Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
T-Natural Killers and Interferon Gamma/Interleukin 4 in Augmentation of Infection in Foot Ulcer in Type 2 Diabetes
Authors Hammad R , Elmadbouly AA , Ahmad IH , Mohammed SA, Farouk N , Futooh Z , Alfy MO , Abozaid S , Mohamed EF , Kotb FM , Abdelbadea A, Seliem N , Elshafei A, Mashaal A
Received 13 February 2021
Accepted for publication 30 March 2021
Published 29 April 2021 Volume 2021:14 Pages 1897—1908
DOI https://doi.org/10.2147/DMSO.S305511
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ming-Hui Zou
Reham Hammad,1 Asmaa A Elmadbouly,1 Inass Hassan Ahmad,2 Shaymaa A Mohammed,1 Nehal Farouk,3 Zahraa Futooh,4 Mohamed Omar Alfy,5 Sarah Abozaid,1 Eman F Mohamed,6 Fatma M Kotb,6 Alzahra Abdelbadea,7 Nora Seliem,7 Ahmed Elshafei,8 Alya Mashaal9
1Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 2Endocrinology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 3Vascular Surgery Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 4General Surgery Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 5General Surgery Department, Al Zahraa University Hospital, Al-Azhar University, Cairo, Egypt; 6Internal Medicine Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 7Biochemistry Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 8Biochemistry and Molecular Biology Department, Faculty of Pharmacy for Boys, Al-Azhar University, Cairo, Egypt; 9Immunology, Zoology & Entomology Department, Faculty of Science for Girls, Al-Azhar University, Cairo, Egypt
Correspondence: Asmaa A Elmadbouly Tel +20 1011504476
Email [email protected]
Background: The link between immune system and type 2 diabetes mellitus (T2DM) pathogenesis attracted attention to demonstrate the role of immune cells and their secreted cytokines in T2DM development and its subsequent foot complications.
Objective: To investigate the relation between T Natural killer cell (TNK) %, Interleukin 4 (IL4) and Interferon gamma (IFN-γ) and diabetic foot infection (DFI) development in patients with diabetic foot ulcer (DFU).
Patients and Methods: Ninety patients with diabetes were included in this work, divided as T2DM group (n=30), DFU group (n=30), and DFI group (n=30). TNK% was detected using flow cytometry. Serum IL4 and IFN-γ were measured by ELISA. Diabetes biochemical parameters were also analyzed.
Results: Significant decrease was detected in TNK% and IFN-γ in DFI group compared to other 2 groups (P< 0.001). Significant decrease was detected in serum levels of IL4 in DFI group compared to T2DM group (P=0.006). IFN-γ/IL4 was significantly decreased in DFI compared to DFU group (P=0.020). There was a significant correlation of TNK% with both IL4 and IFN-γ (r=0.385, P< 0.001; r=0.534, P< 0.001, respectively). Significant negative correlation of TNK% with HbA1c and LDL was revealed (r=− 0.631, P< 0.001; and r=− 0.261, P=0.013, respectively), while a positive correlation was seen with HDL (r=0.287, P=0.006). A significant negative correlation of IL4 with HbA1c was found (r=− 0.514, P< 0.001;. As for IFN-γ, a significant negative correlation with HbA1c and LDL was detected (r=− 0.369, P< 0.001; r=− 0.229, P=0.030). TNK % and IFN-γ level showed negative correlations with disease duration/year (r=− 0.546, P< 0.001; r=− 0.338, P=0.001,respectively).
Conclusion: Decline in TNK frequency has essential role in T2DM pathogenesis and subsequent foot complications. Downregulation of TNK% and IFN-γ level have potential roles in predicting infection of diabetic ulcer and are correlated with disease duration.
