Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
T-Lymphocyte Gene-Regulated CCL5 and Its Association with Extrahepatic Metastasis in Hepatocellular Carcinoma
Authors Dong G, Fan F, He Y, Luo Y, Yu J, Liang P
Received 23 May 2023
Accepted for publication 20 July 2023
Published 2 August 2023 Volume 2023:10 Pages 1267—1279
DOI https://doi.org/10.2147/JHC.S420836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Guoping Dong,1,2 Fangying Fan,1,2 Yao He,3 Yanchun Luo,1 Jie Yu,1,2 Ping Liang1,2
1Department of Interventional Ultrasound, Fifth Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 2Chinese PLA Medical School, Beijing, 100853, People’s Republic of China; 3Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, 100871, People’s Republic of China
Correspondence: Ping Liang; Jie Yu, Department of Interventional Ultrasound, Fifth Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China, Tel +86-10-66939530 ; +86-10-66937963, Email [email protected]; [email protected]
Background: Extrahepatic metastasis in hepatocellular carcinoma (HCC) greatly limits the prognostic survival of HCC patients. Levels of preoperative peripheral lymphocyte subsets and cytokines in the serum for predicting extrahepatic spread of hepatocellular carcinoma are still not common in clinical practice. The aim of this study is to investigate the value and mechanisms of peripheral lymphocyte subsets and cytokines in predicting extrahepatic spread of HCC.
Methods: We used a retrospective design to analyze data pertaining to a total of 380 patients with HCC who were examined for peripheral T-lymphocyte subsets before receiving microwave ablation. We performed Cox regression analysis to screen out independent risk factors and used pathology specimens from the patients and public databases of liver cancer to investigate the correlation between cytokines and intra-tumor immune cells.
Results: The CD4low group had better metastasis-free 1-year, 3-year, and 5-year survival rates compared to the CD4high group (80% vs 69%, 67% vs 51%, and 57% vs 39%, respectively; HR 1.7 (1.2, 2.3), P = 0.0019). Similarly, the CD8high group had better metastasis-free 1-year, 3-year, and 5-year survival rates compared to the CD8low group (65% vs 78%, 46% vs 64%, and 34% vs 54%, respectively; HR 0.6 (0.4, 0.8), P < 0.001). Patients with the CD4high/CD8low phenotype had significantly worse metastasis-free survival times compared to other patients (HR 2.0 (1.5, 2.8), P < 0.001). Additionally, T lymphocyte-specific genes (CD4, CD8) were correlated with CCL5 expression, which was also positively correlated with the level of intra-tumoral infiltrating CD8 T cells and the prognosis of HCC patients.
