Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Systemic Infection Predictive Value of Procalcitonin to Lactic Acid Ratio in Diabetes Ketoacidosis Patients
Authors Huang B , Yang S, Ye S
Received 26 April 2022
Accepted for publication 19 July 2022
Published 22 July 2022 Volume 2022:15 Pages 2127—2133
DOI https://doi.org/10.2147/DMSO.S371437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Bin Huang,1,2,* Shengju Yang,1,* Shandong Ye1
1Department of Endocrinology, The First Affiliated Hospital of USTC, Division of Life Science and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China; 2Research Institution of Diabetes, University of Science and Technology of China, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shandong Ye, Department of Endocrinology, The First Affiliated Hospital of USTC, Division of Life Science and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China, Email [email protected]
Introduction: Early detection of bacterial infections associated with adequate antibiotic treatment is key to improving diabetic ketoacidosis (DKA) outcomes. Our study aimed to investigate the different sepsis markers (including procalcitonin to lactic acid ratio, PLR) to diagnose bacterial infection in patients with DKA within one hour after admission.
Methods: A total of 165 patients diagnosed with DKA were enrolled between July 2014 and July 2018 and divided into an infection group (N =62) and a non-infection group (N=103) based on the positive aetiological tests such as blood culture, sputum culture, urine culture, or definite focus of pulmonary, soft tissue, kidney, etc.
Results: Our findings suggest the following: 1) leucocytes (threshold above 10× 109 /L) and PLR (threshold above 0.438) within one hour after admission can help to identify patients with infection in the context of DKA. 2) A subgroup analysis demonstrated that PLR also has a high diagnostic efficacy for infection in patients with DKA, regardless of the type of diabetes.
Conclusion: This study concludes that leucocyte count (threshold > 10× 109/L) and PLR (threshold above 0.438) show a diagnostic value to help distinguish DKA patients with infection. By combining these two markers, the reduction of antibiotic misuse may be possible.
Keywords: diabetic ketoacidosis, infections, procalcitonin to lactic acid ratio, diagnostic value
Introduction
Diabetic ketoacidosis (DKA) accounts for 4–9% of all hospital discharge summaries in patients with diabetes.1 Despite a 50% drop in mortality since 1980 due to standardised protocols, recent studies still report a mortality rate of approximately 2%–5%.2 Infections and discontinuation of insulin therapy are the most frequent triggering factors. Bacterial infections, most commonly urinary tract infections, followed by pneumonia, account for approximately 50% of ketoacidosis cases.3 In DKA, bacterial infections are reported to increase both the mortality rate and duration of hospital stay.4 Early detection of bacterial infections combined with adequate antibiotic treatment is the key to improving patient outcomes.
Typically, the diagnosis of infection depends primarily on clinical symptoms and culture of secretions. The early sites of infection are usually concealed, and microbiological culture reports take 24–48 hours.5 In addition, patients with DKA can have fever, elevated procalcitonin (PCT) and C-reactive protein (CRP) levels, leukocyte count, and percentage of neutrophils (N%), regardless of infection, making predictions of early infection biased.6 Many patients with clinical suspicion of infection are overtreated with antibiotics, leading to bacteriological resistance. Therefore, there is a clinical need to find a rapid and effective index for the early diagnosis of infections to guide physicians in the appropriate use of antibiotics.
Serum PCT is a precursor of calcitonin without hormonal activity and has been recommended for the diagnosis and treatment of bacterial infections. Nevertheless, some data suggest that stress conditions such as trauma, surgery, shock, and severe hyperglycaemia can elevate levels of PCT, CRP, and white blood cells even in the absence of infection; therefore, caution is advised when using these indicators to diagnose infection.7 DKA is a critical acute complication that causes severe dehydration, resulting in elevated serum lactic acid (Lac) levels; increased Lac levels often indicate hypovolaemia and disorders of microcirculation.8 The potential significance of the PCT to Lac ratio (PLR) may be the relative PCT concentration corrected for hypovolaemia and the stress induced by DKA itself. We conducted a retrospective study in which we attempted to investigate the different sepsis markers (including PLR) to diagnose bacterial infection in patients with DKA within one hour of admission.