Keywords: diabetic foot ulcer, diabetic foot infection, natural killer cell, interferon gamma, interleukin 4
Introduction
Epidemiology studies have shown that diabetes has the highest incidence of any chronic disease worldwide and is a huge threat to human health. Diabetes is clinically divided into types I and II.1 The prevalence of type II diabetes mellitus (T2DM) increases with age across all regions and income groups.2 Still, the prevalence is increasing in young age groups due to unhealthy life routines present since childhood.3
Insulin resistance (IR) associates with T2DM.4 Diabetes-related foot complications are important causes for disability worldwide.5 More than one-third of patients with diabetes worldwide will develop diabetic foot ulcer (DFU), which can progress to diabetic foot infection (DFI), and gangrene, consuming most of the healthcare costs dedicated for patients with diabetes.6 Around 17% of DFI will require amputation.7 Studies showed that patients with diabetes fear amputation more than death.8
The DFI patients will demand extensive debridement, and therapies which results in increasing the time of hospitalization, and the costs of treatment.9 In addition, the recurrence rate is high, reaching 40% within 1 year.10 Patients with DFI have a mortality incidence higher than patients with diabetes and without infection.11
Several factors predispose patients with diabetes to develop DFI, including immunopathology, as well as the potential to mount a normal inflammatory response. Also, due to loss of sweat and oil gland function in DM, the foot becomes dry and cracks more easily, leading to a portal for infection.9
The link between the immune system and diabetes pathogenesis has attracted the attention to highlight the pattern of some immune cells and their secreted cytokines associating T2DM progression.12
T-Natural killer (TNK) cells belong to the heterogeneous T-cell group. These cells recognize the specific lipid molecules presented by the CD1d molecules, as well as the polypeptides presented by non-major histocompatibility complexes. Therefore, TNK cells are also referred to as CD1d-dependent natural killer-like T-cells.13 These cells express the T lymphocyte markers (CD3 and TCR), as well as the NK cellular markers (CD56 and CD16) on their surfaces.14
The TNK cell was noticed to act as a link between innate and adaptive immunity through their secreted cytokines after their major histocompatibility complex (MHC) class I recognizes lipid antigens.15 Also, TNK cells directly act on target cells and regulate their biological functions.13
The TNK cell, if stimulated by lipid antigens, can produce Th1 and Th2 cytokines.16 Those cytokines may initiate cell-mediated immunity or inhibit autoreactive immunity.17 Among these TNK cytokines is interferon-gamma (IFN-γ), which is involved in pro-inflammatory response and is considered as a major player in phagocytosis and opsonization and which may point to refer to a protective role against development of local infection in DFI. Regarding interleukin 4 (IL4), it is reported to be involved in anti-inflammatory responses.18 Hence the rationale of studying the ratio as an indicator of balance between pro-inflammatory/anti-inflammatory secreted cytokines.
Dyslipidemia plays a crucial role in T2DM which is characterized by elevated TG, reduced HDL, and predominant LDL, which also plays a crucial role in development of atherogenesis and foot ulcer development.19 Since TNK cells mostly recognize lipid antigens, an altered lipid metabolic profile will also alter the repertoire of lipid antigens that can potentially affect TNK immune-modulatory function and cytokine secretion.20
This study aimed to investigate the link between circulating TNK % and serum IL4, IFN-γ levels, IFN-γ/IL4 ratio, and the development of DFI in patients with DFU. Also, we wanted to assess their relationship with diabetic biochemical parameters. Finally, we wanted to explore the association between TNK%, IL4, and IFN-γ levels and disease duration in DM patients.
Patients and Methods
Ninety patients with diabetes were enrolled in this cross-sectional study. Patients were divided into a T2DM group (n=30) including recently diagnosed patients, without any foot complications, recruited from Internal Medicine and Endocrinology Departments, a DFU group (n=30) recruited from the Vascular Department, and a DFI group (n=30) recruited from the Surgery Outpatient Clinic. All patients were recruited from Al-Zahraa University Hospital, during the period from September to December 2020.
The study was conducted in accordance with the Declaration of Helsinki. All patients were informed about the purpose of the study and written informed consent was obtained from all participants before proceeding with the study. Approval of the study proposal was granted by the Research Ethics Committee of the Faculty of Medicine for Girls (Cairo), Al-Azhar University (Approval No. 202008358).
