and pembrolizumab. (Table 2)
 |
Table 2 Ongoing Clinical Trials with Sacituzumab-Govitecan (SG) in UC |
Conclusion
In the last five years, solid advances in the treatment of mUC have been achieved, however, this is still a lethal disease. SG has shown encouraging efficacy and is now approved in the metastatic setting in patients previously treated with platinum chemotherapy and checkpoint inhibitors, although promising, responses are not universal. A better comprehension of the mechanisms of resistance and identification of predictive biomarkers is an important step to improve outcomes. Furthermore, new combinations are being explored both in metastatic and in earlier settings, but it might not be enough; therefore, pursuing the development of new Trop-2 target ADC is essential as well as identifying ways to target Trop-2 in different ways.
In conclusion, SG has already entered clinical practice due to its solid efficacy and safety results in treating specific tumor types, including urothelial cancers. However, further preclinical and clinical studies are needed to fully understand and exploit the full therapeutic potential of this ADC.
Disclosure
Dr Mariane S Fontes reports personal fees and travel expenses from Janssen Brazil, Astellas, MSD, AstraZeneca, Zodiac; personal fees from Bayer and Amgen, during the conduct of the study. Dr Daniel Vargas Pivato de Almeida reports personal fees from Astellas, AstraZeneca, Bayer, Eli Lilly, Ipsen, Janssen-Cilag, and Sanofi-Aventis, outside the submitted work. Dr Clarissa Cavalin reports personal fees and/or non-financial support from Bayer, Eli Lilly, AstraZeneca, and Novartis, outside the submitted work. Dr Scott T Tagawa reports grants and/or personal fees from Gilead, Seagen, Janssen, EMD Serono, and Merck, outside the submitted work. In addition, Dr Scott T Tagawa has a patent (biomarkers for sacituzumab govitecan therapy [Immunomedics / Gilead / Weill Cornell) issued to Gilead. The authors report no other conflicts of interest in this work.
References
1. Saginala K, Barsouk A, Aluru JS, et al. Epidemiology of bladder cancer. Med Sci. 2020;8(1):15.
2. Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39(22):2474–2485. doi:10.1200/JCO.20.03489
3. Powles T, Sridhar SS, Loriot Y, et al. Avelumab maintenance in advanced urothelial carcinoma: biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nat Med. 2021;27(12):2200–2211. doi:10.1038/s41591-021-01579-0
4. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi:10.1016/S1470-2045(17)30616-2
5. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi:10.1016/S0140-6736(16)32455-2
6. Cathomas R, Lorch A, Bruins HM, et al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol. 2022;81(1):95–103. doi:10.1016/j.eururo.2021.09.026
7. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–348. doi:10.1056/NEJMoa1817323
8. Yeon SH, Lee HJ. Enfortumab vedotin in advanced urothelial carcinoma. N Engl J Med. 2021;385(1):93.
9. Ponziani S, Di Vittorio G, Pitari G, et al. Antibody-drug conjugates: the new frontier of chemotherapy. Int J Mol Sci. 2020;21(15):5510. doi:10.3390/ijms21155510
10. Hafeez U, Parakh S, Gan HK, et al. Antibody-drug conjugates for cancer therapy. Molecules. 2020;25(20):4764. doi:10.3390/molecules25204764
11. Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016;17(6):e254–e262. doi:10.1016/S1470-2045(16)30030-4
12. Diamantis N, Banerji U. Antibody-drug conjugates – an emerging class of cancer treatment. Br J Cancer. 2016;114(4):362–367. doi:10.1038/bjc.2015.435
13. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18(6):327–344. doi:10.1038/s41571-021-00470-8
14. Baah S, Laws M, Rahman KM. Antibody-drug conjugates – a tutorial review. Molecules. 2021;26(10):2943. doi:10.3390/molecules26102943
15. Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. 2019;394(10200):793–804. doi:10.1016/S0140-6736(19)31774-X
16. Khongorzul P, Ling CJ, Khan FU, et al. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3–19. doi:10.1158/1541-7786.MCR-19-0582
17. Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862. doi:10.1016/S0140-6736(15)60165-9
18. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi:10.1056/NEJMoa1209124
19. Tagawa ST, Petrylak GP, Petrylak DP. TROPHY-U-01 cohort 4: sacituzumab govitecan (SG) in combination with cisplatin (Cis) in platinum (PLT)-naïve patients (pts) with metastatic urothelial cancer (mUC). J Clin Oncol. 2022;40(6_suppl):TPS581–TPS581. doi:10.1200/JCO.2022.40.6_suppl.TPS581
20. Rosenberg JE, Terence TWF, Friedlander TW. Study EV-103: durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J Clin Oncol. 2020;38(15_suppl):5044. doi:10.1200/JCO.2020.38.15_suppl.5044
21. Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol. 2020;38(10):1041–1049. doi:10.1200/JCO.19.02044
22. Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37(29):2592–2600. doi:10.1200/JCO.19.01140
23. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–1135. doi:10.1056/NEJMoa2035807
24. Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–6487. doi:10.1038/sj.onc.1210477
25. Latif Z, Watters AD, Dunn I, et al. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer. 2003;89(7):1305–1309. doi:10.1038/sj.bjc.6601245
26. Patelli G, Zeppellini A, Spina F, et al. The evolving panorama of HER2-targeted treatments in metastatic urothelial cancer: a systematic review and future perspectives. Cancer Treat Rev. 2022;104:102351. doi:10.1016/j.ctrv.2022.102351
27. Sheng X, Yan X, Wang L, et al. Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. 2021;27(1):43–51. doi:10.1158/1078-0432.CCR-20-2488
28. Huayan X, Sheng X, Yan X, et al. A phase II study of RC48-ADC in HER2-negative patients with locally advanced or metastatic urothelial carcinoma,
29. Xinan Sheng LZ, Zhisong H, Guo H, et al. Preliminary Results of a Phase Ib/II Combination Study of RC48-ADC, a Novel Humanized Anti-HER2 Antibody-Drug Conjugate (ADC) with Toripalimab, a Humanized IgG4 mAb Against Programmed Death-1 (PD-1) in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Chicago, USA: JCO; 2022.
