Back to Journals » Journal of Pain Research » Volume 16
Systemic Effects of Perineural Glucocorticoids on Fasting Serum Glucose, Potassium, and White Blood Cell Count in Total Hip Arthroplasty
Authors Sharma A, Dai F, Tseng L , Effraim PR, Zhou B, Schonberger RB, Li J
Received 4 November 2022
Accepted for publication 15 February 2023
Published 18 February 2023 Volume 2023:16 Pages 553—561
DOI https://doi.org/10.2147/JPR.S395336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Avijit Sharma,1 Feng Dai,2 Lanya Tseng,1 Philip R Effraim,1 Bin Zhou,2 Robert B Schonberger,1 Jinlei Li1
1Department of Anesthesiology, Yale University School of Medicine, New Haven, CT, USA; 2Yale Center for Analytical Sciences, Department of Biostatistics, Yale University School of Public Health, New Haven, CT, USA
Correspondence: Jinlei Li, Department of Anesthesiology, Yale University School of Medicine, 20 York Street, New Haven, CT, 06510, USA, Tel +1 917 601 6828, Fax +1 203 785 6664, Email [email protected]
Purpose: Glucocorticoids are commonly used as regional anesthesia adjuvants to improve blockade quality and duration. There are limited data in the literature regarding the potential systemic effects and safety of perineural glucocorticoids. This study examines the effects of perineural glucocorticoids on serum glucose, potassium, and white blood cell count (WBC) in the immediate postoperative period after primary total hip arthroplasty (THA).
Patients and Methods: A retrospective cohort study was carried out at a tertiary academic medical center utilizing electronic health records of 210 patients who underwent THA, for which patients received either a periarticular local anesthetic injection alone (PAI, N=132) or additional peripheral nerve blocks (PNB, N=78) containing 10 mg dexamethasone and 80 mg methylprednisolone acetate (PAI+PNB). The primary outcome was change in serum glucose from a preoperative baseline on postoperative days (POD) 1, 2, and 3. Secondary outcomes included changes in WBC and serum potassium.
Results: The change in serum glucose from baseline was found to be significantly higher in the PAI+PNB group compared to the PAI group on POD 1 (mean difference 19.87 mg/dL, 95% CI [12.42, 27.32]; P< 0.001) and POD 2 (mean difference 17.5 mg/dL, 95% CI [9.66, 25.44], P< 0.001). No significant difference was found on POD 3 (mean difference − 8.18 mg/dL, 95% CI [− 19.07, 2.70], P=0.14). Statistically significant but clinically insignificant differences were detected in serum potassium in the PAI+PNB group compared to the PAI group on POD1 (mean difference 0.16 mEq/L, 95% CI [0.02, 0.30], P=0.03) and WBC on POD 2 (mean difference 3.18 × 1000/mm3, 95% CI [2.14, 4.22], P< 0.001).
Conclusion: Patients who underwent THA and received PAI+PNB with glucocorticoid adjuvants demonstrated higher elevations in serum glucose for the first two PODs compared to patients who received PAI alone. These differences resolved by a third POD and are likely to be of no clinical significance.
Keywords: perineural glucocorticoid, perineural dexamethasone, perineural methylprednisolone acetate, total Hip arthroplasty, fascia plane block, serum glucose
Introduction
Glucocorticoids have been used as local anesthetic adjuvants to prolong the duration and improve the quality of nerve blockade. Dexamethasone sodium phosphate (DXP), a common hydrophilic adjuvant, has rapid onset and prolongs peripheral nerve blockade for hours, while methylprednisolone acetate (MPA, Depo-Medrol) is suggested to prolong nerve blockade for days.1,2 While DXP and MPA have been used in peripheral nerve blocks (PNB) and epidural anesthesia without severe adverse events documented, there are little published data regarding their possible effects on fasting serum glucose (FSG), serum potassium, and white blood cell (WBC) counts following their use as local anesthetic adjuvants. Aberrancies in these lab values in the postoperative period could lead to further workup and unnecessary treatment potentially causing delays to hospital discharge. The primary hypothesis of this study was that a perineural DXP/MPA combination administered through anterior quadratus lumborum (AQL)/lateral femoral cutaneous nerve (LFCN) blocks in total hip arthroplasty (THA) would be associated with modest changes in serum glucose, potassium, and WBC count in the postoperative period but that these changes would not have much clinical significance. To investigate this, a retrospective study was carried out that utilized electronic health records to investigate two commonly used strategies for pain control for THA patients at the study institution: spinal anesthetics with periarticular local anesthetic injection (PAI) alone or PAI in addition to peripheral nerve blocks (PNB) with glucocorticoid adjuvants.
