Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Systematic Review of Physical Activity, Sedentary Behaviour and Sleep Among Adults Living with Chronic Respiratory Disease in Low- and Middle-Income Countries
Authors Jayamaha AR , Jones AV , Katagira W , Girase B , Yusuf ZK , Pina I, Wilde LJ, Akylbekov A, Divall P, Singh SJ , Orme MW
Received 19 October 2021
Accepted for publication 17 January 2022
Published 18 April 2022 Volume 2022:17 Pages 821—854
DOI https://doi.org/10.2147/COPD.S345034
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Akila R Jayamaha,1,2 Amy V Jones,1,3 Winceslaus Katagira,4 Bhushan Girase,5 Zainab K Yusuf,1,3 Ilaria Pina,1,3 Laura J Wilde,1,3 Azamat Akylbekov,6 Pip Divall,7 Sally J Singh,1,3 Mark W Orme1,3
1Department of Respiratory Sciences, University of Leicester, Leicester, UK; 2Department of Health Sciences, KIU, Battaramulla, Sri Lanka; 3Centre for Exercise and Rehabilitation Science, NIHR Leicester Biomedical Research Centre-Respiratory, Leicester, UK; 4Makerere University Lung Institute, Makerere University College of Health Sciences, Mulago Hospital, Kampala, Uganda; 5Family Health, PATH, Delhi, India; 6National Centre for Cardiology and Internal Medicine, Bishkek, Kyrgyzstan; 7University Hospitals of Leicester NHS Trust, Leicester, UK
Correspondence: Akila R Jayamaha, Tel +94759359834, Email [email protected]
Abstract: Physical activity (PA), sedentary behaviour (SB) and sleep are important lifestyle behaviours associated with chronic respiratory disease (CRD) morbidity and mortality. These behaviours need to be understood in low- and middle-income countries (LMIC) to develop appropriate interventions.
Purpose: Where and how have free-living PA, SB and sleep data been collected for adults living with CRD in LMIC? What are the free-living PA, SB and sleep levels of adults living with CRD?
Patients and Methods: The literature on free-living PA, SB and sleep of people living with CRD in LMIC was systematically reviewed in five relevant scientific databases. The review included empirical studies conducted in LMIC, reported in any language. Reviewers screened the articles and extracted data on prevalence, levels and measurement approach of PA, SB and sleep using a standardised form. Quality of reporting was assessed using bespoke criteria.
Results: Of 89 articles, most were conducted in Brazil (n=43). PA was the commonest behaviour measured (n=66). Questionnaires (n=52) were more commonly used to measure physical behaviours than device-based (n=37) methods. International Physical Activity Questionnaire was the commonest for measuring PA/SB (n=11). For sleep, most studies used Pittsburgh Sleep Quality Index (n=18). The most common ways of reporting were steps per day (n=21), energy expenditure (n=21), sedentary time (n=16), standing time (n=13), sitting time (n=11), lying time (n=10) and overall sleep quality (n=32). Studies revealed low PA levels [steps per day (range 2669– 7490steps/day)], sedentary lifestyles [sitting time (range 283– 418min/day); standing time (range 139– 270min/day); lying time (range 76– 119min/day)] and poor sleep quality (range 33– 100%) among adults with CRD in LMIC.
Conclusion: Data support low PA levels, sedentary lifestyles and poor sleep among people in LMIC living with CRDs. More studies are needed in more diverse populations and would benefit from a harmonised approach to data collection for international comparisons.
Keywords: chronic respiratory disease, low- and middle-income countries, physical activity, sedentary behaviour, sleep
Introduction
Chronic respiratory disease (CRD) (chronic diseases of the airways and the other structures of the lungs) is associated with cigarette smoking, fumes from cooking on open stoves, air pollution,1 and pulmonary tuberculosis,2 and is highly prevalent across low- and middle-income countries (LMIC).3 Physical behaviours include all activities over a 24-h period across the movement spectrum, from no/little movement (sleep, sedentary behaviour) to movement of greater intensities (light and moderate-to-vigorous physical activity), which are important modifiable risk factors for CRD morbidity and mortality. Physical activity (PA) is defined as any bodily movement produced by skeletal muscles that results in energy expenditure. Optimising the 24-hour behavioural profile of people remains a global challenge.4
Physical inactivity is as a key risk factor for non-communicable disease, has been estimated to cause 9% of premature deaths worldwide5 and global trends are not on track to meet the global physical activity target, which is 10% relative reduction in the prevalence of insufficient physical activity by 2025.6,7 People living with CRD are not only less physically active than healthy adults,8 but also those living with other chronic diseases.9 Symptoms of CRD, including breathlessness, deconditioning and declining lung function, contribute to a physically inactive lifestyle; linked to an increased risk of hospitalisation10 and premature death.11 Sedentary behaviour (SB) (any waking behaviour characterized by an energy expenditure ≤1.5 metabolic equivalents, while in a sitting, reclining or lying posture)12 comprises the majority of people’s waking day. People living with CRD spend more time sedentary13 which has been associated with premature mortality.14 Poor sleep quality,15 sleep dissatisfaction,16 and inadequate sleep17 contributes significantly to societies’ health burden.18 Sleep disturbances are a common feature of CRD, leading to more severe respiratory symptoms and putting people at a greater risk of an acute exacerbation.19
Whilst distinct behaviours, PA, SB and sleep are intrinsically interrelated components of the 24-hour day, demonstrating the need to examine them in the context of each other rather than in isolation. Recent WHO guidelines for PA and SB20 and Canadian 24-hour movement guidelines21 have started to reflect this notion, but research is almost exclusively from high-income countries (HIC).22 Not least, PA in LMIC is mainly accumulated through transportation and occupation-related activities, contrasting HIC where most PA is undertaken recreationally.23 Economical, societal and cultural diversities in LMIC must be considered in the evaluation and monitoring of physical behaviours.24 A range of measurement options are available to quantify free-living PA, SB and sleep.
By compiling the current evidence on free-living PA, SB and sleep of adults living with CRD in LMIC, this review seeks to highlight research priorities, enabling researchers and clinicians to address important lifestyle health challenges in a more time- and cost-effective manner. Our scoping exercise identified no similar published reviews in LMIC. Similar reviews have been conducted in HIC25,26 which have aided comparisons between our findings from LMIC with findings from HIC. This systematic review addressed the following questions: Where have free-living PA, SB and sleep data been collected for adults living with CRD in LMIC? How have the data been collected? What are the free-living PA, SB and sleep levels of adults living with CRD?