Materials and Methods
Participants
A total of 165 patients diagnosed with DKA were enrolled in this study from the Department of Endocrinology of the First Affiliated Hospital (Anhui Provincial Hospital) of the University of Science and Technology of China (USTC) between July 2014 and July 2018. The study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the Research Ethics Committee, The First Affiliated Hospital of USTC. It was agreed that the requirement for informed consent be waived because this study was designed to collect available data from participants’ medical records retrospectively. DKA was defined as a glucose concentration >11.1 mmol/L, a pH <7.35, or HCO3- <15 mmol/L, and the presence of ketones (either in the blood or urine).9 The diagnosis of infection was based on positive aetiological tests such as blood culture, sputum culture, urine culture, or definite focus of pulmonary, soft tissue, kidney, etc., by computed tomography or ultrasound. Patients were divided into an infection group (N = 62) and a non-infection group (N=103). The exclusion criteria were as follows: 1) complication with rheumatism, haematological system diseases, and mental illness; 2) stress stimulation such as operation and trauma in the past 3 months; 3) severe damage to the liver (defined as total bilirubin >2 times the upper limit of normal and aspartate transaminase/alanine transaminase (AST/ALT) >3 times the upper limit of normal) and kidney function (defined as estimated glomerular filtration rate (eGFR) ≤30 mL/min/1.73 m2); 4) use of antibiotics in the past two weeks; 5) one or more criteria known to increase PCT without any indication of bacterial infection, such as small cell lung cancer, cardiac arrest, heat stroke, medullary thyroid carcinoma, or severe trauma; and 6) inability to communicate using standard methods.
Methods
Data regarding the duration and type of diabetes, along with age, sex, and BMI (body mass index), were obtained from the participants’ medical records. All patients were tested for emergency laboratory data, which included routine blood work (leucocytes, N%, CRP), bacteriology (blood, urine, sputum, wound exudate, liver function (ALT, AST, total bilirubin), renal function (serum creatinine, blood urea nitrogen), inflammatory index (PCT), and a coagulation marker (D-dimer) within 1 hour of admission, and blood gas analysis was performed using a pH blood gas analyser (ABL 50; Radiometer, Copenhagen, Denmark). These results provide both a definite DKA diagnosis and data on lactic acid levels. The PLR was defined as serum PCT divided by lactic acid. The eGFR was calculated as follows: 194 × Cr−1.094 × Age−0.287 (× 0.739 for female patients). Two experienced clinicians reviewed and summarised the data.
Statistical Analysis
We compared the differences in general data, clinical symptoms, and laboratory measures among patients with DKA. Continuous measurements were presented as means (SD) if they were normally distributed or medians [interquartile range (IQR)] if not normally distributed, and categorical variables were described as frequency rates and percentages (%). IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA) was used. Independent tests including the t-test, chi-square test, or Mann–Whitney U-test, were used to compare the two patient groups. Logistic regression analysis was used to calculate the odds ratio (OR) and 95% confidence interval (CI) for the predictive value of DKA infection, and this was determined after adjusting for potential confounding variables. In order to evaluate the efficacy of each index on infection in this category of patients, a receiver operating curve (ROC) was drawn and the area under the curve (AUC) was calculated. Statistical significance was set at p <0.05.
Results
Demographic and Metabolism Characterisation of Study Participants
Data from 165 patients diagnosed with DKA (52.12% male and 28.48% T1DM) were evaluated. The mean age of the population was 45.37±17.48 years. Participant age ranged from 18 to 87 years, and the duration of diabetes in the patients ranged from 0–40 years. The duration of diabetes, levels of leukocytes, N%, CRP, PCT, Lac, PLR, serum creatinine were significantly higher in the infection group than the non-infection group, and lower eGFR was observed in the infection group than in the non-infection group (both p <0.05). There were no significant differences in sex, age, BMI, blood pressure, proportion of fever, type of diabetes, ALT, AST, TBil, D-D, PH value, or HCO3- between the two groups (all p >0.05) (Table 1).
 |
Table 1 Demographic and Metabolism Characterisation of Study Participants |
Adjusted Odds Ratios for Infection in Patients with Diabetic Ketoacidosis
The multivariate logistic regression model was adjusted for all variables showing significant associations in the univariate analysis. The estimated OR for patients in the infection group with leukocytes ≥10 × 109/L compared to those with normal leucocytes (<10 × 109/L) was 4.145 (95% CI: 1.153–14.90, P=0.029). The odds of infection with DKA increased by 7.732 with each IQR unit increase of PLR (Table 2).