Inclusion Criteria
T2DM was diagnosed based on the American Diabetes Association criteria.21 The DFI group included patients with infected ulcers and palpable pulsations in the foot attending surgery outpatient clinic. Diagnosis of DFI was done according to the Infectious Diseases Society of America (IDSA) (the presence of infection is defined by ≥2 classic findings of inflammation or purulence including induration, erythema, raised temperature, increased pain, and purulent discharge).22 Cultures were taken from the base of an appropriately debrided ulcer to yield true pathogens and more accurate results, and to exclude colonizers. All cases with DFI in our study showed clinically evident infection and this may be attributed to excluding patients with ischemia and the absence of its confounding effect on local and systemic inflammatory response.
DFU patients were recruited when there was no evidence of infection. The ulcer was classified according to Wagner classification and arterial duplex ultrasonography and Ankle-brachial index were done for all patients to exclude critical limb ischemia (CLI).
Exclusion Criteria
1) Any history of malignancy, autoimmune disorders, cardiac, liver, renal, pulmonary diseases, or use of anti-inflammatory drugs. 2) Type 1 DM (T1DM). 3) T2DM patients with morbid obesity (BMI>35 kg/m2), as they are usually associated with severe co-morbidities. 4) T2DM patients diagnosed for more than 2 years, to exclude any vascular complications. 5) Patients with T2DM associated with critical limb ischemia (CLI) according to Norgren et al.23
Sample Collection, Processing, and Storage
Each patient provided 10 mL of his or her blood after 12 hours of fasting. Each blood sample was divided into four portions; 1) 4 mL of blood was added into an EDTA tube for assessing glycated hemoglobin (HbA1C) and Complete blood count. 2) 4 mL was added to a serum gel separator tube and centrifuged, then serum was separated, 200 µl of separated serum was stored at −80°C for IL4 and IFN-γ measuring by enzyme-linked immunosorbent assay (ELISA) till the time of assay, while the rest of the serum was used for the measurement of diabetic biochemical parameters. 3) 2 mL blood was added to the heparin tube for flow cytometry assay.
Laboratory Investigations
Complete blood count (Sysmex KX-21, Japan), Hemoglobin A1C (D10, BioRad, France) and diabetic biochemical parameters by chemistry auto-analyzer device (Cobas Integra 400 plus, Roche diagnostics, Germany). Biochemical parameters included serum creatinine and lipid profile (total cholesterol (CHO), triglyceride (TG), low-density lipoprotein (LDL), high-density-lipoprotein (HDL) . Measuring of serum levels of cytokines human IL4 and IFN-γ by ELISA assay using Sinogeneclon Co., Ltd China. ELISA kits (ref. no. SG-10264, Lot. no. 202010) (ref. no., SG-10011. Lot. No. 202011), respectively, according to manufacturer instructions.
Measurement of TNK Cells Frequency in Peripheral Blood by Flow Cytometry Assay
Flow cytometry assay was performed using FACS Calibur (BD Biosciences, San Jose, CA). Cell Quest Pro software (BD Biosciences) was used for data analysis. Isotype control was used for positive cutoff detection. Two tubes were used with 50 µl of fresh blood sample each. 5 µl of cocktail of mouse-stained anti-human controls IgG1 FITC/IgG2a PE (catalog no.34240, lot no.90642) was added to the first tube. 5 µl of FITC-conjugated anti-human CD3/PE-conjugated anti-human CD16+CD56 cocktail (catalog no.95131, lot no.6012680. BD Biosciences, USA) was added to the second tube. All tubes were incubated for 20 minutes. Then, red blood cells (RBCs) were lysed, before sample washing using FACS buffer and centrifugation at 500 g. The identification strategy of TNK in three examples of patients’ groups is illustrated in Figure 1.
Microbiological cultures for deep tissue samples and purulent fluids were performed for all DFI group patients. Both aerobic and anaerobic cultures were done on routine culture media and identification of grown colonies was performed using Vitek 2 (Bio-Mérieux, France).
Plain x-ray was done for suspected cases of osteomyelitis (cases with moderate and severe DFI).
