Back to Journals » International Journal of General Medicine » Volume 14
Systemic Immune-Inflammation Index for Predicting the Prognosis of Critically Ill Patients with Acute Pancreatitis
Authors Zhang D, Wang T, Dong X, Sun L, Wu Q, Liu J, Sun X
Received 15 April 2021
Accepted for publication 28 June 2021
Published 13 August 2021 Volume 2021:14 Pages 4491—4498
DOI https://doi.org/10.2147/IJGM.S314393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Daguan Zhang,1 Tingting Wang,1 Xiuli Dong,1 Liang Sun,1 Qiaolin Wu,2 Jianpeng Liu,3 Xuecheng Sun1
1Department of Gastroenterology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 2Department of Anesthesiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 3Department of Pathology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China
Correspondence: Xuecheng Sun
Department of Gastroenterology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China
Email [email protected]
Background: Systemic immune-inflammation index (SII) has been identified as a prognostic biomarker in various diseases. However, its significance in acute pancreatitis (AP) has not been reported. Therefore, the main aim of this study was to determine the association of SII with clinical outcomes of AP patients, after adjusting for several confounders.
Methods: This retrospective cohort study was conducted using data retrieved from the Medical Information Mart for Intensive Care III database (MIMIC-III). The study only included patients diagnosed with AP. SII was calculated as the platelet counts x neutrophil counts/lymphocyte counts. Cox regression models were employed to assess the impact of SII on the 30- and 90-day mortality of AP patients. Subgroup analysis was carried out to explore the stability of the relationship between SII and AP mortality.
Results: A total of 513 patients were found to be eligible based on the inclusion and exclusion criteria. For 30-day all-cause mortality, in the model adjusted for multiple confounders, the HR (95% CI) for mid-SII group (SII: 75.6− 104.2) and high-SII groups (SII: > 104.2) were 1.29 (0.65, 2.56) and 2.57 (1.35, 4.88), respectively, compared to the low-SII group (SII: < 75.5). A similar trend was observed for 90-day mortality. Subgroup analyses presented a stable relationship between SII and 30-day all-cause mortality of AP patients.
Conclusion: SII is a potentially useful prognostic biomarker for AP. However, prospective studies are needed to confirm this finding.
Keywords: acute pancreatitis, systemic immune-inflammation index, multiparameter intelligent monitoring in intensive care unit, mortality, prognosis
Introduction
Acute pancreatitis (AP) is a disease defined as acute inflammation of the pancreas and is the most common gastrointestinal disease with a mortality rate ranging between 1% and 1.5%.1 Clinically, AP is categorized as mild acute pancreatitis and severe acute pancreatitis (SAP).2 Given the high mortality rate of SAP, it is important to identify its prognostic indicators to improve identification of high-risk patients and hence initiate timely treatment.3
The Acute Physiology and Chronic Health Evaluation II and Bedside Index of Severity has been previously used to assess the severity and prognosis of AP. However, the score requires collection of several parameters, some of which may not be relevant to AP prognosis. This limits its application in early diagnosis of AP severity and prediction of prognosis. Thus, most AP patients are not diagnosed within the optimal time frame for early diagnosis and treatment. A recent study proposed a new prognosis marker, named systemic immune-inflammation index (SII), which is based on neutrophils, lymphocytes, and platelets.4 Previously, SII was only associated with the prognosis of tumor patients, but it has been recently applied in inflammation-linked diseases such as chronic obstructive pulmonary disease5 and antineutrophil cytoplasmic autoantibodies associated vasculitis.6 Multiple types of immune cells such as lymphocytes and neutrophil cells are involved in inflammatory responses.7 Several epidemiological studies have reported that SII is a stronger prognostic index than systemic inflammation response biomarkers such as neutrophil-lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) in some diseases.8,9 Moreover, the NLR, PLR, and MLR indexes only integrate two cell types. However, there are no reports on whether SII can predict the clinical prognosis of AP.
Therefore, this study aimed at investigating the relationship between SII and the outcomes of AP using data retrieved from the Medical Information Mart for Intensive Care III (MIMIC-III) database, after adjusting for potential confounders.
Methods
Data Source
We collected data from the MIMIC-III database, a publicly available critical care database developed by the Massachusetts Institute of Technology.10 The database contains 53,423 patients admitted at Beth Israel Deaconess Medical Center between 2001 and 2012. This database includes high resolution hourly vital signs and waveforms from bedside monitors. It also contains laboratory results, prescriptions, procedure, fluid balance, and free-text interpretations of imaging results. Application of the data retrieved from the database was approved by Massachusetts Institute of Technology and the Institutional Review Boards. All data accessed complies with relevant data protection and privacy regulations.
