Back to Journals » Infection and Drug Resistance » Volume 14
Talaromyces marneffei and Burkholderia cepacia Co-Infection in a HIV-Uninfected Patient with Anti-Interferon-γ Autoantibodies
Authors Zeng W, Qiu Y, Tang M, Zhang H, Pan M, Tang S , Zhang J
Received 29 March 2021
Accepted for publication 2 June 2021
Published 10 June 2021 Volume 2021:14 Pages 2173—2177
DOI https://doi.org/10.2147/IDR.S312042
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Wen Zeng,1,2 Ye Qiu,1,2 Mengxin Tang,1,2 Hui Zhang,2 Mianluan Pan,1 Shudan Tang,2 Jianquan Zhang1
1Department of Respiratory and Critical Medicine, The Eighth Affiliated Hospital, Sun Yat-Sen University, Shenzhen, Guangdong, 518000, People’s Republic of China; 2Department of Respiratory and Critical Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China
Correspondence: Jianquan Zhang
Department of Respiratory and Critical Medicine, The Eighth Affiliated Hospital, Sun Yat-Sen University, Shenzhen, Guangdong, 518000, People’s Republic of China
Tel +8613978123845
Fax +86755-23482484
Email [email protected]
Abstract: A high titer of neutralizing anti-interferon-γ autoantibodies can cause immunodeficiency associated with severe or disseminated infections caused by Talaromyces marneffei in human immunodeficiency virus-negative patients. Herein, we reported a rare case of disseminated Talaromyces marneffei and Burkholderia cepacia infection. The patient’s lungs, lymph nodes, and bronchi were involved, and he had neck abscesses and osteomyelitis. We measured the neutralizing anti-interferon-γ autoantibodies in the peripheral blood and found that the patient had a persistently high positive titer. Despite aggressive treatment, the patient developed disseminated intravascular coagulation and died. Thus, high-titer nAIGAs may be associated with multiple opportunistic, persistent and disseminated infections.
Keywords: Burkholderia cepacia, Talaromyces marneffei, adult immunodeficiency, neutralizing anti-interferon-γ autoantibodies
Introduction
An increasing number of cases with Talaromyces marneffei (TM) infections had been reported in non-human immunodeficiency virus (HIV)-infected patients who had high-titer neutralizing anti–interferon-γ autoantibodies (nAIGAs) in the peripheral blood. In recent years,1 Burkholderia cepacia, a rare human pathogen, was most likely involved in opportunistic infections in immunocompromised hosts.2 Cases of osteomyelitis due to B. cepacia are rarely described.3 Herein, we report a rare case of a patient who had osteomyelitis due to disseminated TM and B. cepacia infection, and the nAIGAs in the peripheral blood had a high positive titer.
Case Report
A 49-year-old man from Guangxi, China, presented to our hospital with a 7-month history of cough, fever, and emaciation and a 1-month history of a mass on the left side of his neck. He had been treated with ceftazidime at another hospital, and his fever had resolved, but the mass on his neck had grown. He had no history of previous medical illness or medication. Physical examination on admission revealed a body temperature of 38°C and two palpable masses, one on the left side of his neck and the other below the right side of his chin. The larger mass was 8 cm in diameter, and the masses were inflamed and sensitive to touch. He had moist rales in the upper lobe of the left lung. Hematology revealed a white blood cell count of 14,200 cells/µL, neutrophil count of 12,200 cells/µL, lymphocyte count of 1.39 cells/µL, platelet count of 167,000/µL, and hemoglobin of 124 g/L. He had a C-reactive protein level of 167.04 mg/L, erythrocyte sedimentation rate of 70 mm/h, and procalcitonin of 0.61 ng/mL. The total CD3+, CD4+, and CD8+ T-lymphocyte counts were 1, 137, 470, and 620 cells/L, respectively. The liver and kidney function test results were normal, and the level of total immunoglobulin was within the normal limit. The galactomannan index was 0.35, and the patient was HIV negative. Computed tomography (CT) of the neck revealed mixed-density lesions on the left side of the neck and in the right submental area (Figure 1A). He was started on ceftazidime and levofloxacin upon admission. Resection of the neck masses revealed that they contained pus and a large amount of hemopurulent necrotic material (Figure 1B and C). A pus sample was sent for culture. B. cepacia was cultured from the mass on the neck and submental pus after 1 week. The results of the drug sensitivity test revealed that the patient was sensitive to meropenem, ceftazidime, levofloxacin, cotrimoxazole, and minocycline. Thus, we continued administration of ceftazidime and levofloxacin. The patient’s fever resolved, and the masses decreased in size. However, he developed a productive cough after 2 weeks. CT showed that the neck masses shrank, but a large exudative consolidation shadow in the left upper lobe and left hilar lymphadenopathy was also noted (Figure 1D). Bronchofibroscopy revealed bronchial nodules in the left upper and lower lobes, obstructing the lumen, and mucosal hyperplasia and hypertrophy (Figure 1E). The left hilar lymph nodes and tracheal nodules were biopsied, and samples were sent for culture. TM (Figure 2) was cultured from a bronchial nodule, left hilar lymph node, and bronchoalveolar lavage fluid. We also sent the left hilar lymph node for next-generation sequencing (NGS) (The Beijing Genomics Institute, China), and The results of NGS was also TM.Thus, the patient was diagnosed with disseminated TM and B. cepacia infection.