 |
Table 2 Mutually Adjusted Odds Ratios for Infection in DKA Patients |
Diagnostic Value of PLR and Leucocytes in DKA Infection
To estimate the predictive value of PLR and leukocytes on DKA infection, the ROC curve of PLR and leukocytes on admission was obtained. For PLR and leukocytes, the AUC was 0.907 (95% CI: 0.8472–0.9659) and 0.809 (95% CI: 0.7409–0.8760), respectively. A PLR with an optimal cut-off of 0.438 led to a sensitivity and specificity of 0.86 and 0.907, respectively (Figure 1).
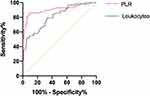 |
Figure 1 Receiver operating characteristics curve of PCT/Lac ratio and WBC. |
Performance of PLR and Leukocytes for the Diagnosis of Infection
The combined diagnostic value of PLR level and leucocytes was further verified. There were 94.12% infection episodes with a PLR and leukocyte count of more than 0.438 and 10×109 /L. The presence of one of these two markers was associated with 51.02% of infection episodes, and 4.88% of infection episodes were associated with a PLR and leukocyte count of less than 0.438 and 10×109 /L, respectively (Figure 2).
 |
Figure 2 PLR and WBC as markers for the diagnosis of DKA infection. |
Diagnostic Value of PLR for DKA Infection in Different Types of Diabetes
A subgroup analysis was performed to estimate the predictive value of PLR for DKA infection in different types of diabetes. For T2DM patients, the AUC was 0.9250 (95% CI: 0.8584–0.9916) and for T1DM patients, the AUC was 0.8662 (95% CI: 0.7449–0.9872), indicating that PLR had a good predictive value (Figure 3).
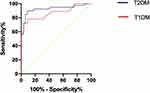 |
Figure 3 Receiver operating characteristics curve of T1DM and T2DM. |
Discussion
The aim of our study was to assess the diagnostic performance of different markers to predict early stages of infection for patients with DKA. Identifying leucocytes (threshold >10× 109 /L) and PLR (threshold >0.438) within one hour of admission can help detect patients with infection in the context of DKA. A subgroup analysis demonstrated that PLR also has a high diagnostic efficacy for infection in patients with DKA, regardless of the type of diabetes.
DKA is a common complication of diabetes and is associated with significant patient morbidity and mortality.10 Despite remarkable progress in hypoglycaemic drugs, monitoring methods, and awareness of this disease, all-cause mortality rates due to DKA, as well as the number of patients who require hospitalisation, have continued to increase in the past 10 years.11 Infection is the most common cause of DKA, and it is directly related to the risk of death. An investigation by 15 hospitals in China revealed that the common causes of DKA were infection (40.1%), discontinuation of hypoglycaemic agents (16.8%), and unknown causes (36.9%).12 Therefore, the early detection of bacterial infections combined with adequate antibiotic treatment is the key to improving patient outcomes. However, DKA can mimic infections; therefore, differentiating between septic and non-septic inflammatory response and/or symptoms may be difficult. Hyperglycemia can stimulates the production and release of cytokines. Plasma CRP, IL-6, IL-1β and TNF-α were elevated without infection in patients with diabetes.13 Previous Studies have shown that CRP is increased in young patients with severe DKA, even in the absence of an infection.14 What is more, unlike patients without DKA, a high frequency of the DKA patients had significant elevations at the time of admission in virtually all cytokines in the study of William H. Hoffman.15 The results of this study found that the levels of N%, leukocytes, and CRP in peripheral blood in DKA with infection were higher than Non-infection patients, which is consistent with those studies above.