Population Selection Criteria
The diagnosis of AP was based on ICD-9 codes (International Classification of Diseases, ninth revision). We only included data from the first intensive care unit (ICU) admission of each patient aged >18 years. The following exclusion criteria was used; 1) stayed in ICU < 2 days, and 2) missing key data.
Data Extraction and Outcomes
Demographic information of patients including age, gender, race, body mass index (BMI), vital signs, laboratory characteristics, comorbidities, and scoring systems were obtained. Vital signs included heart rate, oxygen saturation (SPO2), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Comorbidities included acute kidney injury (AKI), chronic renal disease, chronic liver disease, coronary heart disease (CHD), chronic heart failure (CHF), sepsis, diabetes mellitus (DM), pneumonia, hypertension, alcohol abuse, and depression. Moreover, laboratory characteristics included neutrophil counts, lymphocyte counts, hemoglobin, hematocrit, platelet counts, white blood cell (WBC) counts, albumin, and prothrombin time (PT) over the first 24 h in the ICU. The Simplified Acute Physiology Score II (SAPS II) and Sequential Organ Failure Assessment (SOFA) score were also included.
Lymphocytes, neutrophils and platelet counts were presented as ×109 cells/mm3. SII was calculated as platelet counts * neutrophil counts/lymphocyte counts. The patients were divided into three groups according to the SII score. Primary outcomes were 30- and 90-day mortality. Furthermore, patient mortality information was collected from the social security database.
Statistical Analysis
Normally distributed continuous variables are presented as the mean ± standard deviation (SD) and non-normally distributed continuous variables are shown as the median (interquartile range). Categorical variables are reported as frequencies. Data from independent samples were compared with t-test, whereas count data were compared using the χ2 test or Fisher’s exact tests. The information was graded using Wilcoxon W-test or Kruskal Wallis test. Moreover, Cox proportional hazard regressions were carried out to estimate the association between SII and 30-day and 90-day mortality, and results were presented as hazard ratios (HRs) and 95% confidence interval (CIs). Covariates in model 1 were adjusted for age, sex, and race, while covariates in model 2 were adjusted for age, sex, race, heart rate, sepsis, chronic liver disease, CHF, AKI, CHD, DM, SAPS II score, SOFA score, and alcohol abuse. These covariates were selected according to their associations with the outcomes of interest or a change in effect estimate of more than 10%.11 Stratification analyses were used to examine the effect of SII on different subgroups using different parameters and comorbidities. A two-sided p < 0.05 was considered statistically significant and R software (version 3.6.4) (http://www.R-project.org) was used to carry out all statistical analyses.
Result
Subject Characteristics
In total, 513 patients were found to be eligible according to the inclusion and exclusion criteria. Patients were then divided into three groups, with equal number of cases, based on the SII score; low-SII group, mid-SII group, and high-SII group (SII: <75.5; 75.6 −104.2; and >104.2, respectively). Table 1 summarizes the characteristics of eligible participants. Results indicated that patients in the high-SII group had higher SOFA and SAPS III scores (p < 0.001 for all). They also had lower PT and chronic liver disease. On the other hand, patients with mid-SII scores were older, and had higher hemoglobin and hematocrit levels. Moreover, patients in the high-SII group had higher mean platelet count, neutrophils, and WBC count (p < 0.001 for all).
 |
Table 1 Baseline Characteristics of the Study Population |
Association Between SII and 30- and 90-Day Mortality
Deemed the low-SII group as the reference group, a high-SII was associated with increased mortality in ICU AP patients (Table 2). With regard to 30-day all-cause mortality, unadjusted model results showed that patients in the high-SII group had higher risk of death (HR = 2.78 95% CI, 1.51, 5.12) compared to those in the reference group. After adjusting for age, sex, and race (model 1), the HR (95% CI) for the mid-SII group and high-SII group were 1.27 (0.65, 2.49) and 2.78 (1.49, 5.19), respectively, compared to the reference group. After further adjusting for multiple confounders (model 2), the HR (95% CI) for the mid-SII group and high-SII group were 1.29 (0.65, 2.56) and 2.57 (1.35, 4.88), respectively, compared to the reference group.
 |
Table 2 HRs for All-Cause Mortality Across Groups of SII |
A similar trend was observed for 90-day mortality. The results of Model 1 indicated that the HR (95% CIs) for the mid-SII group and high-SII group were 1.19 (0.66, 2.15) and 3.44 (1.99, 5.94), respectively, compared to the reference (p trend 0.0019). Moreover, the results of model 2 showed that the HR (95% CIs) for the mid-SII group and high-SII group were 1.24 (0.68, 2.25) and 3.19 (1.82, 5.61), respectively, compared to the reference (p trend <0.0001).