Statistical Methods
Statistical package for the Social Sciences (SPSS) version 26 (IBM Corp., Armonk, NY) was used for data coding. Mean and standard deviation were used for data summarization in normally distributed quantitative variables or median and interquartile range for non-normally distributed quantitative variables. Frequencies and relative percentages were used for categorical variables. Comparisons between groups were done using unpaired t-test or analysis of variance (ANOVA) test in quantitative variables with normal distribution while non-parametric Kruskal–Wallis test and Mann–Whitney test were used for non-normally distributed quantitative variables. For comparing categorical data, Chi square (χ2) test was performed. Spearman correlation coefficient was done for correlations between quantitative variables. ROC curve was constructed to detect the best cutoff value of TNK%, IL4, IFN-gamma, IFN-gamma/IL4 for detection of DFI. P-values<0.05 were considered statistically significant.
Results
Ninety patients with diabetes type 2 with a mean±SD age of 51.7±11.51 years were included. Patients were divided into a DFI group (n=30), DFU group (n=30), and T2DM group without complications (n=30). In 100% of the patients, pedal pulses were palpable. In both DFU and DFI groups, ulcers were located below the ankle. No significant difference was seen between mean age values between DFI and DFU groups (P=0.094). Demographic and clinical data of patients are shown in Table 1.
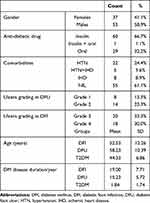 |
Table 1 Demographic and Clinical Data of Study Participants |
Comparative data between the three studied groups are displayed in Table 2.
 |
Table 2 Comparative Laboratory Data Between Groups |
Comparison of TNK% between DFI and DFU groups revealed a significant decrease in DFI group (P<0.001). Also, a comparison between DFI group and T2DM revealed a significant decrease in DFI group (P<0.001), while comparison between the DFU group and T2DM revealed a non-significant difference (P=0.082) (Figure 2A).
 |
Figure 2 (A) Comparison of TNK% in studied groups. (B) Comparison of IFN-gamma/IL4 in studied groups. (C) Comparison of IFN-gamma in studied groups. (D) Comparison of IL4 in studied groups. |
Comparison of IFN-γ/IL4 between the DFI group and DFU revealed a significant decrease in the DFI group (P=0.02), while comparison between the DFI group and T2DM group and comparison between the DFU group and T2DM revealed non-significant differences (P=0.357) and (P=0.748), respectively (Figure 2B).
Comparison of IFN-γ between the DFI group and the other two groups revealed a significant decrease in the DFI group (P<0.001). Comparison between the DFU group and T2DM revealed non-significant differences (P>0.999) (Figure 2C).
The DFI group showed a significant decrease in the IL4 level when compared to T2DM (P=0.006), while no statistical difference was shown in IL4 when compared to DFU (P=0.05). Also, IL4 level showed no significant difference when DFU was compared to T2DM (P>0.999) (Figure 2D).
Correlation Studies of Study Markers in All Study Participants (n=90)
Correlation of TNK% with both IL4 and IFN-γ revealed a significant correlation (r=0.385, P<0.001; and r=0.534; P<0.001, respectively).
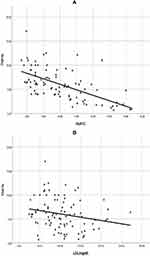
Regarding the metabolic contribution of TNK%, correlation of TNK% with HbA1c and LDL revealed a significant negative correlation (r=−0.631, P<0.001; and r=−0.261, P=0.013, respectively) while a positive correlation was seen with HDL (r=0.287, P=0.006) (Figure 3). No significant correlation was seen with TG or CHO.
Regarding the metabolic contribution of IL4, correlation of IL4% with HbA1c revealed a significant negative correlation with both (r=−0.514, P<0.001, respectively), while no correlation was detected with HDL, LDL, TG, CHO. The IFN-γ correlation with HbA1c and LDL revealed significant a negative correlation with both (r=−0.369, P<0.001; r=−0.229, P=0.03, respectively), while no correlation was seen with HDL, TG, or CHO.
The TNK % showed a significant negative correlation with DM disease duration/year (r=−0.546, P<0.001). Also, IFN-γ level showed a significant negative correlation with DM disease duration/year (r=−0.338, P=0.001). While no significant correlation was seen with IL4 level and disease duration (r=−0.181, P=0.089).