Materials and Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) statement.27 The protocol was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020176196).
The search strategy was developed by an experienced clinical librarian [PD] using appropriate subject headings for each database. Search terms included chronic respiratory disease, “physical* activ*”, “sedent*” and sleep*alongside filters on LMIC (a full search strategy is provided in Appendix A). The strategy was adapted for each database.
Relevant articles were identified through systematic searches in five databases (Ovid-MEDLINE, Cochrane library, Ovid-EMBASE, Ovid-EMCARE and CINAHL). Searches for articles published in peer-reviewed journals were from the inception of each database until 30th January 2020. Global Index Medicus was searched, which included African Index Medicus, Index Medicus for Eastern Mediterranean Region, Index Medicus for South-East Asia Region, Latin Am. and Carib. Center on Health Sci Info, and Western Pacific Region Index Medicus. Reference lists of included articles were searched by hand for additional eligible articles. Studies on adults living with CRD in LMIC (population) which related to PA, SB and sleep (intervention), irrespective of the presence or absence of comparator (control group) which report what, where and how PA, SB and sleep data have been collected (outcome) were included in the review. Eligible studies included cross-sectional, longitudinal and case-control studies. Intervention studies were included if baseline data were presented. No restrictions were placed on sample size or language. The full text of the conference abstracts were requested from the corresponding author and if the authors did not respond, conference abstracts were also considered due to the paucity of the empirical evidence in LMICs.
Search results were screened using Rayyan software.28 Two reviewers independently screened for eligibility by title and abstract [MO, AVJ]. Interrater agreement was 99.6%. Discrepancies between the two reviewers were resolved through discussion and with final decision provided by ZY. The full texts of these potentially eligible articles were then retrieved and independently assessed by two reviewers [MO, ZY]. Discrepancies between the two reviewers were resolved through discussion and final decision provided by SS in the event of no consensus. Interrater agreement was 88.1%. Study authors were asked for full text copies if these could not be retrieved.
Data from included studies were extracted using a bespoke Microsoft Excel spreadsheet which included details on study location,29 study design, participant characteristics, free-living 24-hour physical behaviour measurements and methods, income classification,30 climate classification,31 air quality32 (see Appendix B for full data extraction form/table). Authors of included studies were not contacted in the event of missing data. Data extraction was performed by five authors [AA, ARJ, BG, MO, WK], with double-data extraction for each study. Any conflicts in data extraction were resolved through discussion with final decision provided by MO/SS.
The main summary measures were objectively measured overall free-living PA, SB and sleep. Given the nature of the review, no formal risk of bias assessment was conducted. Instead, indicators of quality of reporting and questionnaire/device deployment were examined. Reporting quality of the included studies was conducted by considering whether key information was provided. The presence or absence of information relating to each of the above items was used to describe the quality of reporting. Reporting quality of the included studies was presented through narrative and table summaries. In accordance with our PROSPERO registration, a meta-analysis was not planned due to anticipated heterogeneity in measurement approaches (Table 1).
 |
Table 1 Summary of the Variables Used to Describe Physical Activity, Sedentary Behaviour and Sleep |
Extracted data and summary measures were stratified into subgroups: Region29 (African, Region of the Americas, South-East Asia, European, Eastern Mediterranean, Western Pacific); income classification (Countries with low-income economies (Gross national income per capita $1045 or less in 2020); lower middle-income economies (GNI per capita between $1046 and $4095) and upper middle-income economies (GNI per capita between $4096 and $12,695) constitute low-and middle-income countries (LMIC)),33 Köppen-Geiger climate classification (Equatorial, Arid, Warm temperature, Snow, Polar),31 air quality levels (PM10: above or below 20µg/m3 annual mean and PM2.5: above or below 10µg/m3 annual mean),34 and measurement approach.
Results
Study Selection and Characteristics
Figure 1 shows the selection process according to the PRISMA statement.27 The initial search retrieved 18,121 records, 153 full texts were screened for eligibility and 89 articles (0.6%) met the criteria for inclusion (Appendix C). Of the 89 articles identified, 78 were articles and 11 were conference abstracts. The full text of the selected conference abstracts were requested from the corresponding authors but none responded. Most studies recruited exclusively COPD (65 studies [73%]) and asthma patients (13 studies [15%]). Four studies (4%) recruited a combined sample of COPD and asthma and two studies (2%) recruited bronchiectasis patients. Excluding studies covering all regions, sample sizes totalled 8986 participants. Most studies were cross-sectional in design (65 studies [73%]), comprised male majority samples (55 studies [62%]) and included data collected in warm temperature climates (45 studies [51%]) (Table 2). Fifteen studies reported having a control group and eight studies compare the patients with healthy control (Appendix D).
 |  |  |  |  |  |  |  |  |  |
Table 2 Characteristics of Individual Studies Included in the Review |
 |
Figure 1 PRISMA flow diagram of study selection. |
Income Classification
Of the 89 articles identified, none were conducted in low-income countries, 12 (13%) were conducted in lower-middle-income countries, 71 (80%) were conducted in upper-middle-income countries (UMIC) and six (7%) spanned income classifications.
WHO Regions
The most prevalent region for included studies was the Region of the Americas (45 studies [51%]; 43 in Brazil) followed by European (11 studies [12%], eight in Turkey) and South-East Asian (10 studies [11%], six in India). Three studies were conducted solely in Africa (all in Nigeria).
The Methods of Physical Behaviour Data Collection
PA (66 studies) was the most commonly measured behaviour; measured solely in 47 studies, in combination with SB in 16 studies, with sleep in one study and with SB and sleep in two studies. Sleep (24 studies) was measured exclusively in 21 studies and SB (20 studies) was measured solely in two studies.
Majority of studies assessed physical behaviours using questionnaires only (52 studies [58%]). Fourteen different questionnaires were used across included studies, with an additional 12 studies reporting specific questions. For sleep, the Pittsburgh Sleep Quality Index (PSQI) was the most common questionnaire (18 studies). For PA/SB, the International Physical Activity Questionnaire (IPAQ) was most common (11 studies). Questions or questionnaires were not provided in four studies. Most studies (58%) did not report the recall period of the questionnaires used and only one study (2%) reported how missing data were handled (Appendix E).