In the subgroup analysis, we did not find differences in the relationship between SII and 30-day mortality across subgroups (Table 3).
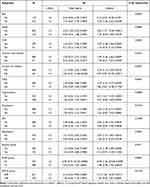 |
Table 3 Subgroup Analysis of the Associations Between 30-Day All-Cause Mortality and the SII |
Discussion
AP is a common disease which begins abruptly, progresses rapidly, and has high mortality and morbidity. Therefore, the condition and prognosis of AP patients should be timely and accurately monitored. There are several methods used to evaluate its prognosis. However, none of these methods is sensitive or specific enough. Recently, various inflammatory markers have been used to predict the prognosis of AP because inflammatory mediators play a critical role in the occurrence of AP. One of the new inflammatory markers is SII, which reflects the immune status. A previous study reported that SII level can reveal inflammation status and is a reproducible biomarker of systemic inflammatory processes.12
This study has shown that SII is significantly associated with mortality of AP after adjusting for multiple variables in various models. Although SII was associated with high mortality, other factors might also affect the result such as age, sex, and diseases. Thus, subgroup analysis was conducted based on age, gender, race, comorbidities, and other factors. Results indicated that there are no statistically significant differences across subgroups, indicating that our findings are reliable. However, we did not elucidate the exact mechanisms underlying the association between SII and mortality of AP patients.
Our findings are based on the principle that neutrophils and lymphocytes are involved in innate and adaptive immune responses in inflammatory disease such as AP. Notably, AP is a highly inflammatory disease. During systemic inflammation, excessive stimulation of leukocytes can invoke the release of inflammatory factors and trigger systemic inflammatory response syndrome. Inflammatory factors cause pancreatic tissue damage or organ failure by chemoattracting a large number of neutrophils to the pancreas.13–15
Inflammatory response may lead to platelet activation. A previous study reported that activated platelets can produce a large number of cytokines and chemokines, thereby promoting activation of neutrophils.16 Activated neutrophils can produce a large number of oxygen-free radicals and proteolytic enzymes, which may damage endothelial cells.17,18 Damage to vascular endothelial cells causes exposure of type I and type III collagen in the sub-endothelial tissue, which leads to platelet adhesion.19 Consequently, adhered platelets begin to release their contents such as thromboxane A2 (TXA2), 5-hydroxytryptamine (5-HT), and adenosine diphosphate (ADP). These pro-coagulant mediators can cause platelet aggregation which can activate the coagulation cascade, ultimately leading to the inflammatory cascade.20
A previous study reported that P-selectin, intercellular adhesion molecule-1 (ICAM-1), and neutrophil activating peptide-2 (CXCL2) promote the effect of mononuclear cell adhesion to endothelial cells.21–23 Moreover, Cloutier et al reported that 5-HT, secreted by platelets, can increase vascular permeability and promotes leukocytes migration.24 Infiltrated leukocytes further increase secretion of chemokines and cytokines, thereby aggravating the inflammatory response. Studies have shown that neutrophil infiltration is the main cause of acute lung injury and ARDS, liver, and kidney injury in AP patients,25 and the decrease of lymphocyte was also confirmed to be closely associated with severity of AP disease.26
Inflammation response causes endothelial cell injury, ischemia-reperfusion injury, and aggravation of AP. After AP development, the storm of pro-inflammatory cytokines leads to SAP and systemic inflammatory response syndrome (SIRS) or multiple organ failure.
Therefore, we suggest that it is feasible to use SII as a systemic inflammatory ischemic index because it can reflect the disease characteristics of AP more comprehensively.
This study has some advantages. To the best of our knowledge, there is no report on the association between SII with the prognosis of AP. Moreover, this study was based on a large cohort of patients, thereby increasing indicating that our results are reliable. However, there were several limitations. Firstly, inherent biases were inevitable given the retrospective and observational nature of this single-center study. Therefore, further well-designed investigations are required to verify our results. In addition, selection bias and confounding bias were inevitable since we only included ICU patients. Secondly, there were cases of missing data despite neutrophils, lymphocytes, and platelet counts being easily obtained. This increases the complexity of analyzing the results, and introduces bias in the results. Finally, SII was calculated based on the values acquired within 24 hours on patients’ admission. As a dynamic index, SII was directly associated with different treatments or stages. We could not determine its optimal detection time window. Dynamic evaluation of SII may make more sense.
