Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Tacrolimus Combined with Corticosteroids Improved the Outcome of CIDP Patients with Autoantibodies Against Paranodal Proteins
Authors Yang M, Xu L, Ji S , Gao H, Zhang Q, Bu B
Received 13 February 2022
Accepted for publication 25 May 2022
Published 16 June 2022 Volume 2022:18 Pages 1207—1217
DOI https://doi.org/10.2147/NDT.S361461
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Meng-ge Yang, Li Xu, Suqiong Ji, Huajie Gao, Qing Zhang, Bitao Bu
Department of Neurology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Bitao Bu, Department of Neurology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Jiefang Street 1095#, Wuhan, 430000, People’s Republic of China, Email [email protected]
Purpose: To investigate the response of tacrolimus to chronic inflammatory demyelinating polyneuropathy (CIDP) with autoantibodies against paranodal proteins, including neurofascin-155 (NF155), contactin-1 (CNTN1) and contactin-associated protein 1 (Caspr1).
Methods: We retrospectively reviewed all CIDP patients who carried anti-NF155, CNTN1 and Caspr1 antibodies and were treated with tacrolimus at Tongji hospital from Jan 2018 to Apr 2021.
Results: There were 58 patients with CIDP and only 9 patients had autoantibodies against paranodal proteins (17.2%). Five of the 9 patients received tacrolimus treatment with an initial dose of 2– 3 mg once daily. One patient with anti-CNTN1 antibody started tacrolimus and corticosteroid treatment, at the first episode and eventually achieved full clinical remission without relapse. Four patients with anti-NF155 or -Caspr1 antibodies experienced relapse during corticosteroids tapering. Then, they were given oral tacrolimus and presented with clinical improvement. During follow-up, only one patient developed worsening weakness due to unreasonable tacrolimus discontinuation. Moreover, 3 patients were successfully withdrawn from corticosteroids and 2 patients took corticosteroids at low maintenance dose (10mg/d) after tacrolimus treatment. No severe adverse events were observed in all the patients.
Conclusion: Patients with autoantibodies against paranodal proteins had a better long-term outcome after adding tacrolimus. Combination therapy with corticosteroids and tacrolimus may be an effective therapeutic regimen.
Keywords: chronic inflammatory demyelinating polyneuropathy, paranodal proteins, tacrolimus, outcome
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an immune-mediated disabling neuropathy, usually causing weakness and sensory disturbances with a progressing or relapsing-remitting course.1 CIDP has been proven to respond well to immune therapies like corticosteroids, intravenous immunoglobulin (IVIG) and plasma exchange (PE).1,2 However, patients with autoantibodies against the paranodal proteins (neurofascin-155 (NF155), contactin-1 (CNTN1) and contactin-associated protein 1 (Caspr1)), are usually resistant to IVIG and only partial response to corticosteroids.3–5 PE and rituximab seem to be effective,6–8 however, PE is not a good choice for maintenance treatment due to invasive procedures, venous access-related complications, high cost and limited availability of equipment.7,8 Rituximab has limited clinical long-term application due to the high cost of regular intravenous administration and the potential higher risk of infection.6,7 To date, oral corticosteroids are still the most widely used maintenance treatment for various subtypes of CIDP, especially in low-income countries, considering economy, relative efficiency and easy access.9 However, the effectiveness, dependence and serious side effects after long-term use of corticosteroid treatment constitute the major concerns for clinicians. Immunosuppressants, such as azathioprine, cyclophosphamide, ciclosporin and mycophenolate mofetil, are still occasionally used after failure of the first-line treatments or as add-on medication.10,11 However, their role in CIDP with autoantibodies against the paranodal proteins is still unknown. Therefore, it is necessary to explore new strategy for the treatment of CIDP with autoantibodies against paranodal proteins.