Of the 36 studies using device-based measurement of physical behaviours, twenty-six studies (72%) used accelerometers and 10 studies (28%) used pedometers. Dynaport activity monitors were used in 15 studies (58% of accelerometer studies) and the SenseWear Armband in seven studies. Five studies (19%) deployed a Dynaport and SenseWear simultaneously. Four studies (15%) did not provide the model of activity monitor used. Most studies (32, 89%) did not report whether participants had access to feedback on their behaviour during the measurement period. Four studies (11%) did not report the number of days participants were asked to wear the device. Most studies (18, 50%) monitored behaviours for two days and six studies (17%) had a seven-day wear protocol. Fifteen studies (42%) did not report how long each day participants were asked to wear a device. Of the 21 studies reporting this information, the majority (18 studies, 86%) asked for 12 hours of wear and during a fixed time period (19 studies, 90%) to be included in analysis, 14 studies (39%) did not report the minimum number of hours each day and minimum number of days the device needed to be worn. Most studies (35, 97%) did not report average wear-time and 34 studies (94%) did not report if or how periods of non-wear were calculated (Appendix F).
Variables to Assess 24-Hour Physical Behaviours
A summary of the variables used to describe physical behaviours is given in Table 1 (full extraction of the variables in Appendix B). The most common PA categories of variables used were steps per day (n=21), energy expenditure (n=21), binary classification of active/inactive (n=19), time spent walking (n=16) and classification of active/inactive using at least three groups (n=16). For SB, the common categories of variables used were sedentary time (n=16), standing time (n=13), sitting time (n=11) and lying time (n=10). Overall sleep quality (n=32), sleep duration (n=14), use of sleep medication (n=11) and sleep disturbances (n=11) were the most commonly used categories of sleep variables.
Levels of Free-Living PA, SB and Sleep
Time spent walking (12 studies; range 36–91min/day) and movement intensity (eight studies; range 0.18–1.9m/s2) measured by Dynaport activity monitor were most common, followed by the number of steps per day measured by Dynaport (six studies; range 2669–6557steps/day) and Yamax (five studies; range 4227–7490 steps/day) and MVPA measured by SenseWear Armband (five studies; range 29–89min/day). Three SB variables, all measured using Dynaport, were directly comparable between studies; comprising sitting time (12 studies; range 283–418min/day); standing time (12 studies; range 139–270min/day); lying time (11 studies; range 76–119min/day). For sleep, PSQI score was provided in 12 studies (range 4–11) and PSQI classification of poor sleep quality in 11 studies (range 33–100%) (Table 3).
 |  |  |  |  |  |  |
Table 3 Summary of the Studies with Comparable Measurement of Physical Activity, Sedentary Behaviour and Sleep |
Sub-Group Analysis of 24-Hour Physical Behaviours
Four studies measuring PA and two studies measuring SB spanned all regions. The Region of the Americas accounted for the majority of studies measuring PA (45 studies [68%]) (Figure 2A) and SB (19 studies [95%]) (Figure 2B). Studies measuring sleep were distributed across regions in small numbers with South-East Asia as the most common (six studies [25%]) (Figure 2C). One study measuring PA included data from all low- and middle- income classifications. No studies solely took place in low-income countries. Most took place in upper-middle income countries (60 [91%] measured PA, 19 [95%] measured SB and 16 [67%] measured sleep). We were unable to classify climate for seven studies measuring PA, two studies measuring SB and one study measuring sleep. Most studies were conducted in warm temperature climates (41 studies [69%] of PA, 17 studies [94%] of SB and six studies of sleep [26%]). There were 52 studies where we were unable to classify air quality (61% measuring PA, 85% measuring SB and 46% measuring sleep). All other studies exceeded the annual 10µg/m3 PM2.5 threshold. Using the annual 20µg/m3 PM10 threshold, 15 studies (58%) measured PA, one study (33%) measured SB and ten studies (77%) measured sleep in areas exceeding the threshold (Table 4).
 |
Table 4 Summary of the Number of Studies Measuring Each Physical Behaviour, Stratified by Region, Income Classification, Measurement Approach, Climate Classification and Air Quality |
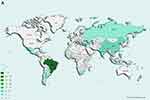 |
Figure 2 (A) Location of studies measuring physical activity. (B) Location of studies measuring sedentary behaviour. (C) Locations of studies measuring sleep. |
 |
Figure 2 Continued. |
 |
Figure 2 Continued |
Discussion
This systematic review provided a comprehensive assessment of the prevalence, levels and measurement approaches of studies assessing 24-hour physical behaviours in people living with CRD in LMIC. The majority of studies examining PA and SB in CRD in LMIC are limited to Brazil (UMIC), whilst studies measuring sleep were more evenly distributed across regions. Questionnaires were more commonly used to measure physical behaviours than device-based methods, with data supporting low physical activity levels, sedentary lifestyles and poor sleep quality for people in LMIC living with CRDs.
Most studies were conducted in upper-middle-income countries with Brazil accounting for almost half. In other regions, Turkey (European), India (South-East Asian) and Nigeria (Africa) had the monopoly of identified articles within their respective regions. No studies were conducted exclusively in low-income countries. Therefore, more studies are needed to compare physical behaviours within and between regions and income classifications. For large cohort studies of general adult populations, such as those included in The Lancet physical inactivity series,5 data were from predominantly HIC in Europe and North America; with data from Africa and Central Asia particularly scarce.35 Efforts to increase the available data on PA, SB and sleep in other LMIC are needed before any reliable prevalence estimates and worthwhile comparisons can be made.
PA was most commonly investigated. There was a relatively equal split of device- and questionnaire-based assessment of PA, with almost exclusively device-based assessment of SB and questionnaire-based assessment of sleep. Compared with a previous systematic review of 76 studies measuring free-living PA in COPD (almost exclusively studies in HIC), the Dynaport activity monitor was more commonly used (42% versus 14%). The disproportionate use of the Dynaport in the included studies from Brazil explains this disparity, whereas the previous review contained a large proportion of studies from HIC. For questionnaire-based PA and SB, we found the IPAQ to be most commonly used; consistent with the evidence base for large epidemiological studies of healthy adult populations.5 IPAQ is commonly used in LMIC for population surveillance, with more than 50 counties adopting it as of 2016,7 but it was developed for individuals aged 15–69 years of age which may not be appropriate for many CRD populations.36 The PSQI was the most common questionnaire in our review with relatively consistent use of the overall PSQI score and classification of poor sleep quality. The PSQI was also more consistently used across regions and currently offers the greatest opportunity for pooled analysis and standardisation. The popularity of the PSQI is supported by a previous review of sleep disorders in COPD.26 Although it is also possible to assess free-living sleep in CRD populations using devices,37,38 the PSQI offers a simple tool to examine sleep quality and duration in LMIC.