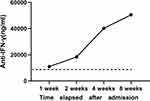
Amphotericin B (50 mg/d) was added as antifungal therapy. We discontinued ceftazidime and levofloxacin after 4 weeks as his neck mass improved. The patient’s cough improved after 1 week. However, he became febrile again, and a new mass appeared on the right side of the neck after 3 weeks. A chest CT scan revealed significant improvement in the pulmonary lesions (Figure 3A). CT of the neck showed an abscess in the posterior pharyngeal wall, a fracture of the 4th cervical vertebra, and a mass on the right side of the neck (Figure 3B and C). Ceftazidime and levofloxacin were added as antibacterial treatment, but there was no obvious treatment effect. The antibiotics were switched to meropenem and cotrimoxazole according to the drug sensitivity results, but the B. cepacia infection remained uncontrolled. He developed acute-onset quadriplegia and underwent an emergency subtotal resection of the 4th cervical vertebra and widening and decompression of the spinal canal. He was transferred to the intensive care unit and treated with meropenem and tigecycline as antibiotics and voriconazole as an antifungal agent. B. cepacia was cultured repeatedly from the blood and pus. The pulmonary lesions were improved, but the neck abscess could not be contained and disseminated widely to multiple body parts (Figure 3D). After 1 week, he developed hepatorenal dysfunction, cardiac insufficiency, and hypotension and became hypoxic. Repeated immune function test revealed CD3+, CD4+, and CD8+ T-lymphocyte counts of 158, 48, and 99 cells/L, respectively. After 2 weeks, he developed disseminated intravascular coagulation and died of B. cepacia sepsis. We considered that the patient might be infected with a special pathogen. According to our experience, we measured the nAIGAs in the peripheral blood at 1 week after admission. Serum AIGA was determined by an enzyme-linked immunosorbent assay kit (USCN Life Science Inc., Wuhan, China) according to the manufacturer’s protocols. We found that he had a persistently high positive titer (11,025 ng/mL), and the antibody titer increased gradually to 50,566 ng/mL at 8 weeks after admission (Figure 4). The positive titer value was based on our previous study.1
Discussion
The high titer of serum nAIGA can cause immunodeficiency associated with severe or disseminated infections caused by nontuberculous mycobacteria, nontyphoidal Salmonella, Burkholderia spp., TM, Cryptococcus neoformans, Histoplasma capsulatum, and the Varicella zoster virus. It is an emerging adult-onset immunodeficiency syndrome in non-HIV-infected patients.4,5 We previously found that up to 60% of HIV-negative patients with TM infection were nAIGA positive.1,6 The positive nAIGA is also the most common immunodeficiency condition in patients with TM infection and is a risk factor for polymicrobial opportunistic infections.5 B. cepacia and TM are rare pathogens that are always involved in opportunistic infections in immunocompromised hosts. Our patient had no previous medical illness, and he was infected by B. cepacia and TM as a result of positive nAIGA. It is extremely rare for a patient to be co-infected with these two rare opportunistic pathogens. Specific human leukocyte antigen class II haplotypes (eg, HLA-DRB1*16:02 and HLA-DQB1*05:02) have been reported to be associated explicitly with AIGAs, especially in Southeast Asia. Further, production of this antibody might be related to the stimulation of pathogen infection.7,8 However, the relationship among the expressions of HLA-DRB1*16:02 and HLA-DQB1*05:02, type of pathogen infection, and AIGA titers remains unclear. Among patients with TM, those with positive nAIGAs relapse more frequently, have more severe infections, and are harder to treat.1,6 Therefore, when HIV-negative hosts, especially those infected by TM with or without other opportunistic infections, develop intracellular opportunistic infections, caution should be taken to rule out whether the immunodeficiency is caused by AIGAs.