Tacrolimus (FK506) is a strong immunosuppressive agent initially used in preventing graft rejection and has been expanding to the fields of various autoimmune diseases.12–15 Moreover, a large number of studies have shown that tacrolimus can promote peripheral nerve regeneration.16–21 The protective effect of tacrolimus on CIDP has been reported in a few cases.22–24 Therefore, we will assess the response of tacrolimus in patients with autoantibodies against paranodal proteins.
Methods
Patients Selection
In this retrospective study, we reviewed all CIDP patients with anti-NF155, CNTN1 and Caspr1 antibodies treated at Tongji hospital from Jan 2018 to Apr 2021. All patients were diagnosed according to the revised diagnostic 2010 criteria of the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS).25 Autoantibodies against paranodal proteins were detected by indirect immunofluorescence test using NF155, CNTN1 or Caspr1-transfected HEK-293 cells, as previously described.26 Tacrolimus was administered firstly at a routine dose of 2–3 mg/day, and then the dose was adjusted based on patient’s condition and tacrolimus trough concentrations.
Follow Up and Outcome Assessment
The patient’s long-term management was achieved by regular out-patient follow-up. The severity of disease was evaluated by the Medical Research Council (MRC) score (0–5) and the Overall Neuropathy Limitation Scale (ONLS) score (0–12).27 The ONLS score is graded from 0 to 5 on the upper limb section and from 0 to 7 on the lower limb section. Grade 0 indicates no limitations and grade 5 or 7 indicates no purposeful movement.27 The clinical outcome was assessed by the Modified Rankin Scale (MRS) score (0–6), and clinical remission was defined as MRS grade score 0–1 or mild symptoms and signs, but no functional limitation. During out-patient follow-up interviews, blood sampling was collected to monitor possible side effects of tacrolimus, including liver injury, nephrotoxicity and granulocytopenia.
Results
We collected 9 patients with autoantibodies against paranodal proteins from 58 patients with CIDP (17.2%). Of the 9 patients, 1 case (1.7%) had anti-CNTN1 IgG4, 5 cases (8.6%) had anti-NF155 IgG4 and 3 cases (5.2%) had anti-Caspr1 IgG. Their demographics and clinical features are described in Table 1. Patients 1, 2, 3, 4 and 5 had received oral tacrolimus as a maintenance therapy. Detailed case information was as follows. Their therapeutic regimens and clinical status are shown in Figure 1, while treatment efficacy, outcome and adverse events are summarized in Table 2. Additionally, patients 6, 7 and 9 were treated with rituximab regularly after plasma exchange or IVIG treatment, while patients 8 was treated with plasma exchange and corticosteroids.
 |
Table 1 Participants’ Demographics and Clinical Features of Patients with Antibodies Against Paranodal Proteins |
 |
Table 2 Treatment Efficacy, Outcome and Adverse Events of Tacrolimus in Patients with Antibodies Against Paranodal Proteins |
Patient 1
A 37-year-old male presented with progressive limbs numbness and weakness for 2.5 months. He had a recent diagnosis of hyperthyroidism and was treated with methimazole. Serum CNTN1 antibodies were positive with titers of 1:10. Neurological examination revealed muscle weakness in the upper and lower limbs, sensory disturbances, sensory ataxia and disappeared tendon reflex. He had difficulty walking and usually required sticks assistance. He was treated with intravenous methylprednisolone (IVMP) and started to take oral prednisone after the end of the course. Moreover, tacrolimus was administrated at day 4 after oral prednisone considering the possibility of relapse. During the 3-years follow-up, he presented with long-lasting improvement and was successfully withdrawn from oral prednisone without any relapse (Figure 1A and B). Moreover, electromyography was performed at the base line and 3, 7, 10, 17, 22 months after treatment. Motor conduction velocity (MCV) and compound muscle action potentials (CMAPs) amplitudes were decreased in median, ulnar and tibial nerves at the peak of illness, which were followed by gradual normalization after treatment (Figure 2A, C and E). Moreover, sensory conduction velocity (SCV) and sensory nerve action potential (SNAP) amplitudes in the median and ulnar nerves, as well as SCV in superficial peroneal nerves showed similar findings (Figure 2B, D and F).