Articles included in our review relied predominantly on summary variables of physical behaviours, such as steps per day, sedentary time and overall sleep quality. The high prevalence of summary variables supports previous work exploring device-based PA, which found 70% of studies used a marker for total activity, with half using step count. Postural assessments such as sitting, standing or lying down were more commonly reported in the included studies using more device-based assessments than previously found (44% versus 20%); due to the exclusive measurement of postures in Brazilian studies.
The commonly reported variables of PA were mostly related to walking (time spent walking and step count) which is easily interpretable and at the heart of clinical practice guidelines for COPD.39 It is estimated that 7000–10,000 steps per day is approximately equivalent to taking part in 30 minutes of daily MVPA9 and data from the included articles support people living with CRD in LMIC to be physically inactive. Indicating a global nature of the problem, prevalence of physical inactivity was found more than twice as high in high-income countries compared to low-income countries.6 However, the considerable variation between studies and locations, such as their measurement and data processing protocols, make it difficult to generalise.
Only the range of PA, SB and sleep levels were presented in the study. The reported study variables were not consistent across the included articles which caused considerable heterogeneity, and it may lead to false conclusions. Pooling the data on various study designs increase the methodological heterogeneity. Studies included in our review suggest people living with CRD in LMIC have worse sleep quality than healthy counterparts across income classifications. We found PSQI classification of poor sleep quality ranged 33–100%; higher than the estimated prevalence of 32.8% (95% confidence interval: 25.9–39.7%) based on a meta-analysis of 19 studies of working-age adults in LMIC40 and from adults residing in high-income European countries.41
The strengths of our systematic review include a pre-defined rigorous search methodology, registered on PROSPERO before study screening, bias assessment, including the use of validated search terms, the comprehensive array of databases searched, the absence of language restrictions and screening included reference lists to reduce selection bias. The data extraction phase was also duplicated to minimise assessor bias and error. However, there may be databases unknown to the authors where additional studies may be published in this area. We were unable to translate two non-English publications (Persian). The climate and air quality classifications were not always possible to identify from information reported in the included studies, limiting subgroup analyses.
Conclusion
Based on 89 articles included in our systematic review of the PA, SB and sleep of people living with CRD in LMIC, we showed considerable inequity between behaviours, geographical locations and income classifications. The majority of studies examining PA and SB in CRD in LMIC were limited to Brazil, whilst studies measuring sleep were more limited and spread across regions. Questionnaires were more commonly used to measure physical behaviours than device-based methods, with summary data supporting low physical activity levels, sedentary lifestyles and poor sleep quality for people in LMIC living with CRDs. There was large variation in measurement approaches and poor quality methodological reporting for all physical behaviours which limited direct comparisons. The lack of standardisation and poor reporting reduces the strength of evidence needed to strengthen the scientific integrity of data and resulting clinical and policy implications to support patients to lead healthier lifestyles. More studies of PA, SB and sleep are needed in a broader range of LMICs. Future work would benefit from a harmonised approach to data collection to ensure international comparisons.
Abbreviations
CRD, Chronic respiratory disease; HIC, High-income countries; LMIC, Low- and middle-income; countries; PA, Physical activity; PRISMA, Preferred reporting items for systematic review and meta‐analysis; PROSPERO, International prospective register of systematic reviews; SB, Sedentary behaviour; UMIC, Upper-middle-income countries; WHO, World health organisation.
Acknowledgments
The authors thank the University Hospitals of Leicester NHS Trust Libraries, British Library and authors of included studies for providing full-text articles. The authors send a special thank you to Dr Xiaorong Ding (Institute of Biomedical Engineering, University of Oxford, UK), Carlos Morgado Areia (Nuffield Department of Clinical Neurosciences, University of Oxford, UK), Daniel Filipe Cunha Taveira (NIHR Leicester Biomedical Research Centre-Respiratory, University of Leicester, UK) and Joao Sousa (NIHR Leicester Biomedical Research Centre-Respiratory, University of Leicester, UK) for extracting data from non-English full-text publications.
Funding
This research was funded by the National Institute for Health Research (NIHR) (17/63/20) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20–31. doi:10.1378/chest.08-2114
2. Amaral AFS, Coton S, Kato B, et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J. 2015;46(4):1104–1112. doi:10.1183/13993003.02325-2014
3. Salvi SS, Manap R, Beasley R. Understanding the true burden of COPD: the epidemiological challenges. Prim Care Respir J. 2012;21(3):249–251. doi:10.4104/pcrj.2012.00082
4. Rollo S, Antsygina O, Tremblay MS. The whole day matters: understanding 24-hour movement guideline adherence and relationships with health indicators across the lifespan. J Sport Health Sci. 2020;9:493–510. doi:10.1016/j.jshs.2020.07.004
5. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Impact of physical inactivity on the world’s major non- communicable diseases. Lancet. 2012;380(9838):219–229. doi:10.1016/S0140-6736(12)61031-9
6. World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. World Health Organization; 2013. Available from: https://apps.who.int/iris/bitstream/handle/10665/94384/?sequence=1.
7. Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 2018;6(10):e1077–e1086. doi:10.1016/S2214-109X(18)30357-7
8. Vorrink SNW, Kort HSM, Troosters T, Lammers J-WJ. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12(1):1–8. doi:10.1186/1465-9921-12-33
9. Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8(1):1–19. doi:10.1186/1479-5868-8-1
10. Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129(3):536–544. doi:10.1378/chest.129.3.536
11. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-2521
12. Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):1–17. doi:10.1186/s12966-017-0525-8
13. Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi:10.1164/rccm.200407-855OC
14. Furlanetto KC, Donária L, Schneider LP, et al. Sedentary behavior is an independent predictor of mortality in subjects with COPD. Respir Care. 2017;62(5):579–587. doi:10.4187/respcare.05306
15. Ohayon M, Wickwire EM, Hirshkowitz M, et al. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health. 2017;3(1):6–19. doi:10.1016/j.sleh.2016.11.006
16. Ohayon MM, Paskow M, Roach A, et al. The National Sleep Foundation’s Sleep Satisfaction Tool. Sleep Health. 2019;5(1):5–11. doi:10.1016/j.sleh.2018.10.003
17. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi:10.1016/j.sleh.2015.10.004
18. Chattu V, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The global problem of insufficient sleep and its serious public health implications. Healthcare. 2019;7(1):1–16. doi:10.3390/healthcare7010001
19. Shorofsky M, Bourbeau J, Kimoff J, et al. Impaired sleep quality in COPD is associated with exacerbations: the CanCOLD cohort study. Chest. 2019;156(5):852–863. doi:10.1016/j.chest.2019.04.132
20. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi:10.1136/bjsports-2020-102955
21. Ross R, Chaput JP, Giangregorio LM, et al. Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2020;45(10):S57–S102. doi:10.1139/apnm-2020-0467
22. Sallis JF, Bull F, Guthold R, et al. Progress in physical activity over the Olympic quadrennium. Lancet. 2016;388(10051):1325–1336. doi:10.1016/S0140-6736(16)30581-5
23. Lambert EV, Kolbe-Alexander T, Adlakha D, et al. Making the case for ‘physical activity security’: the 2020 WHO guidelines on physical activity and sedentary behaviour from a Global South perspective. Br J Sports Med. 2020;54(24):1447–1448. doi:10.1136/bjsports-2020-103524
24. Strain T, Wijndaele K, Garcia L, et al. Levels of domain-specific physical activity at work, in the household, for travel and for leisure among 327 789 adults from 104 countries. Br J Sports Med. 2020;54(24):1488–1497. doi:10.1136/bjsports-2020-102601
25. Byrom B, Rowe DA. Measuring free-living physical activity in COPD patients: deriving methodology standards for clinical trials through a review of research studies. Contemp Clin Trials. 2016;47:172–184. doi:10.1016/j.cct.2016.01.006
26. Garrow AP, Yorke J, Khan N, Vestbo J, Singh D, Tyson S. Systematic literature review of patient-reported outcome measures used in assessment and measurement of sleep disorders in chronic obstructive pulmonary disease. Int J COPD. 2015;10:293–307. doi:10.2147/COPD.S68093
27. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;88:105906. doi:10.1136/bmj.n71
28. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10. doi:10.1186/s13643-016-0384-4
29. World Health Organisation. Definition of regional groupings. Available from: http://www.who.int/healthinfo/global_burden_disease/definition_regions/en/.
30. The World Bank. World Bank Country and Lending Groups. Country Classification. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
31. Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol Zeitschrift. 2006;15(3):259–263. doi:10.1127/0941-2948/2006/0130
32. Peel MC, Finlayson BL, McMahon TA. Updated world map of the Koppen-Geiger climate classification. Hydrol Earth Syst Sci. 2007;11(5):1633–1644. doi:10.1002/ppp.421
33. World Bank. New World Bank Country Classifications by Income Level: 2020–2021; Washington, DC, USA: World Bank; 2020. Available from: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2020-2021.
34. World Health Organisation. WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Global update 2005. Summary of risk assessment; 2005. Available from: https://apps.who.int/iris/handle/10665/69477.
35. Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Physical activity levels of the world’s population Surveillance progress, gaps and prospects. Lancet. 2012;380:247–257. doi:10.1016/S0140-6736(12)60646-1
36. Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-Country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi:10.1249/01.MSS.0000078924.61453.FB
37. Orme MW, Steiner MC, Morgan MD, et al. 24-hour accelerometry in COPD: exploring physical activity, sedentary behavior, sleep and clinical characteristics. Int J COPD. 2019;14:419–430. doi:10.2147/COPD.S183029
38. Spina G, Spruit MA, Alison J, et al. Analysis of nocturnal actigraphic sleep measures in patients with COPD and their association with daytime physical activity. Thorax. 2017;72(8):694–701. doi:10.1136/thoraxjnl-2016-208900
39. Lewthwaite H, Effing TW, Olds T, Williams MT. Physical activity, sedentary behaviour and sleep in COPD guidelines: a systematic review. Chron Respir Dis. 2017;14(3):231–244. doi:10.1177/1479972316687224
40. Simonelli G, Marshall NS, Grillakis A, Miller CB, Hoyos CM, Glozier N. Sleep health epidemiology in low and middle-income countries: a systematic review and meta-analysis of the prevalence of poor sleep quality and sleep duration. Sleep Health. 2018;4(3):239–250. doi:10.1016/j.sleh.2018.03.001
41. Ohayon MM, Reynolds III CF. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Med. 2009;10(9):952–960. doi:10.1016/j.sleep.2009.07.008
42. Adetiloye AO, Erhabor GE, Adewole OO, Awopeju O. The determinants of sleep quality and its impact on exercise capacity of patients with COPD. Afr J Respir Med. 2018;13(2):10–14.
43. Adewole O, Erhabor G, Oguntola O. Sleep pattern, quality and sleepiness among patients with chronic pulmonary parenchyma disease in Nigeria. Sleep Med. 2011;12:S47. doi:10.1016/S1389-9457(11)70173-3
44. Desalu OO, Onyedum CC, Makusidi MA, et al. Physical and socioeconomic impact of asthma in Nigeria: experience of patients attending three tertiary hospitals. Niger J Clin Pract. 2019;22(6):855–861. doi:10.4103/njcp.njcp_294_18
45. Ali Zohal M, Yazdi Z, Kazemifar AM. Daytime sleepiness and quality of sleep in patients with COPD compared to control group. Glob J Health Sci. 2013;5(3):150–155. doi:10.5539/gjhs.v5n3p150
46. Borji M, Salimi E. Comparing the effect of home care nursing and health promotion model on quality of life, sleep quality and life style of patients with asthma: a randomized clinical trial. Iran J Allergy Asthma Immunol. 2018;17(1):150.