TM was cultured from the patient’s hilar lymph node, bronchial nodules, and bronchoalveolar lavage fluid, and antifungal therapy was effective. Therefore, we think that the TM infection involved the lungs, lymph nodes, and bronchi. Although TM can also cause neck abscesses or osteomyelitis, repeated cultures only found B. cepacia. Further, as the initial antibacterial treatment was effective, it is likely that the neck abscesses and osteomyelitis were caused only by the bacterium. B. cepacia can cause disseminated soft tissue infection of the neck, followed by severe sepsis, but it rarely causes suppurative spinal infection or osteomyelitis.2,9
The progressive increase in the patient’s serum nAIGA titer may explain the difficulty in controlling the infection. Although the B. cepacia isolate was a sensitive strain, the patient was treated with systemic antibiotics according to the drug sensitivity test and underwent multiple local surgeries. However, the infection persisted and progressed to sepsis, eventually leading to secondary cellular immune failure and death. Lowering the nAIGA titer may be beneficial for infection control, and a high nAIGA titer may lead to difficult control of the infection.1,6 We suggest that patients with positive nAIGA titers should have an extended course of antibiotics to reduce recurrence. In previous studies, patients showing progressive or relapsed infections despite at least a 3-month or over 1-year course of intensive antimicrobial therapy were treated with rituximab, and favorable clinical outcomes were achieved.10,11 Our patient had a severe infection and had no significant benefit from antibiotic treatment. We are unsure whether this immunosuppressive drug benefitted the patient or exacerbated the infection.
Conclusion
We herein report concurrent TM and B. cepacia infections in a patient with nAIGA-associated adult-onset immunodeficiency. It is unclear how nAIGAs are produced, regulated, or cleared from the peripheral blood. nAIGAs are also strongly associated with disseminated intracellular opportunistic pathogens. Thus, high-titer nAIGAs may be associated with multiple opportunistic infections, persistent infections, and disseminated infections.
Abbreviations
CT, computed tomography; HIV, human immunodeficiency virus; nAIGAs, neutralizing anti–interferon-γ autoantibodies; NGS, next-generation sequencing; TM, Talaromyces marneffei.
Ethics Approval and Informed Consent
Signed consent was obtained for the publication of the case details from the participant. This study was approved by the First Affiliated Hospital of Guangxi Medical University’s Ethical Review Committee (2020.KY-E-139)
Consent for Publication
Signed consent was obtained for the publication of the case details from the participant.
Author Contributions
All authors contributed to study conception, study desing, data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was approved by the Ethical Review Committee of the First Affiliated Hospital of Guangxi Medical University (2018.KY-E-094). The clinical trial was registered on www.clinicaltrials.gov (NCT03819348). Written informed consent was provided by all participants in the prospective cohort study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zeng W, Qiu Y, Tang S, Zhang J, Pan M, Zhong X. Characterization of anti-interferon-γ antibodies in HIV negative patients infected with disseminated Talaromyces marneffei and cryptococcosis. Open Forum Infect Dis. 2019;6:ofz208. doi:10.1093/ofid/ofz208
2. Hammoud M, Fares Y, Atoui R, Dabboucy B. Burkholderia cepacia as a cause of pyogenic spondylodiscitis in immunocompetent patients: a single-institution case series and literature review. J Spine Surg. 2019;5:372–377. doi:10.21037/jss.2019.07.02
3. Chang WS, Ho MW, Lin PC, et al. Clinical characteristics, treatments, and outcomes of hematogenous pyogenic vertebral osteomyelitis, 12-year experience from a tertiary hospital in central Taiwan. J Microbiol Immunol Infect. 2018;51:235–242. doi:10.1016/j.jmii.2017.08.002
4. Chi CY, Lin CH, Ho MW, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore). 2016;95:e3927. doi:10.1097/MD.0000000000003927
5. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725–734. doi:10.1056/NEJMoa1111160
6. Qiu Y, Feng X, Zeng W, Zhang H, Zhang J. Immunodeficiency disease spectrum in HIV-negative individuals with Talaromycosis. J Clin Immunol. 2021;41:221–223. doi:10.1007/s10875-020-00869-5
7. Chi CY, Chu CC, Liu JP, et al. Anti-IFN-γ autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood. 2013;121:1357–1366. doi:10.1182/blood-2012-08-452482
8. Lin CH, Chi CY, Shih HP, et al. Identification of a major epitope by anti-interferon-γ autoantibodies in patients with mycobacterial disease. Nat Med. 2016;22:994–1001. doi:10.1038/nm.4158
9. Tang BS, Chan JF, Chen M, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol. 2010;17:1132–1138. doi:10.1128/CVI.00053-10
10. Laisuan W, Pisitkun P, Ngamjanyaporn P, Suangtamai T, Rotjanapan P. Prospective pilot study of cyclophosphamide as an adjunct treatment in patients with adult-onset immunodeficiency associated with anti-interferon-γ autoantibodies. Open Forum Infect Dis. 2020;7:ofaa035. doi:10.1093/ofid/ofaa035
11. Browne SK, Zaman R, Sampaio EP, et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood. 2012;119:3933–3939. doi:10.1182/blood-2011-12-395707
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.