Conclusion: Both CD4+ and CD8+ T lymphocyte subsets were independent risk factors for extrahepatic metastasis in HCC. Serum CCL5 levels could indicate the infiltration level of intra-tumoral CD8+ T cells and the risk of extrahepatic metastasis in HCC patients, aiding in patient risk stratification for metastasis.
Keywords: cytokine CCL5, extrahepatic metastasis of hepatocellular carcinoma, peripheral lymphatic subset analysis, cytotoxic CD8+ T cells
Graphical Abstract:
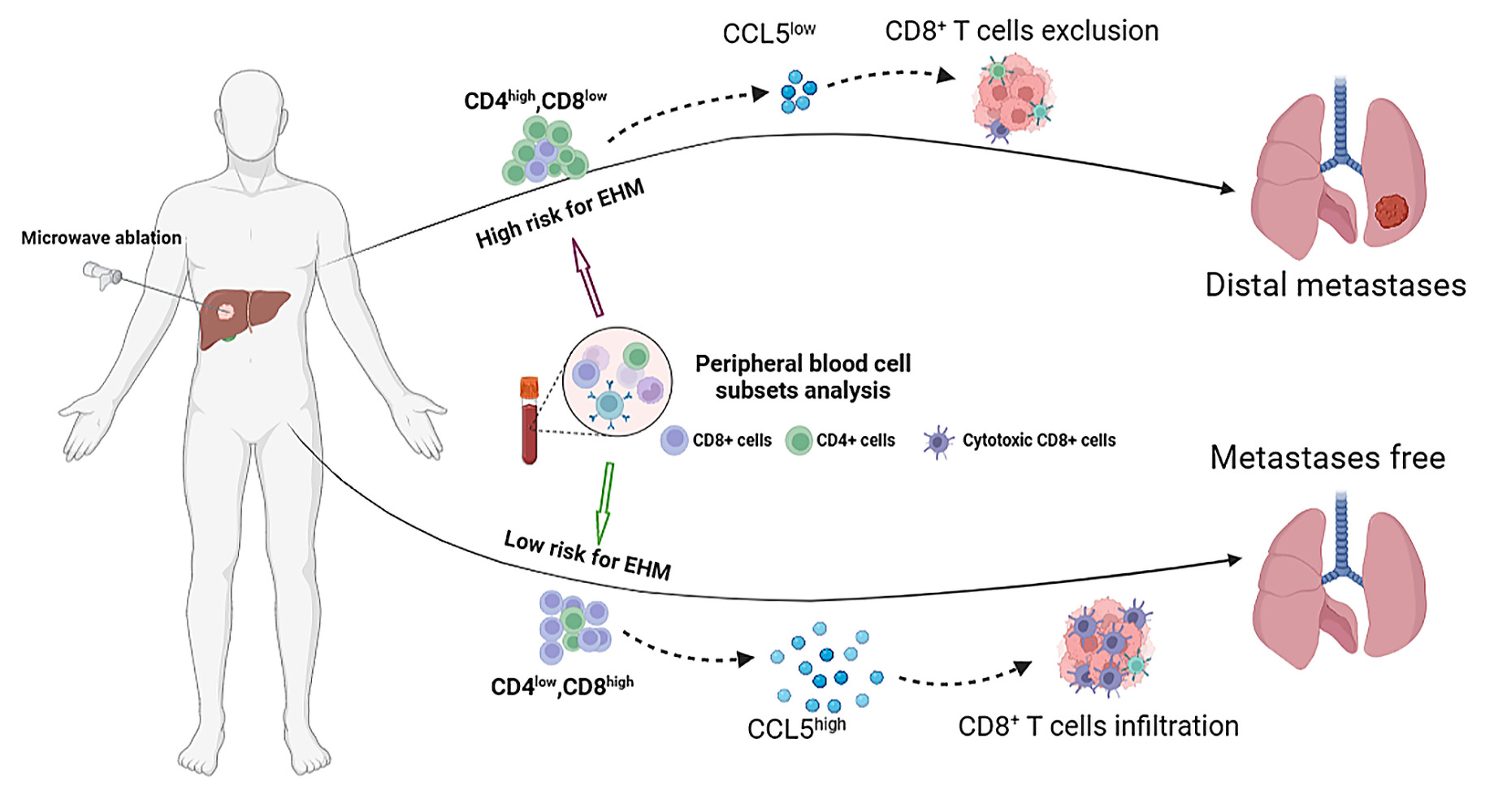
Introduction
Hepatocellular liver cancer is one of the most common malignancies in the world, with its incidence increasing year by year worldwide.1,2 Although five-year survival rates can reach 50–70% after surgery or ablative treatment for early-stage hepatocellular carcinoma (HCC), the high recurrence and metastasis rates still greatly limit the prognostic survival of patients.3–6 Incurability and death in more than 90% of cancer patients was due to metastasis,7,8 and the occurrence of extrahepatic metastasis (EHM) of HCC was up to 37%, which is one of biggest challenge in the management of HCC.9 EHM can occur during all stages of hepatocellular carcinoma progression and involve multiple other organs or tissues through hematogenous or lymphatic metastases. Therefore, management of hepatocellular carcinoma necessitates early diagnosis and treatment of HCC patients with extrahepatic metastasis to effectively improve the prognosis and survival conditions of patients.
Tumor immune escape is an important mechanism for distant tumor implantation. Circulating immune tumor cells are not effectively removed if the balance of the immune environment of the body system is disrupted, for instance, through insufficient killing capacity of CD8+ T-cells or an excessive number of inhibitory CD4+ T-cells. This increases the likelihood that the surviving tumor cells will implant in distant organs. The growth of tumor-specific CD8+ T lymphocytes and the production of interferon-gamma (IFNg) were shown to be suppressed by high doses of IL-15, as reported by Chen et al.10 In addition, circulating Treg cells can modulate the anti-tumor immune function of the body through tumor-specific immune responses, and patients with HCC having low levels of myeloid-derived suppressor cells (MDSC) who were treated with hepatic artery-infusion chemotherapy (HAIC) had better overall survival.11,12 Moreover, incomplete radiofrequency ablation can lead to activation of METTL1 in residual tumor cells, which in turn regulates the transcription levels of TGF-β. This recruitment of PMN-MDSCs subsequently induces HCC recurrence.13 These results suggest that peripheral lymphocyte status is associated with the prognosis of HCC patients. However, it is still unknown whether the peripheral immune status of patients with HCC treated with ablation therapy affects HCC metastasis.