47. Chegeni SP, Gholami M, Azargoon A, Hossein Pour AH, Birjandi M, Norollahi H. The effect of progressive muscle relaxation on the management of fatigue and quality of sleep in patients with chronic obstructive pulmonary disease: a randomized controlled clinical trial. Complement Ther Clin Pract. 2018;31:64–70. doi:10.1016/j.ctcp.2018.01.010
48. Eslaminejad A, Safa M, Ghassem BF, Hajizadeh F, Pashm FM. Relationship between sleep quality and mental health according to demographics of 850 patients with chronic obstructive pulmonary disease. J Health Psychol. 2017;22(12):1603–1613. doi:10.1177/1359105316684937
49. Mahmood ZA, Malghooth ZT. Relationship of hips and knees osteoarthritis with bronchial asthma. Res J Pharm Biol Chem Sci. 2019;10(2):64–70.
50. Moussa SB, Sfaxi I, Tabka Z, Saad HB, Rouatbi S. Oxidative stress and lung function profiles of male smokers free from COPD compared to those with COPD: a case-control study. Libyan J Med. 2014;9(1):23873. doi:10.3402/ljm.v9.23873
51. Moussa SB, Rouatbi S, Saad HB. Incapacity, handicap, and oxidative stress markers of male smokers with and without COPD. Respir Care. 2016;61(5):668–679. doi:10.4187/respcare.04420
52. Mihaltan F, Adir Y, Antczak A, et al. Importance of the relationship between symptoms and self-reported physical activity level in stable COPD based on the results from the SPACE study. Respir Res. 2019;20(1). doi:10.1186/s12931-019-1053-7
53. Mihaltan F, Antczak A, Radulovic V, Chen Y, Alecu S. Physical activity level in stable copd patients from central eastern europe: results from a large observational study. Chest. 2019;156(4):A903. doi:10.1016/j.chest.2019.08.848
54. Vukoja M, Kopitovic I, Milicic D, Maksimovic O, Pavlovic-Popovic Z, Ilic M. Sleep quality and daytime sleepiness in patients with COPD and asthma. Clin Respir J. 2018;12(2):398–403. doi:10.1111/crj.12528
55. Akinci AC, Yildirim E. Factors affecting health status in patients with chronic obstructive pulmonary disease. Int J Nurs Pract. 2013;19(1):31–38. doi:10.1111/ijn.12034
56. Genc A, Ucok K, Gunay E, et al. Effects of long-acting beta-2 agonist treatment on daily energy balance and body composition in patients with chronic obstructive pulmonary disease. Turk J Med Sci. 2012;42:1414–1422.
57. Genc A, Ucok K, Sener U, et al. Association analyses of oxidative stress, aerobic capacity, daily physical activity, and body composition parameters in patients with mild to moderate COPD. Turk J Med Sci. 2014;44(6):972–979. doi:10.3906/sag-1308-65
58. Pehlivan E, Niksarlioglu EY, Balci A, Kilic L. The effect of pulmonary rehabilitation on the physical activity level and general clinical status of patients with bronchiectasis. Turk Thorac J. 2019;20(1):30–35. doi:10.5152/TurkThoracJ.2018.18093
59. Şahin AZ, Dayapoğlu N. Effect of progressive relaxation exercises on fatigue and sleep quality in patients with chronic obstructive lung disease (COPD). Complement Ther Clin Pract. 2015;21(4):277–281. doi:10.1016/j.ctcp.2015.10.002
60. Saglam M, Savci S, Vardar Yagli N, et al. Relationship between obesity and respiratory muscle strength, functional capacity, and physical activity level in patients with chronic obstructive pulmonary disease. Fiz Rehabil. 2013;24(3):157–162.
61. Turan O, Ure I, Turan PA. Erectile dysfunction in COPD patients. Chronic Respir Dis. 2016;13(1):5–12. doi:10.1177/1479972315619382
62. Yilmaz FT, Aydin HT. The effect of a regular walking program on dyspnoea severity and quality of life in normal weight, overweight, and obese patients with chronic obstructive pulmonary disease. Int J Nurs Pract. 2018;24(3):1. doi:10.1111/ijn.12636
63. Lopez Jove OR, Galdames A, Barrionuevo V, et al. Depression in patients with chronic obstructive pulmonary disease (COPD): relationship to dyspnea degrees and impact on quality of life (QOL). Am J Respir Crit Care Med. 2013;187:A2505.
64. Molinas JA, Torrent C, Ardusso LR, Crisci CD, Barayazarra S, Crisci S. Relationship between body mass index and severity of bronchial asthma in adults. Arch Allergy Immunol Clin. 2007;38(1):19–28.
65. Amorim PB, Stelmach R, Carvalho CRF, Fernandes FLA, Carvalho-Pinto R, Cukier A. Barriers associated with reduced physical activity in COPD patients. J Bras Pneumol. 2014;40(5):504–512. doi:10.1590/S1806-37132014000500006
66. Athayde FTS, Vieira DSR, Britto RR, Parreira VF. Functional outcomes in patients with chronic obstructive pulmonary disease: a multivariate analysis. Rev Brasil Fisioter. 2014;18(1):63–71. doi:10.1590/S1413-35552012005000142
67. Borges RC, Carvalho CRF. Physical activity in daily life in Brazilian COPD patients during and after exacerbation. COPD. 2012;9(6):596–602. doi:10.3109/15412555.2012.705364
68. Campos FL, de Bruin PFC, Pinto TF, da Silva FGC, Pereira EDB, de Bruin VMS. Depressive symptoms, quality of sleep, and disease control in women with asthma. Sleep Breathing. 2017;21(2):361–367. doi:10.1007/s11325-016-1422-0
69. Cani KC, Matte DL, Silva IJCS, Gulart AA, Karloh M, Mayer AF. Impact of home oxygen therapy on the level of physical activities in daily life in subjects with COPD. Respir Care. 2019;64(11):1392–1400. doi:10.4187/respcare.06206
70. Cavalcante AM, De Bruin VMS, Pereira EDB, et al. Restless leg syndrome, fatigue and quality of sleep in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:A4533.