Materials and Methods
Selection and Description of Participants
With Yale University institutional review board approval and exempt informed consent to this retrospective cohort study, data was collected from the electronic health records of all patients who underwent THA under spinal anesthesia between July 2017 and July 2019 in a single, academic teaching hospital. All data were immediately de-identified once the initial data collection was complete to maintain patient data confidentiality and compliance with the Declaration of Helsinki. Inclusion criteria were patients with hip osteoarthritis who underwent elective, unilateral, primary THA performed under spinal anesthesia intraoperatively with plain 0.5% spinal bupivacaine. Exclusion criteria were revision THA, bilateral THA, history of ipsilateral hip surgery, or intraoperative general anesthesia. Among the 228 consecutive patients undergoing elective unilateral, primary THA due to osteoarthritis, 18 were excluded (16 patients who received general instead of spinal anesthesia and 2 patients with incomplete postoperative records). Patients were grouped based on whether they received a spinal anesthetic with PAI alone (PAI group) or additionally received the AQL/LFCN block with glucocorticoid adjuvants (PAI+PNB group). The final analysis included 210 patients, with 132 in the PAI group and 78 in the PAI+PNB group. If a patient underwent two qualifying THAs in this time frame (N=28), each case was included. See Figure 1 for a consort diagram illustrating the cohort identification process.
 |
Figure 1 Flowchart of screened and excluded patients. Abbreviations: PAI, periarticular injection; PNB, peripheral nerve block. |
Technical Information
Although this was a retrospective study, standard institutional guidance included instructions for all patients to fast after midnight on evening prior to surgery. At the time of this study, preoperative carbohydrate loading was not part of the protocols for orthopedic procedures at the study institute. All blocks in the study cohort were performed under ultrasound guidance in the preoperative area by a regional anesthesia trained anesthesiologist. The practice in the study institution was to use standard American Society of Anesthesiologists (ASA) monitors and minor sedation consisting of intravenous midazolam and fentanyl as needed. LFCN at the study institution is typically performed supine, while AQL is performed in the lateral decubitus position with operative side up. A total of 40 mL 0.2% ropivacaine, 5 mg of DXP, and 40 mg MPA was used for all AQL blocks, and 20 mL 0.2% ropivacaine, 5 mg of DXP, and 40 mg MPA were used for all LFCN blocks included in this retrospective analysis. Included patients received spinal anesthesia with 2.5–3 mL 0.5% isobaric plain bupivacaine in the operating room followed by propofol infusion for sedation and routine postoperative nausea/vomiting prophylaxis including ondansetron 4 mg for one dose intravenously at the end of the procedure. Postoperatively, institutional care protocols prioritized restarting a full diet postoperatively with resumption of home diabetic medications concurrently. All laboratory data presented were taken from morning blood draws scheduled prior to breakfast delivery.
Outcomes
Data on patient characteristics including age, sex, body mass index (BMI), and ASA physical status, and baseline and postoperative values for three biomarkers (FSG, serum potassium, and WBC count) were collected. Surgical characteristics of anesthesia type, length of surgery, and perioperative adverse events and complications including delayed wound healing, wound infection, systemic infection, reoperation, diabetic ketoacidosis, cerebrovascular events, cardiovascular events, pulmonary complications (pneumonia, unplanned intubation), and falls were collected from the electronic health records for all patients for 90 days. The primary outcome was the difference between the two groups (PAI vs PAI+PNB) in changes in serum glucose levels from baseline to postoperative days 1, 2, and 3. Secondary outcomes were differences between the two groups of patients in change in WBC count and serum potassium from baseline on postoperative days 1, 2, and 3.
Statistical Analysis
All descriptive data are presented as number of observations (%) for categorical variables and mean value and standard deviation (SD) for continuous variables. Continuous variables were compared using the two-sample Welch’s t-test with unequal variances. Categorical data between two groups was compared using the Chi-Square test or Fisher’s exact test if the expected number of events was less than five. The mixed effect model for repeated measure was utilized to compare the difference of each outcome (FSG, potassium, and WBC, respectively) between two groups, in which its preoperative baseline value, time (eg, day 1, 2, and 3), group, and time by group interaction were adjusted as covariates, with an unstructured variance-covariance matrix specified to account for within-subject correlation of repeatedly measured values. The least square means of treatment difference and their 95% confidence intervals (CI) are reported. All statistical tests were performed using the statistical software SAS version 9.4 (Cary, NC). A two-sided p-value of less than 0.05 without adjusting for multiple testing was considered to be statistically significant.