It is well established that cytokines attract immune cells and route them to target organs, thus initiating inflammatory responses and immune surveillance. The role of CCL5 in the progression of hepatocellular carcinoma tumors is still debatable. The expression level of endosialin in HSC is inversely linked with tumor proliferation capability in hepatocarcinogenesis,14 suggesting that CCL5 may adversely control endosialin. Cancer associated-fibroblasts derived CCL5 can extend the cytoplasmic presence of HIF and activate ZEB1, thereby inducing the transformation of tumor cells through epithelial-mesenchymal transition (EMT) and promoting distant metastasis of the tumor.15 Moreover, overexpression of CCL5 in circulating tumor cells drove regulatory T cells to migrate to tumor tissue, assisting the tumor cells in immune escape and distant metastasis.14,16
However, a new study showed that PGLYRP2 could regulate the transcriptional level of CCL5 by directly interacting with the CCL5 promoter region and, in this way, enhancing the anti-tumor immune response of the body.17 In addition, it has been found that the CCL4/CCL5-CCR1/CCR5 pathway can promote the infiltration of γδ T cells from peripheral blood or tumor peripheral areas into tumor tissues, thus playing an anti-tumor role.18 Finally, in a β-catenin-driven HCC model, re-expression of CCL5 in tumor cells was able to increase the numbers of intra-tumoral dendritic cells and antigen-specific CD8 T cells, thereby restoring the immune surveillance function of the body.19
The presence of extrahepatic metastasis in HCC patients is crucial for selecting the appropriate clinical treatment approach. Timely and effective assessment of the risk of extrahepatic metastasis in HCC patients is an important prerequisite for improving patient prognosis and overall survival. In this study, we systematically evaluated the clinical value of peripheral immune status for the assessment of extrahepatic metastases in HCC. In addition, the level of serum CCL5 is related to the function and status of tumor-infiltrating T lymphocytes in HCC patients undergoing thermal ablation. This suggests that serum CCL5 may potentially serve as a promising indicator for evaluating the risk of extrahepatic metastasis in HCC ablation patients.
Materials and Methods
Patients
This single-center research comprised 380 blood samples from patients having curative thermal ablation of HCC treated between April 2010 and December 2021. Through retrospective chart analysis, we collected data from primary laboratory tests, such as the definition of peripheral lymphocyte subsets and the levels of serum cytokines, alpha fetoprotein (AFP), albumin, platelets (PLTs), hemoglobins (HBs), and prothrombin, and extracted demographic information such as gender, age, tumor size, hepatitis virus history, Child-Pugh score, Barcelona Clinical Liver Cancer (BCLC) stage, and long-term follow-up survival data. Blood was collected from patients only after they signed an informed consent form, in accordance with the Declaration of Helsinki standards. The exclusion criteria of this retrospective study are patients who were lost to follow-up, patients with missing laboratory examination data, and patients with extrahepatic metastasis prior to treatment.
Follow-Up
Within a month after undergoing thermal ablation, all patients treated for HCC underwent either ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MRI) to assess the efficacy of the procedure and ensure that all malignant lesions had been eradicated. Extrahepatic metastasis (EHM) was the study endpoint, defined as the appearance of new tumor lesions outside of the liver detected by imaging. Patients were closely followed up every three to six months, and those suspected of having distant metastases were required to provide diagnostic imaging results from their local hospital.
Analysis of Peripheral Lymphatic Subsets
In this retrospective study, patients with HCC had two to three milliliters of their fasting venous blood taken and placed in ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes before undergoing thermal ablation. For the analysis of peripheral lymphocyte subsets, the corresponding antibodies were added as per the manufacturer’s instructions, and these included anti-human CD45-AmCyan-A, CD3-APC-Cy7A, CD4-PE-Cy7, CD8-PerCP-Cy5-5-A, CD16-FITC-A, CD19-PerCP-Cy5, CD25-APC-A, CD28-PE-A, CD45RA-FITC, CD45RO-APC-A, and CD56-PE-A (Beckman Coulter, Inc., BD Bioscience). Fluorescence-activated cell sorting (FACS) data were analyzed using FlowJo software (Tree Star, Inc.).
Subgrouping
Patients with hepatocellular carcinoma who were included in this retrospective study were categorized according to the analysis of peripheral lymphocyte subpopulations. The CD4high group was defined as individuals with a CD4 cell percentage greater than 25, while the CD4low group consisted of those with a CD4 cell percentage less than or equal to 25. Similarly, the CD8high group was identified by a CD8 lymphocyte percentage greater than 30, and the CD8low group included individuals with a cell percentage less than or equal to 30. Additionally, individuals in the CD4high CD8low group were characterized as those with a CD4 cell percentage greater than 25 and a CD8 lymphocyte percentage less than or equal to 30.
Cytokine Assay
Blood samples were collected using EDTA anticoagulation tubes and then centrifuged at 2000 rpm for 10 minutes at room temperature. We used the Bio-Plex 200 system (Bio-Plex Pro Human Cytokine Grp I Panel 27-Plex; Bio-Rad, USA) as specified by the manufacturer of the commercial kit. The procedure is detailed in a previous study.20 In this study, we detected a total of 27 cytokines, including IL-1β, IL-1RA, IL-6, IL-8, IL-17A, IL-10, and TNF-α (immunomodulatory cytokines); FGF-basic, VEGF, G-CSF, and GM-CSF (growth cytokines); IFN-γ, IL-2, IL-4, IL-5, IL-7, IL-13 IL-12p70, IL-15, and IP-10 (helper T cell-associated cytokines); IL-9, MCP-1, RANTES, MIP-1α, MIP-1β, PDGF-BB, and Eotaxin.