30. Madison RW, Gupta SV, Elamin YY, et al. Urothelial cancer harbours EGFR and HER2 amplifications and exon 20 insertions. BJU Int. 2020;125(5):739–746. doi:10.1111/bju.15006
31. Anderson MG, Falls HD, Mitten MJ, et al. Targeting multiple EGFR-expressing tumors with a highly potent tumor-selective antibody-drug conjugate. Mol Cancer Ther. 2020;19(10):2117–2125. doi:10.1158/1535-7163.MCT-20-0149
32. A study evaluating the safety, pharmacokinetics, and anti-tumor activity of ABBV-321 in subjects with advanced solid tumors associated with overexpression of the epidermal growth factor. Receptor (EGFR).
33. Cubas R, Zhang S, Li M, et al. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9(1):253. doi:10.1186/1476-4598-9-253
34. Avellini C, Licini C, Lazzarini R, et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget. 2017;8(35):58642–58653. doi:10.18632/oncotarget.17407
35. Rodon J, Xue LJ, Xue J, et al. An open-label, global, first-in-human study of SKB264 in patients with locally advanced or metastatic solid tumors. Ann Oncol. 2021;32:S585. doi:10.1016/j.annonc.2021.08.1036
36. Brunner A, Verdorfer PM, Verdorfer I, Tzankov A, Mikuz G, Ensinger C. EpCAM is predominantly expressed in high grade and advanced stage urothelial carcinoma of the bladder. J Clin Pathol. 2008;61(3):307–310. doi:10.1136/jcp.2007.049460
37. Kowalski M, Guindon J, Brazas L, et al. A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guérin. J Urol. 2012;188(5):1712–1718. doi:10.1016/j.juro.2012.07.020
38. Dickstein R, Cowan B, Cowan B, et al. LBA27 phase 3 study of vicinium in Bcg-unresponsive non-muscle invasive bladder cancer: initial results. J Urol. 2018;199(4S). doi:10.1016/j.juro.2018.03.099
39. Morrison K, Challita-Eid PM, Raitano A, et al. Development of ASG-15ME, a novel antibody–drug conjugate targeting SLITRK6, a new urothelial cancer biomarker. Mol Cancer Ther. 2016;15(6):1301–1310. doi:10.1158/1535-7163.MCT-15-0570
40. Petrylak D, Sonpavde HE, Sonpavde G, et al. Interim analysis of a phase I dose escalation trial of the antibody drug conjugate (ADC) AGS15E (ASG-15ME) in patients (Pts) with metastatic urothelial cancer (mUC). Ann Oncol. 2016;27:vi269. doi:10.1093/annonc/mdw373.08
41. Calvo E, Dowlati HA, Dowlati A. 555TiP A first-in-human trial of the integrin beta-6-targeted antibody–drug conjugate, SGN-B6A, in patients with advanced solid tumors. Ann Oncol. 2021;2:S613. doi:10.1016/j.annonc.2021.08.1077
42. Dong P, Xiong Y, Yue J, et al. B7H3 as a promoter of metastasis and promising therapeutic target. Front Oncol. 2018;8:264. doi:10.3389/fonc.2018.00264
43. Johnson ML, Piha-Paul DT, Piha-Paul SA, et al. 513O A phase I/II multicenter, first-in-human study of DS-7300 (B7-H3 DXd-ADC) in patients (pts) with advanced solid tumors. Ann Oncol. 2021;32:S583–S585. doi:10.1016/j.annonc.2021.08.1035
44. Vranic S, Gatalica Z. Trop-2 protein as a therapeutic target: a focused review on Trop-2-based antibody-drug conjugates and their predictive biomarkers. Bosn J Basic Med Sci. 2022;22(1):14–21. doi:10.17305/bjbms.2021.6100
45. Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9(48):28989–29006. doi:10.18632/oncotarget.25615
46. Guerra E, Trerotola M, Aloisi AL, et al. The Trop-2 signalling network in cancer growth. Oncogene. 2013;32(12):1594–1600. doi:10.1038/onc.2012.151
47. Xu P, Zhao Y, Liu K, et al. Prognostic role and clinical significance of trophoblast cell surface antigen 2 in various carcinomas. Cancer Manag Res. 2017;9:821–837. doi:10.2147/CMAR.S147033
48. Syed YY. Sacituzumab govitecan: first approval. Drugs. 2020;80(10):1019–1025. doi:10.1007/s40265-020-01337-5