Results
Baseline Group Characteristics
The patient demographic data showed no statistically significant differences in age, sex, BMI, or ASA physical status between the PAI+PNB group and the PAI group (Table 1). There were no type I Diabetes Mellitus (DM) patients involved. More patients with Type II DM were found in the PAI group than the PAI + PNB group. The distribution of diabetic status was statistically significant between the two groups (3% for IDDM II and 4% for NIDDM II, respectively, in the PAI+PNB group vs 6% and 13%, respectively, in the PAI alone group, P=0.04). There were no significant differences in the preoperative laboratory values of glucose, potassium, and WBC count between the two groups.
 |
Table 1 Summary of Patient Demographics and Baseline Characteristics |
Fasting Serum Glucose
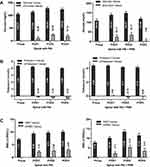
Figure 2 shows the trend of postoperative absolute values of these three biomarkers and their changes from baseline on POD 1, 2, and 3 before covariate adjustment. After adjusting for covariate effects (Table 2 and Figure 3), the mean fasting glucose level of the PAI+PNB group increased by 27.54 mg/dL, 95% CI [21.45, 33.64] from the preoperative baseline value on POD 1, which was significantly higher compared to the increase of 7.67 mg/dL, 95% CI [3.42,11.93], from the baseline in the PAI group. This resulted in a mean difference of 19.87 mg/dL (95% CI [12.42, 27.32], P<0.001) between the groups. The difference was also found to be statistically significant on POD 2 (mean difference 17.5 mg/dL, 95% CI [9.66, 25.44], P<0.001), but was not found on POD 3 (mean difference −8.18 mg/dL, 95% CI [−19.07, 2.70], P=0.140).
 |
Table 2 Comparison of Changes in Glucose, Potassium, and WBC Count from Preoperative Baseline in the PAI and PAI+PNB Groups |
Serum Potassium
For the secondary outcome serum potassium level, the difference in changes from baseline between the PAI+PNB and PAI group on POD 1 was statistically significant but clinically insignificant (mean difference 0.16 mEq/L, 95% CI [0.02, 0.30], P=0.025). No statistically significant difference was found on POD 2 and POD 3 (Figure 3 and Table 2).
White Blood Cell Count
The difference in WBC changes from baseline between the PAI+PNB and PAI group demonstrated a small but statistically significant difference at POD 2 (mean difference 3.18 × 1000/mm3, 95% CI [2.14, 4.22], P<0.001). No statistically significant differences in WBC count were found on either POD 1 (mean difference 0.28 × 1000/mm3, 95% CI [−0.58, 1.13], P=0.53) or POD 3 (mean difference 0.64, 95% CI [−0.42, 1.69], P=0.24) (Figure 3 and Table 2).
Discussion
Perineural glucocorticoid use has gained popularity due to the suggestion that it provides longer duration of analgesic effects of nerve blocks without the adverse effects of systemic glucocorticoid administration.3,4 However, the actual systemic effects of perineural administration of glucocorticoids in real-world practice remain poorly studied and largely unknown.5 This study presents an analysis of the effects of perineural glucocorticoids on serum glucose, potassium, and WBC count, which are subjects of clinical importance that have not been well-described in the literature. Prolonged abnormal values of these parameters can lead to further unnecessary workup, prolonged hospitalizations, and increased medical costs. This analysis finds that among hip arthroplasty patients who receive glucocorticoids through peripheral nerve blocks as compared to similar patients without such treatment, there is in fact a small relative, clinically insignificant, increase from baseline in serum glucose levels during the first two postoperative days. The data suggests that while systemic effects of glucocorticoids within peripheral nerve blocks can be detected, the magnitude and duration of their systemic effects is unlikely to change clinical decision-making regarding their use in nerve blocks.6 In secondary analyses, effects on serum potassium were marginal and seen on POD 1 only, and differences in the degree of WBC count elevation from baseline between the two groups were seen only on POD 2.