Statistical Analysis
We used the EMpowerRCH software for statistical analysis in this study. In order to study the influence of peripheral lymphocyte subsets on extrahepatic metastasis-free survival in HCC patients undergoing ablation therapy, we included 15 variables factors related to peripheral immune function status. Univariate Cox regression analysis was performed to examine the relationship between the 15 variables and survival with liver extrahepatic metastasis. Results indicated that three variables showed significant associations (p<0.05). Following this, a multivariate analysis was conducted on these three variables, revealing that two variables remained statistically significant and could be considered as independent factors. Student’s t-test was used to analyze continuous variables, and the chi-square test or Fisher’s exact test were used for categorical variables. Kaplan-Meier survival curves were plotted to assess survival differences between patients with different peripheral lymphoid subset immune function phenotypes. Spearman correlation analysis was performed to evaluate the relationship between CCL2, CCL5, and the characteristics of CD4 and CD8. A P value < 0.05 was considered statistically significant.
Single-Cell Sequencing
In this research, sequencing information was acquired using the liquid droplet-based scRNA-seq method. Tissues were processed using a single-cell platform that included GemCode gel beads, microarrays, and library preparation kits (10X Genomics, Pleasanton). The number of cells loaded per patient was 1*105. The cells were then separated into individual cells with emulsifiers and subjected to cell lysis and RNA-barcoded reverse transcription in the GemCode instrument, followed by amplification, shearing and 3′ adaptor and sample index attachment. Library sequencing was done on the Illumina Hiseq 4000.
Results
Baseline Characteristics of Patients
Only 380 patients with HCC treated with microwave ablation (original cohort, n = 315; validation cohort, n = 65) were included in this retrospective study (Figure 1) after excluding 548 non-HCC patients, 104 patients lost to follow-up, 44 patients with no laboratory data, and 16 patients with extrahepatic metastases prior to treatment. The baseline characteristics and clinical characteristics of the original cohort (median age: 58.8 years; interquartile range: 32–89 years; n = 315) and validation cohort patients (median age: 59.5 years; interquartile range: 34–80 years; n = 65) are summarized in Table 1. The findings suggest that there were no statistically significant disparities observed in terms of lifestyle habits, BMI indices, tumor markers, blood examinations, comorbidities, and physical functional status between the two cohorts of patients. Therefore, all baseline characteristics of the two cohorts were comparable (P > 0.05).
 |
Table 1 Patient Characteristics in Primary and Validation Cohort |
 |
Figure 1 Flowchart of data sampling. |
Preoperative identification of peripheral lymphatic subgroups was performed on 315 patients who had had microwave ablation for hepatocellular cancer; of them, 180 (57.14%) had extrahepatic metastases. Moreover, among patients with extrahepatic metastases, there was a higher proportion of patients in BCLC stage B when compared to patients with no extrahepatic metastases (37 of 180 [20.6%] vs 14 of 135 [10.4%], respectively; P = 0.015) and more tumor cells (1.9 ± 1.6 vs 1.4 ± 0.8, respectively; P = 0.015, Table S1).
To comprehensively analyze the systemic immune status of patients with and without EHM, laboratory test results linked to peripheral immune status were compared between the two groups. There were no statistically significant differences between the groups in terms of total leukocyte or lymphocyte, neutrophil granulocyte, or platelet counts. The values of serum AFP, hemoglobin, and prothrombin were also comparable between the two groups (Table S2). Overall, the baseline levels were generally consistent between the two groups of patients with EHM and non-EHM HCC in the original cohort.
Independent factors associated with extrahepatic metastasis of hepatocellular carcinoma:
A common contributor to EHM in individuals with hepatocellular carcinoma is a compromised anti-tumor immune response. To some extent, the effectiveness of an organism’s defenses against tumors may be reflected in the ratio of peripheral immune cells. We investigated the possible relationship between peripheral immune lymphoid subsets and extrahepatic metastasis to better understand the impact of systemic immunological features of the organism on the incidence of extrahepatic metastasis in HCC patients.
Univariate Cox regression analysis revealed that the ratio of three T lymphocytes, CD4, CD8, and Treg, was associated with the occurrence of extrahepatic metastasis in HCC patients (P < 0.05, Table 2). We used the fitting curve to calculate the optimal cut-off values for CD4, CD8, and Treg in the study (Figure S1). Multivariate Cox regression model analysis showed that CD4 and CD8 were independent factors for the occurrence of extrahepatic metastasis in HCC patients (Table 2). In conclusion, we found that the ratio of peripheral immune CD4+ T cells was a risk factor for the occurrence of extrahepatic metastasis in HCC patients, while the ratio of CD8+ T cells was a protective factor.