Conclusions
A high SII was associated with increased mortality in ICU AP patients. Thus, SII is a potentially useful prognostic biomarker of AP. However, our findings should be further evaluated using prospective studies with longer follow up.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254–272.e211. doi:10.1053/j.gastro.2018.08.063
2. Goyal H, Guerreso K, Smith B, et al. Severity and outcomes of acute alcoholic pancreatitis in cannabis users. Transl Gastroenterol Hepatol. 2017;2:60. doi:10.21037/tgh.2017.06.03
3. Mutinga M, Rosenbluth A, Tenner SM, Odze RR, Sica GT, Banks PA. Does mortality occur early or late in acute pancreatitis? Int J Pancreatol. 2000;28(2):91–95. doi:10.1385/IJGC:28:2:091
4. Hu B, Yang X-R, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
5. Zuo H, Xie X, Peng J, Wang L, Zhu R. Predictive value of novel inflammation-based biomarkers for pulmonary hypertension in the acute exacerbation of chronic obstructive pulmonary disease. Anal Cell Pathol (Amst). 2019;2019:5189165.
6. Kim Y, Choi H, Jung SM, Song JJ, Park Y-B, Lee S-W. Systemic immune-inflammation index could estimate the cross-sectional high activity and the poor outcomes in immunosuppressive drug-naïve patients with antineutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton). 2019;24(7):711–717. doi:10.1111/nep.13491
7. Pan Q-X, Su Z-J, Zhang J-H, Wang C-R, Ke S-Y. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. Onco Targets Ther. 2015;8:1375–1385. doi:10.2147/OTT.S82437
8. Cem M, Semra P, Tuba KK, Ali Y. Systemic immune-inflammation index predicting survival outcome in patients with classical Hodgkin lymphoma. Biomark Med. 2019;13:1565–1575.
9. Fumihiro S, Hiroaki T, Yuka K, et al. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer (Amsterdam, Netherlands). 2019;136:45–51. doi:10.1016/j.lungcan.2019.08.006
10. Saeed M, Villarroel M, Reisner AT, et al. Multiparameter intelligent monitoring in intensive care II: a public-access intensive care unit database. Crit Care Med. 2011;39(5):952–960. doi:10.1097/CCM.0b013e31820a92c6
11. Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14.
12. Gorgel SN, Akin Y, Koc EM, Kose O, Ozcan S, Yilmaz Y. Retrospective study of systemic immune-inflammation index in muscle invasive bladder cancer: initial results of single centre. Int Urol Nephrol. 2019;52:469–473.
13. Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–439. doi:10.1038/nri2779
14. Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602–611. doi:10.1038/ni.2921
15. Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988;3(2–3):105–112.
16. Duchene J, von Hundelshausen P. Platelet-derived chemokines in atherosclerosis. Hamostaseologie. 2015;35(2):137–141. doi:10.5482/HAMO-14-11-0058
17. Khan Z, Shen XZ, Bernstein EA, et al. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood. 2017;130(3):328–339. doi:10.1182/blood-2016-11-752006
18. Hildebrand F, Giannoudis P, Kretteck C, Pape HC. Damage control: extremities. Injury. 2004;35(7):678–689. doi:10.1016/j.injury.2004.03.004
19. Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96(12):1233–1239. doi:10.1161/01.RES.0000171805.24799.fa
20. Thomas MR, Storey RF. Effect of P2Y12 inhibitors on inflammation and immunity. Thromb Haemost. 2015;114(3):490–497. doi:10.1160/TH14-12-1068
21. Sreeramkumar V, Adrover JM, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science (New York, NY). 2014;346(6214):1234–1238. doi:10.1126/science.1256478
22. Hoth JJ, Wells JD, Hiltbold EM, McCall CE, Yoza BK. Mechanism of neutrophil recruitment to the lung after pulmonary contusion. Shock (Augusta, Ga). 2011;35(6):604–609. doi:10.1097/SHK.0b013e3182144a50
23. Halai K, Whiteford J, Ma B, Nourshargh S, Woodfin A. ICAM-2 facilitates luminal interactions between neutrophils and endothelial cells in vivo. J Cell Sci. 2014;127(Pt 3):620–629.
24. Cloutier N, Pare A, Farndale RW, et al. Platelets can enhance vascular permeability. Blood. 2012;120(6):1334–1343. doi:10.1182/blood-2012-02-413047
25. Yu C, Merza M, Luo L, Thorlacius H. Inhibition of Ras signalling reduces neutrophil infiltration and tissue damage in severe acute pancreatitis. Eur J Pharmacol. 2015;746:245–251. doi:10.1016/j.ejphar.2014.11.020
26. Pinhu L, Qin Y, Xiong B, You Y, Li J, Sooranna SR. Overexpression of Fas and FasL is associated with infectious complications and severity of experimental severe acute pancreatitis by promoting apoptosis of lymphocytes. Inflammation. 2014;37(4):1202–1212. doi:10.1007/s10753-014-9847-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