49. Goldenberg DM, Sharkey RM. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: a case study of anti-TROP-2 sacituzumab govitecan. MAbs. 2019;11(6):987–995. doi:10.1080/19420862.2019.1632115
50. Goldenberg DM, Cardillo TM, Govindan SV, et al. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496–22512. doi:10.18632/oncotarget.4318
51. Cardillo TM, Govindan SV, Sharkey RM, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26(5):919–931. doi:10.1021/acs.bioconjchem.5b00223
52. Ocean AJ, Starodub AN, Bardia A, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics. Cancer. 2017;123(19):3843–3854. doi:10.1002/cncr.30789
53. U.S. Food and Drug Administration (FDA). TRODELVY (sacituzumab govitecan-hziy) for injection, for intravenous use: US Prescribing Information; 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761115s009lbl.pdf.
54. Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32(6):746–756. doi:10.1016/j.annonc.2021.03.005
55. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi:10.1056/NEJMoa2028485
56. FDA, F.g.a.a.t.s.g.-h.f.m.t.n.b.c; 2022. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sacituzumab-govitecan-hziy-metastatic-triple-negative-breast-cancer.
57. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer | FDA; 2022. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer.
58. Marmé F, Cortes J. Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2022;40(29):3365–3376. doi:10.1200/JCO.22.01002
59. Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab govitecan, a novel antibody – drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer. 2016;14(1):e75–9. doi:10.1016/j.clgc.2015.10.002
60. Tagawa ST, Lam FB, Lam ET, et al. Sacituzumab govitecan (IMMU-132) in patients with previously treated metastatic urothelial cancer: results from a phase I/II study. J Clin Oncol. 2019;37(7_suppl):354. doi:10.1200/JCO.2019.37.7_suppl.354
61. Grivas P, Bellmunt TS, Bellmunt J, et al. TROPiCS-04: study of sacituzumab govitecan in metastatic or locally advanced unresectable urothelial cancer that has progressed after platinum and checkpoint inhibitor therapy. J Clin Oncol. 2021;39(6_suppl):TPS498. doi:10.1200/JCO.2021.39.6_suppl.TPS498
62. Spring LM, Nakajima E, Hutchinson J, et al. Sacituzumab govitecan for metastatic triple-negative breast cancer: clinical overview and management of potential toxicities. Oncologist. 2021;26(10):827–834. doi:10.1002/onco.13878
63. Coates JT, Sun S, Leshchiner I, et al. Parallel genomic alterations of antigen and payload targets mediate polyclonal acquired clinical resistance to sacituzumab govitecan in triple-negative breast cancer. Cancer Discov. 2021;11(10):2436–2445. doi:10.1158/2159-8290.CD-21-0702
64. Chou J, Trepka K, Sjöström M, et al. TROP2 Expression across molecular subtypes of urothelial carcinoma and enfortumab vedotin-resistant cells. Eur Urol Oncol. 2022. doi:10.1016/j.euo.2021.11.005
65. Loriot Y, Petrylak BA, Petrylak DP. Efficacy of sacituzumab govitecan (SG) by trophoblast cell surface antigen 2 (Trop 2) expression in patients with metastatic urothelial cancer (mUC). Ann Oncol. 2021;32:S712–S713. doi:10.1016/j.annonc.2021.08.096
66. Carmon KS, Azhdarinia A. Application of Immuno-PET in antibody-drug conjugate development. Mol Imaging. 2018;17:1536012118801223. doi:10.1177/1536012118801223
67. Petros Grivas DP, Park CH, Barthélémy P. TROPHY-U-01 Cohort 3: sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) who progressed after platinum (PLT)-based regimens. J Clin Oncol. 2022;40:434. doi:10.1200/JCO.2022.40.6_suppl.434
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