 |
Table 2 Univariate and Multivariate Analyses of Prognostic Factors Associated with EHM |
Effect of EHM-Related Risk Factors on Clinical Outcomes in HCC Patients
To further investigate the effect of peripheral CD4 and CD8 lymphatic subsets on the clinical outcome of HCC patients with hepatocellular carcinoma, we analyzed the levels of peripheral CD4 and CD8 subsets in HCC patients for the survival time without extrahepatic metastasis among different groups of HCC patients. The 1-, 3-, and 5-year metastasis-free survival rates in the CD4high and CD4low groups were 69% vs 80%, 51% vs 67%, and 39% vs 57%, respectively. The median progression-free survival was 38.4 months vs 85.7 months in the CD4high and CD4low groups, respectively (HR 1.7 (1.2, 2.3), P = 0.0019, Figure 2). In addition, the 1-, 3-, and 5-year metastasis-free survival was 78% vs 65%, 64% vs 46%, and 54% vs 34% in the CD8high and CD8low groups, respectively (HR 0.6 (0.4, 0.8), P < 0.001, Figure 2, Table S3). The median progression-free survival was 77.7 months vs 28.8 months in the CD8high and CD8low groups, respectively. This implies that a high ratio of CD4 T cells or a low level of CD8 T cells in peripheral blood indicates a poor prognosis for extrahepatic metastasis-free survival in HCC patients.
 |
Figure 2 Kaplan-Meier analyses for MFS according to CD4+ cell ratio (A), CD8+ cell ratio (B), and combined (CD4+/CD8+) cell ratio (C) in the primary cohort. |
Next, based on the ratios of CD4 and CD8, we divided the original cohort into four subset groups: CD4low/CD8high group, CD4low/CD8low group, CD4high/CD8low group, and CD4high/CD8high group. Using the Kaplan-Meier (KM) survival curve analysis, we found that the CD4high/CD8low group (immunosuppressed) had the worst prognosis of all four groups (HR 2.0 (1.5, 2.8), P < 0.001), indicating that patients with the lymphocytic phenotype of CD4high/CD8low had a higher incidence of extrahepatic metastases (Figure 2).
To further validate the above results, we reenrolled 65 patients for extrahepatic metastasis-free survival curve analysis. Similarly, in the validation cohort, patients in the CD4low and CD8high groups had better metastasis-free survival than the CD4high and CD8low groups (P < 0.05). Also, the CD4high/CD8low group had the worst prognosis for metastasis-free survival (Figure S2). Hence, we also conducted a comparative analysis of the overall survival (OS) outcomes among HCC patients with different peripheral immune functional statuses. The results consistently demonstrated that patients with an immunosuppressive phenotype (CD4high, CD8low groups) exhibited worse overall survival prognosis (Figure S2). Thus, our results indicated that HCC patients with an immunosuppressive phenotype (CD4high/CD8low) had higher risks of extrahepatic metastasis after receiving microwave ablation.
T-lymphocyte gene-regulated CCL5 expression was downregulated in patients with a high risk of extrahepatic metastasis:
Lymphocyte proliferation, differentiation, and circulation are largely controlled by cytokines. Cytokines either inhibit or promote tumor growth, and they are secreted by a wide variety of cells, including immune cells, endothelial cells, and many others. Furthermore, cytokines have been linked to the invasive and metastatic potential of tumor cells. In this study, we measured the levels of serum cytokines in 65 patients with HCC in the validation group. We found that CCL5, CCl2, and GCSF were significantly downregulated in the immunosuppressed patient group (P < 0.05, Figure S3 and S4A).
We analyzed the disease-free survival and overall survival of HCC patients with different expression levels of CCL5, CCl2, and GCSF using the RNA-data sequencing library of the Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia2.cancer-pku.cn/) to further investigate the effects of these three cytokines on the prognostic survival of patients with hepatocellular carcinoma.21 We divided the patients with HCC into high-expression and low-expression groups based on the levels of CCL5, CCl2, and GCSF genes. Although the expression levels of these three genes had no effect on the overall survival (OS) of HCC patients (P > 0.05), HCC patients with high CCL5 expression had better disease-free survival time compared with those with low CCL5 expression (P < 0.05), while the expression levels of CCL2 and GCSF had no significant effect on the disease-free survival of HCC patients (Figure S4B and S4C).
Next, we investigated whether T lymphocyte-specific genes (CD4/CD8) could regulate the expression of CCL5. The results of Spearman correlation analysis confirmed that the expression level of CCL5 was mono-correlated with the T lymphocyte-specific gene signature cluster (CD4/CD8) (Figure S4D), suggesting that T lymphocyte-specific genes might regulate the expression level of CCL5.
CCL5 affected the targeting infiltration of CD8 T cells into tumor tissues:
To further investigate how T-lymphocyte-regulated CCL5 affects extrahepatic metastasis in HCC patients, we used publicly available databases to assess the correlation between CCL5 expression levels and the intra-tumor infiltration capacity of CD8 lymphocytes. The CIBERSORT score can be used to evaluate the correlation of specific genes with the infiltration level of intra-tumoral immune cells.22 As shown in Figure 3A, the expression level of CCL5 was positively correlated with the infiltration number of CD8 T lymphocytes in the tumors. Meanwhile, survival analysis showed that patients with high CD8 expression likewise had better prognostic survival (P = 0.029).