PCT is one of the major relevant markers for the diagnosis of bacterial infections because it is related to bacterial load and infection severity.16 However, current studies suggest that PCT may be a secondary inflammatory mediator, which amplifies and aggravates the inflammatory response, but it is not the initiating factor.17 In non-infected patients, PCT levels may be increased due to various stress conditions, such as trauma, stroke, and severe hyperglycaemia.18 Anno et al showed that PCT levels were significantly elevated in patients with DKA even in the absence of infection.19 It has been speculated that metabolic cytokine storms and some signal transductions are associated with such elevations; however, the exact mechanism remains unknown. Combined with the above results, this suggests that the assessment of infection in patients with DKA by PCT alone is of limited value. Our logistic analysis also indicated that PCT was not an independent diagnostic factor of DKA infection. Lac is often used as an indicator of tissue hypoperfusion and hypoxia, which may indicate the severity of the disease, but which it has little to do with the presence or absence of bacterial infection.20 DKA is a critical acute complication that causes severe body fluid loss, resulting in reduced tissue perfusion, thus leading to the formation of Lac.21 In the DKA state, the concentration of Lac can be used for assessing body fluid volume and stress intensity. The study by Wei Xu demonstrated that more total leukocytes were significantly correlated with DKA, and leukocyte counts can add valuable information to indicate the presence of hyperglycaemic crisis and acute infection.22 Combinations of different indicators could be helpful to clinicians in making clinical diagnosis or prediction. The potential significance of the PLR may be the relative PCT concentration corrected for hypovolaemia and the stress induced by DKA itself. This study shows that leucocytes (threshold >10× 109 /L) and PLR (threshold >0.438) may help identify early stages of infection in patients with DKA, and combining these two markers may help with specificity. Although DKA usually occurs in patients with T1DM, patients with T2DM are also susceptible to DKA under stressful conditions such as trauma, surgery, or infections.23 DKA may be the first manifestation of unrecognised T1DM or T2DM. Magliano confirm that patients with diabetes have an increased mortality from a range of infections, compared with the general population, and that the increased risk appears to be greater for type 1 than type 2 diabetes.24 Considering that patients with type 1 diabetes are often induced by autoimmune response, we conducted a subgroup analysis on whether such abnormalities would affect PLR values under infection conditions. The analysis demonstrated that PLR also has a high diagnostic efficacy for infection in patients with DKA, regardless of the type of diabetes, proving the applicability of this novel factor.
This study has several limitations. First, the proven bacterial infection was defined by a positive bacterial culture, which may have induced bias. Second, considering that this study was a retrospective study with a Chinese population, the sample size was relatively small; thus, the results may not be widely applicable. Finally, unmeasured confounding factors might not have been fully addressed.
Conclusions
In conclusion, our study indicates that leucocyte count (threshold >10×109/L) and PLR (threshold >0.438) have diagnostic value in identifying DKA patients with infection. Combining these two markers may help reduce the misuse of antibiotics.
Declarations
The study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the Research Ethics Committee, The First Affiliated Hospital of USTC. It was agreed that the requirement for informed consent be waived because this study was designed to collect available data from participants’ medical records retrospectively.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the national natural science foundation of China (81800713) and local scientific and technological development project guided by central government of China (no. 2017070802D147).
Disclosure
The authors declare that they have no competing interests.
References
1. Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2:95–108. doi:10.2165/00024677-200302020-00003
2. Zaynab A, Hakam H. Precipitating factors, outcomes, and recurrence of diabetic ketoacidosis at a university hospital in Damascus. Avicenna J Med. 2015;5:11–15. doi:10.4103/2231-0770.148503