Patient 2
A 57-year-old male was admitted with progressive limbs numbness and weakness and neuropathic pain for 30 days. Anti-Caspr1 antibodies were found to be positive (serum, 1:1000; cerebrospinal fluid (CSF), 1:10). His weakness gradually improved from grade 4 to grade 5 after IVMP and oral prednisone. Neuropathic pain was relieved after oral pregabalin. However, his symptoms worsened again and gradually developed unstable walking, deep sensory disturbances and tremor in his right hand in the second months after discharge. Although oral prednisone (40mg/d) has been resumed, his symptoms did not improve but continued to worsen. Then, tacrolimus was given at a dose of 3 mg daily and prednisone was tapered gradually. After 6 months of follow-up, the patient’s weakness and sensory disturbances gradually relieved and only the tremor in his right hand remained (Figure 1C and D).
Patient 3
A 22-year-old male admitted with limbs numbness and weakness (especially the lower limbs) for 8 months. He was initially diagnosed with Miller-Fisher syndrome, and IVMP and IVIG completely relieved his dysphagia, hoarseness and diplopia. However, his limb weakness worsened and developed ataxia and upper limbs tremor at 8th months of oral prednisone tapering. Then, tacrolimus was administered and he improved moderately, although obvious lower limb weakness persisted (MRC grade 4) (Figure 1E and F). Two years after tacrolimus treatment, hidden serum autoantibodies were confirmed to be anti-NF155 IgG4 positive (1:100). He was finally diagnosed with CIDP based on clinical characteristics. For better outcomes, several intravenous rituximab and PE courses were also tried but had poor responses.
Patient 4
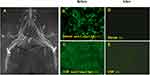
A 37-year-old female was admitted with progressive numbness and weakness (especially the lower limbs) for 15 days. She had a Guillain Barré syndrome (GBS) medical history one year ago but was relieved after treatment. Anti-Caspr1 IgG autoantibodies were positive (serum, 1:32; CSF, 1:1) (Figure 3B and C) and magnetic resonance imaging (MRI) showed significant peripheral nerves hypertrophy (Figure 3A). She presented with a severe disability and required a wheelchair at onset. Corticosteroids and IVIG effectively relieved his symptoms. Unfortunately, she worsened again after oral prednisone and three courses of IVIG. Although serological and CSF analysis showed that the anti-Caspr1 antibody has changed from positive to negative (Figure 3D and E), oral tacrolimus was still added considering the possibility of recurrent attacks. During the next 18 months of follow-up, she presented with long-lasting improvement and left only mild weakness of the lower limbs (MRC grade 5-) (Figure 1G and H).
Patient 5
An 18-year-old male suffered from limbs weakness and numbness (especially the lower limbs) for 6 months. He was firstly diagnosed with GBS and treated with IVMP and oral prednisone after IVIG failed 4 months ago. His MRC score recovered from grade 3+ to 5-; however, the symptoms worsened after oral prednisone was completely withdrawn. Then, oral prednisone was resumed and tacrolimus was administered. His weakness improved significantly, numbness disappeared, and prednisone was successfully discontinued over the next year. At the 24th month of tacrolimus treatment, he discontinued treatment without medical advice and soon experienced weakness worsen. Moreover, a serological analysis found positive anti-NF155 IgG4 antibodies. Considering that this patient was resistant to IVIG in the previous episodes, IVMP and tacrolimus were given in this episode (Figure 1I and J). Although there is still a slight disability (abnormal gait and hand tremor) 9 months later, he could complete activities of daily living independently (MRS score 2).