Subsequently, by using a publicly available dataset on the Gene Expression Omnibus (GEO) repository and the median expression value of CCL5 as the cut-off point, we divided patients with HCC into the CCL5high group and the CCL5low group. We found that patients with high CCL5 expression had more M1-type macrophages and CD8T cells in their tumor tissues (Figure 3B). In addition, we used unsupervised clustering analysis and cell type identification to detect the expression levels of CCL5 in different immune cell types. The results of single-cell sequencing revealed that T lymphocytes with high CCL5 expression were more likely to exhibit a functional phenotype of cytotoxicity, such as GZMK+ CD8+ T cells, GZLY+ CD8+ T cells, CD8+ memory T cells, as well as CD8+ effector T cells (Figure 3C). To sum up, our data analysis revealed that CCL5 can promote pro-inflammatory cells to infiltrate tumor tissue.
Finally, to further confirm the recruitment of serum CCL5 on CD8+ T cells of tumor tissues, immunohistochemical staining was performed on the specimens sampled from the two groups of HCC patients in the validation cohort with high and low expression of CCL5.The immunohistochemical results showed that compared with patients with low CCL5 expression, patients with high CCL5 expression had more CD3+ T and CD8+ T lymphocytes (Figure 3D and E), suggesting that HCC patients with high CCL5 expression had stronger anti-tumor immune responses after treatment with microwave ablation. In summary, our results confirmed that CCL5 regulates the infiltration status of cytotoxic CD8+ T cells within the tumor, which may explain why HCC patients with high CCL5 have low risks of extrahepatic metastasis.
Discussion
There is growing evidence to show that early diagnosis of extrahepatic metastases can effectively enhance the treatment strategy and clinical management of patients with hepatocellular carcinoma, which is crucial to improving their prognostic survival.23,24 The true percentage of patients with extrahepatic metastases was close to 68%, as evidenced by autopsy results.9 However, only 13–36% of HCC patients with distant metastases are clearly diagnosed with imaging. Biopsy of tissue specimens in patients with hepatocellular carcinoma is clinically challenging, and there is an urgent need for identifying markers in the circulatory system that can reflect the risk of extrahepatic metastasis. Cytotoxic CD8+ T lymphocytes, which are key killer cells for the antitumor response, and auxiliary CD4+ T lymphocytes, which have immunomodulatory functions, can balance the inflammatory response of the organism through cell-cell interactions or cytokine secretion.25 Tumor cells can escape immune surveillance and implant into distant organ tissues through hematogenous metastasis when the immune macroenvironmental state is dysregulated or the balance of the inflammatory response is disrupted.26,27
Most of the current research focuses on building predictive models to assess the occurrence of extrahepatic metastasis after hepatocellular carcinoma (HCC) treatment.28 Zhou et al found that neutrophils, prothrombin time, tumor number, and size are independent factors for HCC patients who develop extrahepatic metastasis.29 Additionally, another study discovered that the serum factor IL-17A is specifically detected in HCC patients with extrahepatic metastasis.30 These findings suggest a potential link between peripheral immune status and the occurrence of extrahepatic metastasis in HCC patients. In the present study, we analyzed peripheral blood samples of HCC patients treated with thermal ablation and found that peripheral blood lymphocyte subsets, CD4+ T cells and CD8+ T cells, were strongly associated with clinical outcomes of EHM in HCC patients. In addition, the TCGA database allowed us to draw the conclusion that CD8 expression was also positively correlated with prognostic survival in HCC patients. We then defined patients with the peripheral immune phenotype CD4high/CD8low as having immunosuppression, and KM survival curve survival analysis showed that HCC patients with systemic immunosuppressive phenotypes had higher risks of extrahepatic metastases.
A longitudinal study showed that circadian rhythms of the immune system affect the prognosis of melanoma patients treated with immunotherapy.31 In addition, Breanna et al found that the systemic immune state is adjustable, and that tumor progression dynamically reshapes the composition and function of the immune macroenvironment.32 These research studies suggest that the dynamic changes in the immune functional state of the organism are correlated with the progression and metastasis of tumors. Our findings suggest that preoperative peripheral blood T lymphocytes can be a useful indicator for stratifying risk of extrahepatic metastases.
Although the status of the peripheral immune macroenvironment can indeed influence the occurrence of extrahepatic metastasis in hepatocellular carcinoma, its impact on the overall survival of HCC patients is very limited. In particular, our earlier research findings demonstrated that Huaier granules, a traditional Chinese medicine used clinically as an immunomodulator, could enhance the anti-tumor immune response and reduce the recurrence in patients with early hepatocellular carcinoma following thermal ablation.33 In addition, Sophora granules were also found to improve the functional status of peripheral immune cell subsets, such as increasing the numbers of CD3+ T cells, CD4+ T cells, and NK cells.34 Results of a national multicenter study demonstrated that Sophora granules used as adjuvant therapy significantly reduced extrahepatic recurrence in patients with hepatocellular carcinoma after curative hepatectomy.35 In conclusion, immunomodulators can improve the peripheral immune macroenvironment in patients with hepatocellular carcinoma, thus reducing the risk of extrahepatic metastasis.