71. Cetlin AA, Gutierrez MR, Bettiol H, Barbieri MA, Vianna EO. Influence of asthma definition on the asthma-obesity relationship. BMC Public Health. 2012;12:844. doi:10.1186/1471-2458-12-844
72. Coelho CM, Campos LA, Pereira FO, et al. Objectively measured daily-life physical activity of moderate-to-severe Brazilian asthmatic women in comparison to healthy controls: a cross-sectional study. J Asthma. 2018;55(1):73–78. doi:10.1080/02770903.2017.1306547
73. Coelho CM, Reboredo MM, Valle FM, et al. Effects of an unsupervised pedometer-based physical activity program on daily steps of adults with moderate to severe asthma: a randomized controlled trial. J Sports Sci. 2018;36(10):1186–1193. doi:10.1080/02640414.2017.1364402
74. Da Costa CC, Bordin CD, Roberto CJP, Lermen C, Colombo C, Machado DSR. The repercussions of a pulmonary rehabilitation program on the level of physical activity in patients with chronic obstructive pulmonary disease. Rev Inspirar Movimento Saude. 2012;4(5):34–37.
75. Cukier A, Godoy I, Costa CHD, et al. Symptom variability over the course of the day in patients with stable COPD in Brazil: a real-world observational study. J Bras Pneumol. 2020;46(3):e20190223. doi:10.36416/1806-3756/e20190223
76. Felcar JM, Probst VS, De Carvalho DR, et al. Effects of exercise training in water and on land in patients with COPD: a randomised clinical trial. Physiotherapy. 2018;104(4):408–416. doi:10.1016/j.physio.2017.10.009
77. Filippin TR, Ariza D, Beloto A-CNDO. Correlation of the bod mortality predictor index in physically active and sedentary chronic obstructive pulmonary diseased patients. Rev Inspirar Movimento Saude. 2011;3(4):30–35.
78. Fonseca FR, Karloh M, De araujo CLP, Dos santos K, Mayer AF. Nutritional status and its relationship with different dimensions of functional status in patients with chronic obstructive pulmonary disease. Rev Nutr. 2016;29(5):635–644. doi:10.1590/1678-98652016000500002
79. Freitas PD, Silva AG, Ferreira PG, et al. Exercise improves physical activity and comorbidities in obese adults with asthma. Med Sci Sports Exerc. 2018;50(7):1367–1376. doi:10.1249/MSS.0000000000001574
80. Furlanetto KC, Pinto IFS, Sant’Anna T, Hernandes NA, Pitta F. Profile of patients with chronic obstructive pulmonary disease classified as physically active and inactive according to different thresholds of physical activity in daily life. Braz J Phys Ther. 2016;20(6):517–524. doi:10.1590/bjpt-rbf.2014.0185
81. Furlanetto KC, Demeyer H, Sant’anna T, et al. Physical activity of patients with COPD from regions with different climatic variations. COPD. 2017;14(3):276–283. doi:10.1080/15412555.2017.1303039
82. Gulart AA, Munari AB, Klein SR, Venancio RS, Alexandre HF, Mayer AF. The London Chest Activity of Daily Living scale cut-off point to discriminate functional status in patients with chronic obstructive pulmonary disease. Braz J Phys Ther. 2019;24(3):264–272. doi:10.1016/j.bjpt.2019.03.002
83. Hernandes NA, Teixeira DC, Probst VS, Brunetto AF, Ramos EMC, Pitta F. Profile of the level of physical activity in the daily lives of patients with COPD in Brazil. J Bras Pneumol. 2009;35(10):949–956. doi:10.1590/S1806-37132009001000002
84. Jardim JR, Piazza M, Carvalho AK, Ivanaga IT, Nascimento OA. Assessment of physical activity by pedometer in patients with chronic obstructive pulmonary disease (COPD) In an emerging country. Am J Respir Crit Care Med. 2011;183(1):A2034.
85. Karloh M, Araujo CLP, Gulart AA, Reis CM, Steidle LJM, Mayer AF. The Glittre-ADL test reflects functional performance measured by physical activities of daily living in patients with chronic obstructive pulmonary disease. Braz J Phys Ther. 2016;20(3):223–230. doi:10.1590/bjpt-rbf.2014.0155
86. Landal AC, Monteiro F, Hevely BC, Kanesawa LM, Hernandes N, Pitta F. Factors associated with improved body composition in individuals with COPD after physical training. Fisioter mov. 2014;27(4):633–641. doi:10.1590/0103-5150.027.004.AO15
87. Lanza FC, Castro RAS, de Camargo A, et al. COPD assessment test (CAT) is a valid and simple tool to measure the impact of Bronchiectasis on affected patients. COPD. 2018;15(5):512–519. doi:10.1080/15412555.2018.1540034
88. Lopes AC, Xavier RF, Pereira AC AC, et al. Identifying COPD patients at risk for worse symptoms, HRQoL, and self-efficacy: a cluster analysis. Chronic Illn. 2019;15(2):138–148. doi:10.1177/1742395317753883
89. Mantoani LC, Hernandes N, Guimarães M, Vitorasso RL, Probst VS, Pitta F. Does the BODE index reflect the level of physical activity in daily life in patients with COPD? Braz j Phys Ther (Impr). 2011;15(2):131–137. doi:10.1590/S1413-35552011000200008
90. Monteiro F, Camillo CA, Vitorasso R, et al. Obesity and physical activity in the daily life of patients with COPD. Lung. 2012;190(4):403–410. doi:10.1007/s00408-012-9381-0
91. Morita AA, Silva LKO, Bisca GW, et al. Heart rate recovery, physical activity level, and functional status in subjects with COPD. Respir Care. 2018;63(8):1002–1008. doi:10.4187/respcare.05918
92. Munari AB, Gulart AA, Dos Santos K, Venâncio RS, Karloh M, Mayer AF. Modified Medical Research Council Dyspnea Scale in GOLD classification better reflects physical activities of daily living. Respir Care. 2018;63(1):77–85. doi:10.4187/respcare.05636
93. Nobeschi L, Zangirolami-Raimundo J, Cordoni PK, et al. Evaluation of sleep quality and daytime somnolence in patients with chronic obstructive pulmonary disease in pulmonary rehabilitation. BMC Pulm Med. 2020;20(1):1–7. doi:10.1186/s12890-020-1046-9
94. Nunes DM, Mota RMS, Machado MO, Pereira EDB, de Bruin VMS, de Bruin PFC. Effect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary disease. Braz J Med Biol Res. 2008;41(10):926–931. doi:10.1590/S0100-879X2008001000016
95. Nyssen SM, Santos J, Barusso MS, Delfino de OA
96. Pitta F, Breyer M, Hernandes NA, et al. Comparison of daily physical activity between COPD patients from Central Europe and South America. Respir Med. 2009;103(3):421–426. doi:10.1016/j.rmed.2008.09.019
97. Probst VS, Kovelis D, Hernandes N, Camillo CA, Cavalheri V, Pitta F. Effects of 2 exercise training programs on physical activity in daily life in patients with COPD. Respir Care. 2011;56(11):1799–1807. doi:10.4187/respcare.01110
98. Reboredo MDM, Lucinda LM, Yecker GD, et al. Barriers to physical activity in patients with chronic respiratory diseases. Am J Respir Crit Care Med. 2018;197:A2174.