Treatment Efficacy, Outcome and Adverse Events
At the last follow-up, 3 patients reached clinical remission (MRS score 0–1) and 2 patients achieved partial clinical remission (MRS score 2). Four cases of 5 patients experienced relapses before tacrolimus treatment. Inversely, none of them relapsed after tacrolimus treatment, except for one patient who completely discontinued tacrolimus without medical advice. Additionally, 3 patients were successfully withdrawn from corticosteroids and 2 patients took corticosteroids at low maintenance dose (10mg/d) during follow-up. In terms of adverse events, patient 5 developed mild but acceptable hand tremors and patient 2 had mild slight transferase elevation (less than 3 times the normal value). No severe adverse events were observed in all the patients.
In the small cohort, patient 6 who carried anti-NF155 IgG4 and received PE treatment developed severe bacteremia and thrombosis in the right internal jugular vein and right subclavian vein. For the next 3 months, he received rituximab treatment and reached partial clinical remission after follow-up for 18 months (MRS: from 4 to 2). Patients 7 and 8 harboring anti-NF155 IgG4 were resistant to IVIG. Then, patient 7 was treated with regular rituximab and he was completely recovered after 6 months although suffered from a transient skin rash during the treatment (MRS: from 2 to 0). Patients 8 was treated with PE and oral prednisone and his symptoms were significantly relieved after 3 months of follow-up (MRS: from 2 to 1). Patient 9 harboring anti-Caspr1 IgG were treated with IVIG, rituximab, IVMP, and oral corticosteroids. Unfortunately, the disease was still gradually progressive and eventually, he died of respiratory failure at 4 months after onset.
Discussion
CIDP is mediated by T-cell immunity and B-cell immunity. T cells response not only destroys the blood-nerve barrier by producing a variety of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interferon γ (IFN-γ) and interleukin-2 (IL-2), but also promotes the activation of B cells and the production of antibodies.1,28 Anti-NF155, CNTN1 and Caspr1 antibodies are pathogenic antibodies.28 Moreover, there are significant differences in pathogenesis, clinical features and therapeutic options between patients with and without these antibodies.28 Therefore, the latest revised EAN/PNS guideline (2021) has suggested to name these conditions “auto-immune nodopathies” rather than CIDP variants.10 In our study, it can be observed that patients with autoantibodies against NF155, CNTN1 and Caspr1 had a male predominance (male: female: 8:1) and remarkably increased CSF total protein, usually above 2000 mg/dl (normal value: 150–450 mg/dl). Moreover, patients with anti-NF155 IgG4 showed a younger age at the onset (median: 17; range: 10–34), which was consistent with previous reports.29
Tacrolimus is a well-known immunosuppressant that mainly inhibits T lymphocyte activation and T lymphocytes-derived cytokines production, such as TNF-α, IFN-γ and IL-2.30 Suppressed T cells further affect B cell proliferation, leading to a reduction in the production of antibodies.31 Our data showed that patients had a better long-term outcome after adding tacrolimus. The efficacy of the agent in other antibody-dependent autoimmune diseases, such as myasthenia gravis (MG) and neuromyelitis optica spectrum disorder (NMOSD), could also explain the rationale for tacrolimus in treating CIDP with antibodies to paranodal proteins.14,15
Glial NF155 binds to axonal Caspr1/CNTN1 to form axon–glia junctions, which are the most important functional structure of the paranodes.7,32 Autoantibodies against NF155, CNTN1 and Caspr1 were predominantly of IgG4 subclass. Moreover, Anti-NF155 IgG4, anti-CNTN1 IgG4 and anti-Caspr1 IgG4 can target the paranodal region and dismantle the axo-glial interactions, leading to nerve conduction defects.28,29,33,34 Furthermore, these autoantibodies are closely related to demyelination and early axonal involvement.34,35 In our study, all patients with anti-NF155, CNTN1 and Caspr1 antibodies presented with severe demyelination and axonal damage in both sensory and motor nerves (Table 1).