The significance of the CCL5-CCR5 signaling axis in the occurrence, progression, and metastasis of tumors remains controversial. Human HCC tissues have been shown to have elevated levels of CCR5 and CCL5 and that CCL5 induces the proliferation of CCR5-expressing cancer cells.14,36,37 In this context, elevated levels of serum CCL4 and CCL5 in patients with liver cirrhosis may indicate the presence of HCC.38 However, a recently published study showed that γδ T cell-specific gene expression was positively correlated with the levels of CCL4, CCL5, and CCR5 in patients with HCC and that γδ T cells were recruited from peripheral blood or peritumoral regions to tumor tissues to clear tumor cells via the interaction of CCL4/CCL5 with their receptors.11 In addition, NLRC3 (the NLR Family CARD Domain-Containing Protein 3) was a positive predictor of survival outcome in HCC patients,39 as it was involved in CD8+ T cell infiltration and was positively correlated with CCL5 expression. Similarly, in this study, we found that the levels of CCL5 were monotonically correlated with T lymphocyte-specific genes (CD4/CD8), which may suggest that peripheral blood lymphocytes are the main source of serum CCL5 and that serum CCL5 levels can partially reflect the macroscopic environment of immune function status.
Our study still has some limitations. First, we analyzed only preoperative peripheral blood lymphocyte subsets in this study. Although our aim was to explore whether the state of the systemic immune macroenvironment affects the occurrence of EHM in HCC patients, a longitudinal study that includes the dynamics of peripheral blood lymphocyte subsets could more accurately reflect the potential correlation. Second, this study is a single-center retrospective study with a small sample size, especially with a relatively small validation group and limited patient clinical information. In addition, we determined the correlation between CCL5 and EHM through bioinformatics techniques using publicly available databases. Therefore, our results may not be generalizable to all clinical situations. Third, using public databases like TCGA & GEO for clinical research also has limitations. For instance, data in public databases often comes from various studies and laboratories, leading to potential differences in data quality. Additionally, the samples in public databases may not represent the diversity and breadth of the entire population. Finally, we did not include some emerging immune cell markers such as PDCD1, TOX, CTLA4, LAYN, GZMK, and CCR7 in our study since the functional definition of peripheral lymphocyte subsets is not well-established. Moreover, we have not adequately discussed the distribution and role of immune cell subsets in the tumor microenvironment.
Conclusions
In summary, we found that specific immune cells (CD4+ and CD8+ T cells) in the peripheral blood can predict the risk of HCC spreading outside the liver. We also observed a connection between immune function and cancer spread, mediated by serum CCL5. Higher levels of CCL5 were associated with better patient outcomes, likely due to its role in promoting immune cells to infiltrate the tumor tissue and prevent metastasis. These findings have important implications for assessing the risk of cancer extrahepatic spread in HCC patients.
Abbreviations
HCC, hepatocellular carcinoma; EHM, extrahepatic metastasis; MVI, Microvascular invasion; MFS, metastasis-free survival; DFS, disease-free survival; OS, overall survival; PBMCs, peripheral blood mononuclear cells; Tregs, regulatory T cells; BCLC, Barcelona Clinic Liver Cancer; EDTA, Ethylene Diamine Tetraacetic Acid; HBV, hepatitis B virus; HCV, hepatitis C virus; CTCs, circulating tumor cells; AFP, alpha fetoprotein; IL, interleukin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; WBC, white blood cell; PLT, platelet; ALB, albumin; HR, hazard ratio; TME, tumor microenvironment.
Ethics Approval Statement
This study was reviewed by the Ethics Committee of Fifth Medical Center of Chinese PLA General Hospital. Since this study was a retrospective study, the patient data used was kept confidential throughout, and it would not cause any harm to patients, so the informed consent was waived.