These findings also highlight the importance of defining a clinically significant difference when evaluating statistical findings of clinical studies. The concept of minimal clinically important difference (MCID) has drawn significant attention in the medical literature as it is increasingly appreciated that statistical significance alone is not sufficient to inform clinical decision-making. While there are no formal MCID thresholds for perioperative serum glucose elevations, it is generally accepted that with the exception of IDDM I, insulin treatment in the acute perioperative period should be reserved for patients who reach a serum glucose of 180 mg/dL and above, and glucose levels ranging from 140 to 170 mg/dL have been associated with the lowest risk of adverse perioperative outcomes.7,8 The additional increase in serum glucose observed in the PAI+PNB group compared to the PAI alone group was 19.87 mg/dL with 95% CI [12.42, 27.32] on POD 1 and 17.5 mg/dL with 95% CI [9.66, 25.34] on POD 2. A meta-analysis by Toner et al6 found a similar increase of 20.0 mg/dL in perioperative glucose concentration after elective noncardiac surgery during which systemically administered glucocorticoids in doses up to 22 mg of DXP were administered. This meta-analysis of 56 trials, including 5607 patients, determined the increase in glucose by 20.0 mg/dL to be clinically insignificant, and similarly in this study, the relative glucose elevation in the PAI+PNB group is considered clinically insignificant.
Literature regarding glucocorticoid effects in hip arthroplasty patients is limited. Intravenous glucocorticoids have been shown to be associated with transient serum glucose elevation for up to 6 hours in total knee or total hip replacement,9,10 but epidurally administered MPA has not been associated with significant glycemic effects even in diabetic patients.11 Similarly, prior studies comparing perineural and intravenous DXP did not reveal significant differences in glycemic control.12,13 This study used doses of glucocorticoids commonly used in previous studies including DXP 5 mg in the peripheral nerve blocks14 and MPA 40 mg in epidural injection, as previous studies suggest these doses do not raise serum glucose levels to problematic levels.15
In addition, what exactly constitutes a problematic elevation of serum glucose is controversial. Hyperglycemia has been implicated as a contributor to surgical site infection (SSI), and some studies have identified glucocorticoid administration as an independent risk factor for SSI following surgery.14,16 However, a recent meta-analysis suggests that perioperative glucocorticoids are not associated with wound infection or poor wound healing at doses equivalent to 2 to 100 mg of DXP.6 A study on a cohort of 314 diabetic and nondiabetic total knee and hip arthroplasty patients showed that on the first night after surgery, up to 28.7% patients had a serum glucose of 180 mg/dL or higher, and on average serum glucose changed from 105.7 mg/dL (SD 2.1) preoperatively to 142.9 mg/dL (SD 4.3) in the evening following the surgery.17 The Centers for Disease Control (CDC) recommends that perioperative blood glucose levels should be kept <200 mg/dL in patients with and without diabetes to prevent surgical site infections. This level is much higher than the peak fasting serum glucose level of around 149 mg/dL seen in this study.16,18
Regarding the secondary endpoints, it is well described that hyperglycemia may be accompanied by changes in concentrations of various electrolytes.19–23 In this study, there was a small but statistically significant increase in potassium level on POD 1 in the PAI+PNB group, but the absolute values were well within normal physiological limits of 3.5–5.1 mEq/L (Figure 2).
There are limited studies on either the effects of glucocorticoids or the effects of total joint arthroplasty itself on WBC count.24 A study of 14,277 primary unilateral and bilateral total hip arthroplasty and total knee arthroplasty cases found postoperative leukocytosis in 38% of patients.25 The average increase in postoperative WBC count was approximately 3 × 1000/mm3 during the first 2 postoperative days, which is consistent with the findings of this study (Table 2 and Figure 3).25 It is notable in this study that there was an increase in WBC count from baseline in the PAI+PNB group of 5.31 × 1000/mm3 on POD 2 (Table 2 and Figure 3) with a peak absolute WBC count of around 13 × 1000/mm3 (Figure 2). This moderate WBC elevation is worthy of further study, as even though elevations of this magnitude are non-specific, they could raise concerns about immediate peri-prosthetic infections.25 It should be noted that the modest leukocytosis associated with glucocorticoids, as seen in this study, typically does not present challenges in early identification of infection, as glucocorticoid-induced leukocytosis is not associated with left shift, a significant increase in band neutrophils, or fever, unlike an active infection.26 In the event of active infection with concurrent glucocorticoids usage, a further increase in WBC count would likely be observed.27 The potential risks and benefits of the administration of an immunosuppressive drug like glucocorticoids to patients undergoing total joint replacement surgery is a matter of ongoing debate, and the findings presented in this study, though suggestive of the safety of perineural glucocorticoid usage, cannot definitively rule out the potential for adverse immunosuppressive effects with accompanying increased risk of infection.