Clinical improvement was observed in all patients treated with tacrolimus and corticosteroids, suggesting that the combination therapy with corticosteroids and tacrolimus may be an effective regimen. Moreover, patients (1, 2 and 4) who received oral tacrolimus within three months of onset seemed to have better clinical outcomes than those (patients 3 and 5) who started tacrolimus treatment later. Notably, patient 1 started oral tacrolimus at first attack and eventually achieved full clinical remission without relapse. Electromyography in patient 1 also disclosed that decreased MCV, CMAP, SCV and SNAP, gradually returned to a normal level. Early use of tacrolimus seems to benefit these patients, which may be associated with the neuroprotective effects of tacrolimus.18 Tacrolimus has been proven to have a neuroprotective effect on multiple nerve injury models, especially axonal regeneration.16,17,36,37 Moreover, the optimal timing of tacrolimus administration was up to 3 days before or within 10 days after nerve reconstruction.18,19
Like other immunosuppressors, tacrolimus is often initiated when corticosteroid treatment is insufficient or relapse following treatment. Given IVIG resistance, partial response to corticosteroids and a better prognosis after adding tacrolimus, early use of the combination of tacrolimus and corticosteroids may benefit patients with autoantibodies against the paranodal proteins. More importantly, tacrolimus may be an effective maintenance treatment, which can prevent recurrence and facilitate corticosteroid withdrawal.
Oral tacrolimus was generally well tolerated in the 5 patients with no severe side effects, in contrast to catheter-related bacteremia and venous thrombosis in patients who received plasma exchange treatment, just like the patient 6. However, tacrolimus-related neurotoxicities, such as hand tremors and headache, remain a concern for clinicians.38 In our study, one patient developed tacrolimus-related hand tremor, but he did not discontinue tacrolimus treatment due to significant clinical improvement. In fact, our group has applied tacrolimus to the long-term immunotherapy for several autoimmune diseases considering its good effectiveness and safety, including NMOSD, neuromyotonia, immune-mediated necrotizing myopathy and neuronal surface antibody-mediated autoimmune encephalitis.12–14,39 Furthermore, neurotoxicity was rarely observed and only one patient with NMOSD occurred hand tremor.14
Conclusions
Our study showed that tacrolimus could improve the long-term outcome of CIDP patients with autoantibodies against paranodal proteins by preventing relapses and reducing corticosteroid dosage. Early use of tacrolimus seems to benefit patients due to its neuroregenerative effects. Tacrolimus, an effective and easily accessible immunosuppressant, could be a good choice for treating CIDP with autoantibodies against paranode proteins, in combination with corticosteroids. However, our study is limited by the small sample retrospective design and no comparison of the titers of antibodies before and after treatment. There were differences in therapeutic regimens in different cases because of differences in individual compliance with medical suggestions. Therefore, large-sample prospective controlled studies are still needed to further confirm our initial observation and clarify changes in the titers of autoantibodies.
Ethics Declarations
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of the Tongji hospital of Tongji Medical College. Written informed consent has been provided by the patients or their legal guardian/next of kin to have the case details and any accompanying images published.