The ROC curve output data of study markers as predictors of infection in diabetic ulcer are demonstrated in Table 3 and Figure 4.
 |
Table 3 Output Data of ROC Curve for Discriminative Ability of Study Markers as Predictors of Infection in Diabetic Ulcer |
 |
Figure 4 ROC curve for TNK%, IL4, IFN-gamma, IFN-gamma/IL4 as discriminators of infection between DFU and DFI. |
Discussion
The relationship between the immune system and diabetes pathogenesis has attracted extensive attention.24 DM is characterized by exhaustion of glycolytic ability due to prolonged hyperglycemia. Prolonged hyperglycemia leads to increased cytotoxic mediator secretion and inflammatory cytokines upon infection, a phenomenon known as diabetic chronic inflammation.25
In the current study, comparison of TNK% in DFI and DFU groups revealed a significant decrease in TNK% in the DFI group. Also, the comparison between the DFI group and T2DM revealed a significant decrease in the DFI group while comparison between the DFU group and T2DM revealed non-significant differences. These findings highlight an immunoregulatory function for TNK cells which agreed with Gómez-Díaz et al,26 who found that TNK abnormalities associate with type 1 diabetes progression. Van-Kaer and Wu27 stated that invariant T natural killer (iTNK) cells comprise most of the TNK cells. They are important players in immune regulation as they promote self-tolerance, in addition to their cytotoxic properties. Tard et al16 reported that iTNK cell defects are associated with T2DM development as iTNK are rapidly able to produce IL4 and IFN-γ after the stimulation of their TCR in addition to their cytotoxic ability and added that iTNK can induce anergy of pathogenic T-cells.
Comparison of IFN-γ between the DFI group and DFU and T2DM groups revealed a significant decrease in the DFI group (P<0.001). This finding agreed with the findings of Sunandhakumari et al 18 that IFN-γ is involved in triggering phagocyte-dependent inflammation through macrophage activation, which is responsible for complement fixation and opsonization. Also, Mahmoud et al28 proved that declining of IFN-γ predicts infection in foot ulcer and added that IR shifts T-helper differentiation toward Th2 in the expense of Th1 which is concerned with cytotoxic response. Also, increased IL6 in DM suppresses IFN-γ gene expression during T-cell activation, which does not allow T1 differentiation.
Tsiavou et al29,30 and Kartika et al documented the reduction in IFN-γ production in T2DM. Also, Schmohl et al31 reported that IFN-γ was not detected in wound fluid extracted from DFU with or without infection, whereas 100% recovery was found for IFN-γ during wound recovery.
This low IFN-γ level detected in association with infection in DFI group can explain the increased susceptibility to local infection, as IFN-γ is concerned with boosting microbicidal functions through NADPH oxidase and nitric oxide (NO) synthase production. Both are major players in bacterial and fungal killing. Also, IFN-γ reinforces MHCII expression, which is crucial for Ag presentation.32 Also, Foss-Freitas et al33 reported lower IFN-γ levels in the T2DM group compared to the normal control and proposed that IFN-γ improves the capacity of granulocyte activation and phagocytic capacity, reducing the susceptibility to infections.
Comparison of IFN-γ between the DFU group and T2DM revealed non-significant differences. This was not in line with Theocharidis et al34, who observed inhibition of IFN-γ in DFU when compared to T2DM due to dysregulation of biological processes that included cell movement of monocytes, migration of dendritic cells, and chemotaxis of antigen-presenting cells pointing to an impaired migratory profile of immune cells in DM skin and proposed that up-regulation of IFN-γ is a sign of healing onset in DFU.
Also, Xu et al35 suggested that IFN-γ may correlate with DFUs onset but reported a significant upregulation in IFN-γ extracted from the skin tissue surrounding the ulcer in DFU when compared to the control group, using Western blot analysis. This could be explained by differences in techniques used and sample analyzed for IFN-γ level as we tested its serum level using the ELISA technique.