3. Mahesh MG, Prasad SR, Chandra BS, et al. The study of different clinical pattern of diabetic ketoacidosis and common precipitating events and independent mortality factors. J Clin Diagn Res. 2017;11:OC42–OC46. doi:10.7860/JCDR/2017/25347.9760
4. Liu WY, Lin SG, Wang LR, et al. Platelet-to-lymphocyte ratio: a novel prognostic factor for prediction of 90-day outcomes in critically ill patients with diabetic ketoacidosis. Medicine. 2016;95(4):e2596. doi:10.1097/MD.0000000000002596
5. Mervyn S, Deutschman CS, Warren SC, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. doi:10.1001/jama.2016.0287
6. Yu Y, Li HJ. Diagnostic and prognostic value of procalcitonin for early intracranial infection after craniotomy. Braz J Med Biol Res. 2017;50(5):e6021. doi:10.1590/1414-431x20176021
7. Jian K, Ping G, Xiao-Dong Z, et al. Early differential value of plasma presepsin on infection of trauma patients. Shock. 2019;52:362–369. doi:10.1097/SHK.0000000000001269
8. Prapai D, Karn W, Kevalee U, et al. Isolated methylmalonic acidemia with unusual presentation mimicking diabetic ketoacidosis. J Pediatr Endocrinol Metab. 2016;29:373–378. doi:10.1515/jpem-2015-0228
9. Nathan K, Simona G, Schunk JE, et al. Clinical trial of fluid infusion rates for pediatric diabetic ketoacidosis. N Engl J Med. 2018;378:2275–2287. doi:10.1056/NEJMoa1716816
10. Savage MW, Dhatariya KK, Kilvert A, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabet Med. 2011;28:508–515. doi:10.1111/j.1464-5491.2011.03246.x
11. Cheng YC, Huang CH, Lin WR, et al. Clinical outcomes of septic patients with diabetic ketoacidosis between 2004 and 2013 in a tertiary hospital in Taiwan. J Microbiol Immunol Infect. 2016;49(5):663–671. doi:10.1016/j.jmii.2014.08.018
12. Xu Y, Bai J, Wang G, et al. Clinical profile of diabetic ketoacidosis in tertiary hospitals in China: a multicentre, clinic-based study. Diabet Med. 2016;33(2):261–268. doi:10.1111/dme.12820
13. Gao L, Liu X, Zhang D, et al. Early diagnosis of bacterial infection in patients with septicopyemia by laboratory analysis of PCT, CRP and IL-6. Exp Ther Med. 2017;13(6):3479–3483. doi:10.3892/etm.2017.4417
14. Kyriaki K, Evangelia K, Soultana G, et al. Cytokine response to diabetic ketoacidosis (DKA) in children with type 1 diabetes (T1DM). Endocr J. 2011;58:1045–1053. doi:10.1507/endocrj.EJ11-0024
15. Hoffman WH, Lynne BC, Waller JL, et al. Cytokine response to diabetic ketoacidosis and its treatment. Clin Immunol. 2003;108:175–181. doi:10.1016/S1521-6616(03)00144-X
16. Siyuan Y, Toshimi K, Ryuji U, et al. Diagnostic potential of presepsin in bacterial infection following hepato-biliary-pancreatic surgery: a prospective observational study. J Hepatobiliary Pancreat Sci. 2020;27(10):756–766. doi:10.1002/jhbp.802
17. Maria AA, Marco DR, Piersandro S, et al. A 2020 review on the role of procalcitonin in different clinical settings: an update conducted with the tools of the Evidence Based Laboratory Medicine. Ann Transl Med. 2020;8:610. doi:10.21037/atm-20-1855
18. Xingjie L, Jing Y, Wenjuan H, et al. [Risk factors analysis of acute respiratory distress syndrome in intensive care unit traumatic patients]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:978–982. Chinese. doi:10.3760/cma.j.issn.2095-4352.2018.010.015
19. Anno T, Shigemoto R, Kawasaki F, et al. Marked elevation of plasma procalcitonin levels in patients with diabetic ketoacidosis: a possible useful diagnostic biomarker. Diabetes Metab. 2019;46(6):504–505.
20. Sameh S, Naglaa M, Amr M, et al. Venous glucose, serum lactate and base deficit as biochemical predictors of mortality in patients with polytrauma. Ulus Travma Acil Cerrahi Derg. 2016;22:29–33. doi:10.5505/tjtes.2015.96832
21. Matthew C, Thompson AD, DePiero Andrew D. Is lactic acidosis predictive of outcomes in pediatric diabetic ketoacidosis? Am J Emerg Med. 2020;38:329–332. doi:10.1016/j.ajem.2019.158449
22. Wei X, Hai-feng W, Shao-gang M, et al. Correlation between peripheral white blood cell counts and hyperglycemic emergencies. Int J Med Sci. 2013;10:758–765. doi:10.7150/ijms.6155
23. Kamata Y, Takano K, Kishihara E, et al. Distinct clinical characteristics and therapeutic modalities for diabetic ketoacidosis in type 1 and type 2 diabetes mellitus. J Diabetes Complicat. 2017;31(2):468–472. doi:10.1016/j.jdiacomp.2016.06.023
24. Josephine MD, Harding JL, Kerryn C, et al. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care. 2015;38:1274–1280. doi:10.2337/dc14-2820
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