Acknowledgments
The authors thank Kindstar Global company for testing the autoantibodies against paranode proteins.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number: 81873758).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Rodriguez Y, Vatti N, Ramirez-Santana C, et al. Chronic inflammatory demyelinating polyneuropathy as an autoimmune disease. J Autoimmun. 2019;102:8–37. doi:10.1016/j.jaut.2019.04.021
2. Lehmann HC, Burke D, Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2019;90(9):981–987. doi:10.1136/jnnp-2019-320314
3. Querol L, Devaux J, Rojas-Garcia R, Illa I. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol. 2017;13(9):533–547. doi:10.1038/nrneurol.2017.84
4. Vural A, Doppler K, Meinl E. Autoantibodies against the node of Ranvier in seropositive chronic inflammatory demyelinating polyneuropathy: diagnostic, pathogenic, and therapeutic relevance. Front Immunol. 2018;9:1029. doi:10.3389/fimmu.2018.01029
5. Ogata H, Yamasaki R, Hiwatashi A, et al. Characterization of IgG4 anti-neurofascin 155 antibody-positive polyneuropathy. Ann Clin Transl Neurol. 2015;2(10):960–971. doi:10.1002/acn3.248
6. Querol L, Rojas-Garcia R, Diaz-Manera J, et al. Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm. 2015;2(5):e149. doi:10.1212/NXI.0000000000000149
7. Bunschoten C, Jacobs BC, Van den Bergh PYK, Cornblath DR, van Doorn PA. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019;18(8):784–794. doi:10.1016/S1474-4422(19)30144-9
8. Mehndiratta MM, Hughes RA, Pritchard J. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2015;(8):CD003906. doi:10.1002/14651858.CD003906.pub4
9. van Lieverloo GGA, Peric S, Doneddu PE, et al. Corticosteroids in chronic inflammatory demyelinating polyneuropathy. J Neurol. 2018;265(9):2052–2059. doi:10.1007/s00415-018-8948-y
10. Van den Bergh PYK, van Doorn PA, Hadden RDM, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force-second revision. J Peripher Nerv Syst. 2021;26(3):242–268. doi:10.1111/jns.12455
11. Rajabally YA. Unconventional treatments for chronic inflammatory demyelinating polyneuropathy. Neurodegener Dis Manag. 2017;7(5):331–342. doi:10.2217/nmt-2017-0017
12. Liu C, Ji S, Bi Z, Shang K, Gao H, Bu B. Tacrolimus as a therapeutic option in patients with acquired neuromyotonia. J Neuroimmunol. 2021;355:577569. doi:10.1016/j.jneuroim.2021.577569
13. Feng F, Li Y, Ji S, Wang Q, Bu B. Tacrolimus combined with corticosteroids effectively improved the outcome of a cohort of patients with immune-mediated necrotising myopathy. Clin Exp Rheumatol. 2019;37(5):740–747.
14. Chen B, Wu Q, Ke G, Bu B. Efficacy and safety of tacrolimus treatment for neuromyelitis optica spectrum disorder. Sci Rep. 2017;7(1):831. doi:10.1038/s41598-017-00860-y
15. Bi Z, Cao Y, Lin J, et al. Long-term improvement in a Chinese cohort of glucocorticoid-resistant childhood-onset myasthenia gravis patients treated with tacrolimus. Front Neurol. 2022;13:820205. doi:10.3389/fneur.2022.820205
16. Tajdaran K, Chan K, Shoichet MS, Gordon T, Borschel GH. Local delivery of FK506 to injured peripheral nerve enhances axon regeneration after surgical nerve repair in rats. Acta Biomater. 2019;96:211–221. doi:10.1016/j.actbio.2019.05.058
17. Gold BG, Storm-Dickerson T, Austin DR. The immunosuppressant FK506 increases functional recovery and nerve regeneration following peripheral nerve injury. Restor Neurol Neurosci. 1994;6(4):287–296. doi:10.3233/RNN-1994-6404
18. Saffari TM, Bedar M, Zuidam JM, et al. Exploring the neuroregenerative potential of tacrolimus. Expert Rev Clin Pharmacol. 2019;12(11):1047–1057. doi:10.1080/17512433.2019.1675507
19. Yan Y, Sun HH, Hunter DA, Mackinnon SE, Johnson PJ. Efficacy of short-term FK506 administration on accelerating nerve regeneration. Neurorehabil Neural Repair. 2012;26(6):570–580. doi:10.1177/1545968311431965