The DFI group showed a significant decrease in IL4 level when compared to T2DM, while no difference was seen in IL4 when the DFI group was compared to the DFU group. IL4 is concerned with M2 macrophage stimulation. M2 macrophages enhance insulin sensitivity, while M1 enhance IR.36 Also, the decrease of TNK cells in DFI also enhances the M1 phenotype switch.37
Comparison of IFN-γ/IL4 between the DFI group and DFU revealed a significant decrease in the DFI group while comparison between the DFI group and T2DM and comparison between the DFU group and T2DM revealed non-significant differences (Figure 2B). These findings highlight the role of IFN-γ/IL4 ratio in the development of infection in foot ulcers. As all cases of DFI had ulcer grades of 3 or 4 while DFU had ulcer grades of 1 or 2, this ratio may also play a role in ulcer grade progression.
The TNK % showed a negative correlation with DM disease duration/year. Correlation of TNK% with HbA1c and LDL revealed a significant negative correlation, while a positive correlation was seen with HDL (Figure 3). These observed findings strengthen the theory of the regulatory role of TNK cells in T2DM.
Correlation of serum level of IL4 with HbA1c revealed a significant negative correlation. These data agreed with that of Yang et al,38 which stated that the IL4 improves insulin sensitivity and glucose tolerance. This regulatory role was attributed to the ability of IL4 to suppress the production of the cytokines enhancing the IR as IL-6 and TNF-α.39
Surprisingly, IFN-γ revealed a significant negative correlation with HbA1c and LDL. This was in contrast to Kartika et al30, who demonstrated that IFN-γ is known to stimulate proinflammatory macrophage of M1 phenotype which potentiates the development of T2DM.
Correlation of TNK% with both IL4 and IFN-γ revealed a significant positive correlation. This agreed with Tard et al16, who reported that under the stimulation of antigens, TNK can interact with immune systems through the production of IL4 and IFN-γ cytokines.
The TNK % and IFN-γ level both showed a significant negative correlation with DM disease duration/year (r=−0.546, P<0.001; r=−0.338, P=0.001, respectively). This could highlight the link between TNK % and its secreted IFN-γ along DM progression with time.
Output data of ROC curves demonstrated in Table 3 and Figure 4 reveled that downregulation of TNK% and IFN-γ level have a role in occurrence of infection in DFU with IFN-γ being the more sensitive, which could be attributed to the protective role of IFN-γ in reducing the susceptibility to infections, as mentioned above.33 While TNK% are more specific, these given results introduce both TNK% and IFN-γ as potential immune therapy agents.
Study Limitations
Due to funding limitations the current study did not assess the level of intracellular production of IL4 and IFN-γ cytokines in TNK using flow cytometry to ascertain that their origin is the TNK. This point should be covered in future studies. Still, the presence of a positive correlation between the TNK% and both cytokines determine the relationship between them and linking TNK mediated T2DM pathogenesis and progression of DFI, through the production of IL4 and IFN-γ cytokines. Also, due to a lack of financial resources, frequency of TNK after appropriate management of infection was not studied, which should also be covered in future studies.
Conclusion
1) Decline in TNK frequency plays a role in T2DM pathogenesis and augmentation of subsequent foot complications.
2) Downregulation of TNK% and IFN-γ level have a potential role in occurrence of infection of diabetic ulcer with IFN-γ being the more sensitive, with TNK% being more specific.
3) Altered IL4 level has less role in augmentation of infection in foot ulcer than IFN-γ.
4) The TNK% and IFN-γ are downregulated in T2DM in a disease duration dependent manner.
5) The role of IFN-γ level downregulation is more sensitive and specific in augmentation of foot ulcer infection than altered IFN-γ/IL4 ratio.
6) The TNK and IFN-γ are potential agents for immune therapy to prevent foot complications in T2DM.
Disclosure
The authors declare that they did not receive any fund for this work and no conflicts of interest.