Limitations of this study include the retrospective nature of the study, with results subject to various biases including selection bias. The study is also from a single center and includes moderate sample size, raising questions about generalizability and reproducibility. Although ASA Physical Status was similar between the two groups, there was a notable difference in diabetic status between groups, with 6% vs 3% having IDDM II and 13% vs 4% having NIDDM II in the PAI alone group vs PAI+PNB group, respectively. This difference is possibly reflective of clinicians’ hesitancy to administer glucocorticoids to patients with preexisting glycemic control issues. While it cannot be certain how this observed imbalance in groups may have biased the results, it is reassuring to note that a previous study by Even et al15 showed the extent of serum glucose elevation through epidurally injected glucocorticoids was not related to pre-procedural diabetic control as indicated by hemoglobin A1c levels. In addition, some data points for this study were missing for various reasons, such as patient discharges prior to all data points being collected. However, these incomplete sets of lab values were accounted for in the adjusted analyses when determining changes in glucose, potassium, and WBC count (see Table 2 and Figure 3), and the analyses showed primary and secondary outcomes similar to those calculated with the unadjusted data (see Figure 2). A final limitation of the analysis is that the adjustment for multiple testing was not prespecified, so the findings should be interpreted cautiously as hypothesis-generating rather than as definitive evidence of the effects of perineural glucocorticoid adjuvants in hip arthroplasty patients. As is the case with many retrospective observational findings, a prospectively randomized controlled trial would be needed to delineate the existence and extent of any systemic effects of perineural glucocorticoids on serum glucose, potassium, and WBC count.
Conclusion
This study compared changes in fasting serum glucose, as well as potassium levels and WBC count, between THA patients who either received or did not receive motor-sparing fascial plane peripheral nerve blocks containing perineural glucocorticoids. Our findings demonstrate that transient changes in these parameters were unlikely to be of clinical significance in this population.
Acknowledgments
- The authors would like to thank the entire team of total joint arthroplasty surgeons at Yale University, especially Drs. Lee Rubin, Michael Leslie, Mary O’Connor, Daniel Wiznia, Diren Arsoy, David Gibson, Michael Baumgartner, Joseph Wu, Michael Luchini, and Philip Luchini, who contributed their patients for the study.
- The authors would like to thank Mr. Richard Hintz and Mrs. Soundari Sureshanand at JDAT-Research, YCCI at Yale University School of Medicine for electronic medical record data acquisition.
Funding
This publication was made possible by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Disclosure
Dr. Schonberger reports owning stock in Johnson & Johnson and also reports that Merck, Inc. has provided support to Yale University for a study on which Dr. Schonberger is a co-investigator unrelated to the present work. The authors report no other conflicts of interest in this work.
References
1. Kirkham KR, Jacot-Guillarmod A, Albrecht E. Optimal dose of perineural dexamethasone to prolong analgesia after brachial plexus blockade: a systematic review and meta-analysis. Anesth Analg. 2018;126(1):270–279. doi:10.1213/ANE.0000000000002488
2. An K, Elkassabany NM, Liu J. Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in a mouse sciatic nerve block model. PLoS One. 2015;10(4):e0123459. doi:10.1371/journal.pone.0123459
3. Baeriswyl M, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Efficacy of perineural vs systemic dexamethasone to prolong analgesia after peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2017;119(2):183–191. doi:10.1093/bja/aex191
4. Zorrilla-Vaca A, Li J. Dexamethasone injected perineurally is more effective than administered intravenously for peripheral nerve blocks: a meta-analysis of randomized controlled trials. Clin J Pain. 2018;34(3):276–284. doi:10.1097/AJP.0000000000000519
5. Hewson D, Bedforth N, McCartney C, Hardman J. Dexamethasone and peripheral nerve blocks: back to basic (science). Br J Anaesth. 2019;122(4):411–412. doi:10.1016/j.bja.2019.02.004