Funding
National Scientific Foundation Committee of China (Grant Nos.82102043, 92159305, 82030047 and 81971625).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–261. doi:10.1016/j.jhep.2019.08.025
2. European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
3. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
4. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
5. Yu J, Cheng ZG, Han ZY, et al. Period-dependent survival benefit of percutaneous microwave ablation for hepatocellular carcinoma: a 12-year real-world, multicentric experience. Liver Cancer. 2022;11(4):341–353. doi:10.1159/000522134
6. Sun J, Guo R, Bi X, et al. Guidelines for diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus in China (2021 Edition). Liver Cancer. 2022;11(4):315–328. doi:10.1159/000523997
7. Ganesh K, Massague J. Targeting metastatic cancer. Nat Med. 2021;27(1):34–44. doi:10.1038/s41591-020-01195-4
8. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi:10.1126/science.1203543
9. Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. doi:10.1148/radiology.216.3.r00se24698
10. Cheng L, Du X, Wang Z, et al. Hyper-IL-15 suppresses metastatic and autochthonous liver cancer by promoting tumour-specific CD8+ T cell responses. J Hepatol. 2014;61(6):1297–1303. doi:10.1016/j.jhep.2014.07.004
11. Mizukoshi E, Yamashita T, Arai K, et al. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65(6):715–725. doi:10.1007/s00262-016-1837-2
12. Bonertz A, Weitz J, Pietsch DH, et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119(11):3311–3321. doi:10.1172/JCI39608
13. Zeng X, Liao G, Li S, et al. Eliminating METTL1-mediated accumulation of PMN-MDSCs prevents hepatocellular carcinoma recurrence after radiofrequency ablation. Hepatology. 2023;77(4):1122–1138. doi:10.1002/hep.32585
14. Mohs A, Kuttkat N, Reißing J, et al. Functional role of CCL5/RANTES for HCC progression during chronic liver disease. J Hepatol. 2017;66(4):743–753. doi:10.1016/j.jhep.2016.12.011
15. Xu H, Zhao J, Li J, et al. Cancer associated fibroblast-derived CCL5 promotes hepatocellular carcinoma metastasis through activating HIF1alpha/ZEB1 axis. Cell Death Dis. 2022;13(5):478. doi:10.1038/s41419-022-04935-1
16. Sun YF, Wu L, Liu SP, et al. Dissecting spatial heterogeneity and the immune-evasion mechanism of CTCs by single-cell RNA-seq in hepatocellular carcinoma. Nat Commun. 2021;12(1):4091. doi:10.1038/s41467-021-24386-0
17. Yang Z, Feng J, Xiao L, et al. Tumor-derived peptidoglycan recognition protein 2 predicts survival and antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71(5):1626–1642. doi:10.1002/hep.30924
18. Zhao N, Dang H, Ma L, et al. Intratumoral γδ T-cell infiltrates, chemokine (C-C Motif) ligand 4/chemokine (C-C Motif) ligand 5 protein expression and survival in patients with hepatocellular carcinoma. Hepatology. 2021;73(3):1045–1060. doi:10.1002/hep.31412
19. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. doi:10.1158/2159-8290.CD-19-0074
20. Fan F, Dong G, Han C, et al. Peripheral immune factors aiding clinical parameter for better early recurrence prediction of hepatocellular carcinoma after thermal ablation. Int J Hyperthermia. 2023;40(1):2172219. doi:10.1080/02656736.2023.2172219
21. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
22. Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–W514. doi:10.1093/nar/gkaa407
23. Gao Q, Zhou J, Wang XY, et al. Infiltrating memory/senescent T cell ratio predicts extrahepatic metastasis of hepatocellular carcinoma. Ann Surg Oncol. 2012;19(2):455–466. doi:10.1245/s10434-011-1864-3
24. Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85(9):1198–1200. doi:10.1046/j.1365-2168.1998.00846.x
25. Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21(1):3–10. doi:10.1016/j.cytogfr.2009.11.002
26. Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647. doi:10.1038/s41577-018-0044-0
27. Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531(7593):253–257. doi:10.1038/nature16969
28. Kang HJ, Kim H, Lee DH, et al. Gadoxetate-enhanced MRI features of proliferative hepatocellular carcinoma are prognostic after surgery. Radiology. 2021;300(3):572–582. doi:10.1148/radiol.2021204352
29. Zhou L, Ren L, Yu W, et al. Construction and validation of a prediction model of extrahepatic metastasis for hepatocellular carcinoma based on common clinically available data. Front Oncol. 2022;12:961194. doi:10.3389/fonc.2022.961194
30. Lee HL, Jang JW, Lee SW, et al. Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci Rep. 2019;9(1):3260. doi:10.1038/s41598-019-40078-8
31. Wang Z, Yu XL, Zhang J, et al. Huaier granule prevents the recurrence of early-stage hepatocellular carcinoma after thermal ablation: a cohort study. J Ethnopharmacol. 2021;281:114539. doi:10.1016/j.jep.2021.114539
32. Qian DC, Kleber T, Brammer B, et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 2021;22(12):1777–1786. doi:10.1016/S1470-2045(21)00546-5
33. Allen BM, Hiam KJ, Burnett CE, et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat Med. 2020;26(7):1125–1134. doi:10.1038/s41591-020-0892-6
34. Lu MQ, Kong QZ, Lu HD, et al. Effect of Huaier granule on T lymphocyte subsets in patients after breast cancer surgery. Maternal Child Health Care China. 2016;31(20):4125–4127. Chinese.
35. Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006–2016. doi:10.1136/gutjnl-2018-315983
36. Singh SK, Mishra MK, Rivers BM, Gordetsky JB, Bae S, Singh R. Biological and clinical significance of the CCR5/CCL5 axis in hepatocellular carcinoma. Cancers. 2020;12(4):883. doi:10.3390/cancers12040883
37. Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376. doi:10.1155/2014/292376
38. Sadeghi M, Lahdou I, Oweira H, et al. Serum levels of chemokines CCL4 and CCL5 in cirrhotic patients indicate the presence of hepatocellular carcinoma. Br J Cancer. 2015;113(5):756–762. doi:10.1038/bjc.2015.227
39. Wang C, Shi J, Xu J, et al. NLRC3 high expression represents a novel predictor for positive overall survival correlated with CCL5 and CXCL9 in HCC patients. Front Oncol. 2022;12:815326. doi:10.3389/fonc.2022.815326
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