References
1. Zamstein O, Sheiner E, Wainstock T, Landau D, Walfisch A. Maternal gestational diabetes and long-term respiratory related hospitalizations of the offspring. Diabetes Res Clin Pract. 2018;140:200–207. doi:10.1016/j.diabres.2018.03.050
2. Aboelnasr MS, Shaltout AK, AlSheikh MR, Abdelhameed AH, Elrefaey W. Diabetic kidney disease in patients newly diagnosed with type-2 diabetes mellitus: incidence and associations. Saudi J Kidney Dis Transpl. 2020;31(1):191–199. doi:10.4103/1319-2442.279940
3. Kivelä J, Wikström K, Virtanen E, et al. Obtaining evidence base for the development of feel4Diabetes intervention to prevent type 2 diabetes–a narrative literature review. BMC Endocr Disord. 2020;20(1):1–24. doi:10.1186/s12902-019-0468-y
4. Bedewi MA, Elsifey AA, Naguib MF, et al. Ultrasonographic measurement of femoral cartilage thickness in type II diabetic patients. Medicine (Baltimore). 2020;99(14):e19455. doi:10.1097/MD.0000000000019455
5. Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global disability burdens of diabetes-related lower-Extremity complications in 1990 and 2016. Diabetes Care. 2020. doi:10.2337/dc19-1614
6. Eren MA, Güneş AE, Kırhan İ, Sabuncu T. The role of the platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in the prediction of length and cost of hospital stay in patients with infected diabetic foot ulcers: a retrospective comparative study. Acta Orthop Traumatol Turc. 2020;54(2):127–131. doi:10.5152/j.aott.2020.02.518
7. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. doi:10.1186/s13047-020-00383-2
8. Wukich DK, Raspovic KM, Suder NC. Patients with diabetic foot disease fear major lower-extremity amputation more than death. Foot Ankle Spec. 2018;11(1):17–21. doi:10.1177/1938640017694722
9. Perera D, Kleinstein SE, Hanson B, et al. Impaired host response and the presence of Acinetobacter baumannii in the serum microbiome of type-II diabetic patients. iScience. 2020;24(1):101941. doi:10.1016/j.isci.2020.101941
10. Huang ZH, Li SQ, Kou Y, Huang L, Yu T, Hu A. Risk factors for the recurrence of diabetic foot ulcers among diabetic patients: a meta‐analysis. Int Wound J. 2019;16(6):1373–1382. doi:10.1111/iwj.13200
11. Malanda B, Burgaz C, Njenga E, et al. Diabetes care and education training audit for primary care physicians - Results from IDF Diab-CET Kenya study needs assessment survey. Diabetes Res Clin Pract. 2020;159:108012. doi:10.1016/j.diabres.2020.108012
12. Hammad RH, El-Madbouly AA, Kotb HG, Zarad MS. Frequency of circulating B1a and B2 B-cell subsets in Egyptian patients with type 2 diabetes mellitus. Egypt J Immunol. 2018;25(1):71–80.
13. Lv X, Gao Y, Dong T, Yang L. Role of natural killer T (NKT) cells in type II diabetes-induced vascular injuries. Med Sci Monit. 2018;24:8322. doi:10.12659/MSM.912446
14. de Mingo Pulido Á, de Gregorio E, Chandra S, et al. Differential role of cathepsins S and B in hepatic APC-mediated NKT cell activation and cytokine secretion. Front Immunol. 2018;9:391. doi:10.3389/fimmu.2018.00391
15. East JE, Kennedy AJ, Webb TJ. Raising the Roof: the preferential pharmacological stimulation of Th1 and th2 responses mediated by TNK cells. Med Res Rev. 2014;34(1):45–76. doi:10.1002/med.21276
16. Tard C, Rouxel O, Lehuen A. Regulatory role of natural killer T cells in diabetes. Biomed J. 2015;38(6):484–495. doi:10.1016/j.bj.2015.04.001
17. Hung JT, Huang JR, Yu AL. Tailored design of NKT-stimulatory glycolipids for polarization of immune responses. J Biomed Sci. 2017;24(1):22. doi:10.1186/s12929-017-0325-0
18. Sunandhakumari VJ, Sadasivan A, Koshi E, Krishna A, Alim A, Sebastian A. Effect of non-surgical periodontal therapy on plasma levels of IL-17 in chronic periodontitis patients with well controlled Type-II diabetes mellitus-A clinical study. Dent J (Basel). 2018;6(2):19. doi:10.3390/dj6020019
19. Chakraborty M, Singh P, Dsouza JMP, Pethusamy K, Thatkar PV, Family Med J. Fasting and postprandial lipid parameters: a comparative evaluation of cardiovascular risk assessment in prediabetes and diabetes. Prim Care. 2020;9(1):287–292.