6. Toner AJ, Ganeshanathan V, Chan MT, Ho KM, Corcoran TB. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2017;126(2):234–248. doi:10.1097/ALN.0000000000001466
7. Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: an update. Anesthesiology. 2017;126(3):547–560. doi:10.1097/ALN.0000000000001515
8. Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010;112(4):860–871. doi:10.1097/ALN.0b013e3181d3d4b4
9. Lunn TH, Kristensen BB, Andersen L, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth. 2011;106(2):230–238. doi:10.1093/bja/aeq333
10. Lindberg-Larsen V, Kehlet H, Bagger J, Madsbad S. Preoperative high-dose methylprednisolone and glycemic control early after total hip and knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Anesth Analg. 2018;127(4):906–913. doi:10.1213/ANE.0000000000003591
11. Zufferey P, Bulliard C, Gremion G, Saugy M, So A. Systemic effects of epidural methylprednisolone injection on glucose tolerance in diabetic patients. BMC Res Notes. 2011;4:552. doi:10.1186/1756-0500-4-552
12. Morales-Munoz C, Sanchez-Ramos JL, Diaz-Lara MD, Gonzalez-Gonzalez J, Gallego-Alonso I, Hernandez-Del-Castillo MS. Eficacia analgesica de una dosis unica de dexametasona perineural en el bloqueo ecoguiado del nervio femoral en cirugia de protesis total de rodilla [Analgesic effect of a single-dose of perineural dexamethasone on ultrasound-guided femoral nerve block after total knee replacement]. Rev Esp Anestesiol Reanim. 2017;64(1):19–26. Spanish. doi:10.1016/j.redar.2016.05.006
13. Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgery? Intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine. 2016;95(23):e3828. doi:10.1097/EJA.0b013e328319c09b
14. Nazar CE, Lacassie HJ, Lopez RA, Munoz HR. Dexamethasone for postoperative nausea and vomiting prophylaxis: effect on glycaemia in obese patients with impaired glucose tolerance. Eur J Anaesthesiol. 2009;26(4):318–321. doi:10.1097/EJA.0b013e328319c09b
15. Even JL, Crosby CG, Song Y, McGirt MJ, Devin CJ. Effects of epidural glucocorticoid injections on blood glucose levels in patients with diabetes mellitus. Spine. 2012;37(1):E46–E50. doi:10.1097/BRS.0b013e31821fd21f
16. Wegener JT, Kraal T, Stevens MF, Hollmann MW, Kerkhoffs G, Haverkamp D. Low-dose dexamethasone during arthroplasty: what do we know about the risks? EFORT Open Rev. 2016;1(8):303–309. doi:10.1302/2058-5241.1.000039
17. Varady NH, Schwab PE, Jones T, Collins JE, Fitz W, Chen AF. Optimal timing of glucose measurements after total joint arthroplasty. J Arthroplasty. 2019;34(7S):S152–S158. doi:10.1016/j.arth.2019.01.004
18. Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–791. doi:10.1001/jamasurg.2017.0904
19. Guyton AC, Hall JE. Textbook of Medical Physiology.
20. Pasternak JJ, McGregor DG, Lanier WL. Effect of single-dose dexamethasone on blood glucose concentration in patients undergoing craniotomy. J Neurosurg Anesthesiol. 2004;16(2):122–125. doi:10.1097/00008506-200404000-00003
21. Görges M, Poznikoff AK, West NC, Brodie SM, Brant RF, Whyte SD. Effects of dexmedetomidine on blood glucose and serum potassium levels in children undergoing general anesthesia: a secondary analysis of safety endpoints during a randomized controlled trial. Anesth Analg. 2019;129(4):1093–1099. doi:10.1213/ANE.0000000000004154
22. Park SY, Kim TJ, Kim MJ. Acute hyperkalemia induced by hyperglycemia in non-diabetic patient. Korean J Anesthesiol. 2011;61(2):175–176. doi:10.4097/kjae.2011.61.2.175
23. Goldfarb S, Cox M, Singer I, Goldberg M. Acute hyperkalemia induced by hyperglycemia: hormonal mechanisms. Ann Intern Med. 1976;84(4):426–432. doi:10.7326/0003-4819-84-4-426
24. Peng CT, Lin HC, Lin YJ, Tsai CH, Yeh TF. Early dexamethasone therapy and blood cell count in preterm infants. Pediatrics. 1999;104(3 Pt 1):476–481. doi:10.1542/peds.104.3.476
25. Deirmengian GK, Zmistowski B, Jacovides C, O’Neil J, Parvizi J. Leukocytosis is common after total Hip and knee arthroplasty. Clin Orthop Relat Res. 2011;469(11):3031–3036. doi:10.1007/s11999-011-1887-x
26. Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773–778. doi:10.1016/0002-9343(81)90363-6
27. Frenkel A, Kachko E, Cohen K, Novack V, Maimon N. Estimations of a degree of glucocorticoid induced leukocytosis in patients with acute infections. Am J Emerg Med. 2018;36(5):749–753. doi:10.1016/j.ajem.2017.10.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.