20. Davis B, Hilgart D, Erickson S, et al. Local FK506 delivery at the direct nerve repair site improves nerve regeneration. Muscle Nerve. 2019;60(5):613–620. doi:10.1002/mus.26656
21. Saffari TM, Chan K, Saffari S, et al. Combined local delivery of tacrolimus and stem cells in hydrogel for enhancing peripheral nerve regeneration. Biotechnol Bioeng. 2021;118(7):2804–2814. doi:10.1002/bit.27799
22. Bukhari S, Bettin M, Cathro HP, Gwathmey K, Gautam J, Bowman B. Anti-neurofascin-associated nephrotic-range proteinuria in chronic inflammatory demyelinating polyneuropathy. Kidney Med. 2020;2(6):797–800. doi:10.1016/j.xkme.2020.06.016
23. Ahlmén J, Andersen O, Hallgren G, Peilot B. Positive effects of tacrolimus in a case of CIDP. Transplant Proc. 1998;30(8):4194. doi:10.1016/S0041-1345(98)01389-X
24. Zhu WJ, Da YW, Chen H, et al. Tacrolimus treatment for relapsing-remitting chronic inflammatory demyelinating polyradiculoneuropathy: two case reports. World J Clin Cases. 2022;10(5):1709–1715. doi:10.12998/wjcc.v10.i5.1709
25. Van den Bergh PYK, Hadden RDM, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur j Neurol. 2010;17(3):356–363. doi:10.1111/j.1468-1331.2009.02930.x
26. Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology. 2014;82(10):879–886. doi:10.1212/WNL.0000000000000205
27. Graham RC, Hughes RA. A modified peripheral neuropathy scale: the overall neuropathy limitations scale. J Neurol Neurosurg Psychiatry. 2006;77(8):973–976. doi:10.1136/jnnp.2005.081547
28. Tang L, Huang Q, Qin Z, Tang X. Distinguish CIDP with autoantibody from that without autoantibody: pathogenesis, histopathology, and clinical features. J Neurol. 2020;268:2757–2768. doi:10.1007/s00415-020-09823-2
29. Devaux JJ, Miura Y, Fukami Y, et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology. 2016;86(9):800–807. doi:10.1212/WNL.0000000000002418
30. Li Y, Guptill JT, Russo MA, et al. Tacrolimus inhibits Th1 and Th17 responses in MuSK-antibody positive myasthenia gravis patients. Exp Neurol. 2019;312:43–50. doi:10.1016/j.expneurol.2018.11.006
31. Broen JCA, van Laar JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat Rev Rheumatol. 2020;16(3):167–178. doi:10.1038/s41584-020-0374-8
32. Rasband MN, Peles E. Mechanisms of node of Ranvier assembly. Nat Rev Neurosci. 2021;22(1):7–20. doi:10.1038/s41583-020-00406-8
33. Cortese A, Lombardi R, Briani C, et al. Antibodies to neurofascin, contactin-1, and contactin-associated protein 1 in CIDP: clinical relevance of IgG isotype. Neurol Neuroimmunol Neuroinflamm. 2020;7(1):e639. doi:10.1212/NXI.0000000000000639
34. Pascual-Goni E, Fehmi J, Lleixa C, et al. Antibodies to the Caspr1/contactin-1 complex in chronic inflammatory demyelinating polyradiculoneuropathy. Brain. 2021;144(4):1183–1196. doi:10.1093/brain/awab014
35. Koike H, Kadoya M, Kaida KI, et al. Paranodal dissection in chronic inflammatory demyelinating polyneuropathy with anti-neurofascin-155 and anti-contactin-1 antibodies. J Neurol Neurosurg Psychiatry. 2017;88(6):465–473. doi:10.1136/jnnp-2016-314895
36. Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107(6):1419–1429. doi:10.1097/00006534-200105000-00016
37. Daneri-Becerra C, Patino-Gaillez MG, Galigniana MD. Proof that the high molecular weight immunophilin FKBP52 mediates the in vivo neuroregenerative effect of the macrolide FK506. Biochem Pharmacol. 2020;182:114204. doi:10.1016/j.bcp.2020.114204
38. Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13(5):313–326. doi:10.1111/j.1432-2277.2000.tb01004.x
39. Liu C, Ji S, Gao H, et al. Efficacy of tacrolimus as long-term immunotherapy for neuronal surface antibody-mediated autoimmune encephalitis. Ther Adv Chronic Dis. 2022;13:20406223211063055. doi:10.1177/20406223211063055
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.