20. Tiwary S, Berzofsky JA, Terabe M. altered lipid tumor environment and its potential effects on NKT cell function in tumor immunity. Front Immunol. 2019;10:2187. doi:10.3389/fimmu.2019.02187
21. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes. Diabetes Care. 2020;43(1):S14–S31. doi:10.2337/dc20-S002
22. Lipsky BA, Senneville É, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(S1):e3280. doi:10.1002/dmrr.3280
23. Norgren L, Hiatt WR, Dormandy JA, et al.; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26(2):81–157.
24. Prattichizzo F, De Nigris V, Spiga R, et al. Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res Rev. 2018;41:1–7.
25. Moura J, Madureira P, Leal EC, Fonseca AC, Carvalho E. Immune aging in diabetes and its implications in wound healing. Clin Immunol. 2019;200:43–54.
26. Gómez-Díaz RA, Aguilar MV, Meguro EN, et al. The role of natural killer T (NKT) cells in the pathogenesis of type 1 diabetes. Diabetes Rev. 2011;7(4):278–283. doi:10.2174/157339911796397839
27. Van Kaer L, Wu L. Therapeutic potential of invariant natural killer T cells in autoimmunity. Front Immunol. 2018;9:519. doi:10.3389/fimmu.2018.00519
28. Mahmoud MA, Ghareeb DA, Sahyoun HA, Elshehawy AA, Elsayed MM. In vivo interrelationship between insulin resistance and interferon gamma production: protective and therapeutic effect of berberine. Evid Based Complement Alternat Med. 2016;2016:2016. doi:10.1155/2016/2039897
29. Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA. Intracellular IFN-γ production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res. 2004;24(7):381–387. doi:10.1089/1079990041535665
30. Kartika R, Purnamasari D, Pradipta S, Larasati RA, Wibowo H. Impact of low interferon-γ and il-10 levels on tnf-α and il-6 production by PHA-induced pbmcs in type 2 diabetes mellitus. J Inflamm Res. 2020;13:187. doi:10.2147/JIR.S245064
31. Schmohl M, Beckert S, Joos TO, Königsrainer A, Schneiderhan-Marra N, Löffler MW. Superficial wound swabbing: a novel method of sampling and processing wound fluid for subsequent immunoassay analysis in diabetic foot ulcerations. Diabetes Care. 2012;35(11):2113–2120. doi:10.2337/dc11-2547
32. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon‐γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189.
33. Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. Effect of metabolic control on interferon-gamma and interleukin-10 production by peripheral blood mononuclear cells from type 1 and type 2 diabetic patients. Braz J Med Biol Res. 2007;40(5):671–677. doi:10.1590/S0100-879X2007000500010
34. Theocharidis G, Baltzis D, Roustit M, et al. Integrated skin transcriptomics and serum multiplex assays reveal novel mechanisms of wound healing in diabetic foot ulcers. Diabetes. 2020;69(10):2157–2169. doi:10.2337/db20-0188
35. Xu S, Weng X, Wang Y, et al. Screening and preliminary validation of T lymphocyte immunoregulation-associated long non-coding RNAs in diabetic foot ulcers. Mol Med Rep. 2019;19(3):2368–2376. doi:10.3892/mmr.2019.9877
36. Chang YH, Ho KT, Lu SH, Huang CN, Shiau MY. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin 4. Int J Obes. 2012;36(7):993–998. doi:10.1038/ijo.2011.168
37. Tateya S, Kim F, Tamori Y. Recent advances in obesity-induced inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013;4:93. doi:10.3389/fendo.2013.00093
38. Yang CP, Shiau MY, Lai YR, et al. Interleukin-4 boosts insulin-induced energy deposits by enhancing glucose uptake and lipogenesis in hepatocytes. Oxid Med Cell Longev. 2018;2018:6923187. doi:10.1155/2018/6923187
39. Shih YL, Ho KT, Tsao CH, et al. Role of cyotkines in metabolism and type 2 diabetes mellitus. Int J Biomed Lab Sci. 2013;(2):1–6.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.