99. Rocha FR, Brüggemann A, Karla V, et al. Diaphragmatic mobility: relationship with lung function, respiratory muscle strength, dyspnea, and physical activity in daily life in patients with COPD. J Bras Pneumol. 2017;43(1):32–37. doi:10.1590/s1806-37562016000000097
100. Rodrigues ESR, Moreira RDF, Rezende AAB, Costa LD. Sedentarism and smoking in patients with cardiovascular, respiratory and orthopedic diseases. Rev Enfermagem Ufpe. 2014;8(3):591–599.
101. Schneider PL, Couto FK, Aparecida HN, Pitta F. Does the wearing time of motion sensor interfere with the choice of physical activity in daily life outcomes of COPD patients? Fisioter Pesquisa. 2018;25(1):43–48. doi:10.1590/1809-2950/16768425012018
102. Silva DR, Coelho AC, Dumke A, et al. Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care. 2011;56(7):961–968. doi:10.4187/respcare.01056
103. Simon KM, Hass AP, Zimmermman JL, Carpes MF. BODE mortality prognostic index and physical activity in chronic obstructive pulmonary patients. Rev bras med esporte. 2009;15(1):19–22. doi:10.1590/S1517-86922009000100004
104. Tavares MG, Nascimento ACSD, Ferraz MCCN, Medeiros RABD, Cabral PC, Burgos MGPDA. Overweight and obesity in patients with chronic obstructive pulmonary disease. Braspen J. 2017;32(1):58–62.
105. Vitorasso R, Camillo CA, Cavalheri V, et al. Is walking in daily life a moderate intensity activity in patients with chronic obstructive pulmonary disease? Eur J Phys Rehabil Med. 2012;48(4):587–592.
106. Xavier R, Lopes AC, Pereira ACAC, et al. Factors associated with daily life physical activity in Brazilian COPD patients. Eur Respir J. 2016;48:PA1889.
107. Yunus FM, Khan S, Mitra DK, Mistry SK, Afsana K, Rahman M. Relationship of sleep pattern and snoring with chronic disease: findings from a nationwide population-based survey. Sleep Health. 2018;4(1):40–48. doi:10.1016/j.sleh.2017.10.003
108. Bhuker M, Kapoor S. Environmental and occupational respiratory diseases-1043. Effects of socioeconomic status, life style patterns and demographic factors. World Allergy Organ J. 2013;6:P42. doi:10.1186/1939-4551-6-S1-P42
109. De S. Subjective assessment of quality of sleep in chronic obstructive pulmonary disease patient and its relationship with associated depression. Lung India. 2012;29(4):332–335. doi:10.4103/0970-2113.102808
110. Gupta R, Ulfberg J, Allen RP, Goel D. Comparison of subjective sleep quality of long-term residents at low and high altitudes: SARAHA study. J Clin Sleep Med. 2018;14(1):15–21. doi:10.5664/jcsm.6870
111. Panigrahi MK, Padhan M, Mohapatra PR. Relationship between sleep quality, asthma control and quality of life in adults with asthma. Am J Respir Crit Care Med. 2018;197:A1089. doi:10.1164/rccm.201708-1728OC
112. Poongadan MN, Gupta N, Kumar R. Lifestyle factors and asthma in India - a case-control study. Pneumonol Alergol Pol. 2016;84(2):104–108. doi:10.5603/PiAP.2016.0008
113. Sharma K, Jain A, Goyal V, et al. Depression and physical activity impairment in COPD subjects. J Clin Diagn Res. 2019;13(7):OC16–22.
114. Ulfathinah A, Rachmi SF, Indracahyani A. Characteristics affecting sleep quality of COPD patients. Enferm Clin. 2019;29:30–35. doi:10.1016/j.enfcli.2019.04.005
115. Widyastuti K, Makhabah DN, Rima A, Sutanto YS, Suradi S, Ambrosino N. Home based pulmonary rehabilitation with pedometers in Indonesian COPD patients. Eur Respir J. 2017;50:PA777.
116. Chanthitivech N, Sirichana W. Cardiopulmonary exercise testing parameters and level of daily physical activity correlation in stable COPD patients. Eur Respir J. 2017;50:PA2250.
117. Chunrong J, Rongchang C. Factors associated with impairment of quadriceps muscle function in Chinese patients with chronic obstructive pulmonary disease. PLoS One. 2014;9(2):e84167. doi:10.1371/journal.pone.0084167
118. Ding B, Small M, Bergstrom G, Holmgren U. A cross-sectional survey of night-time symptoms and impact of sleep disturbance on symptoms and health status in patients with COPD. Int J COPD. 2017;12:589–599. doi:10.2147/COPD.S122485
119. Tao Y, Wang L, Dong X, et al. Psychometric properties of the physical activity scale for the elderly in Chinese patients with COPD. Int J COPD. 2017;12:105–114. doi:10.2147/COPD.S120700
120. Wang L, Nygardh A, Zhao Y, Martensson J. Self-management among patients with chronic obstructive pulmonary disease in China and its association with sociodemographic and clinical variables. Appl Nurs Res. 2016;32:61–66. doi:10.1016/j.apnr.2016.05.001
121. Wang L, Tao Y, Dong X, et al. Demographic, health behavioral, and selfmanagement abilities associated with disease severity among patients with chronic obstructive pulmonary disease: an exploratory study. Int J Nurs Pract. 2017;23(1):e12509. doi:10.1111/ijn.12509
122. Wang S, Wu Y, Ungvari GS, et al. Sleep duration and its association with demographics, lifestyle factors, poor mental health and chronic diseases in older Chinese adults. Psychiatry Res. 2017;257:212–218. doi:10.1016/j.psychres.2017.07.036
123. Zheng L, Wang A. Application effect of rational emotional behavior therapy for home chronic obstructive pulmonary disease patients with depression. Chin Nurs J. 2019;33:1135–1140.
124. Vancampfort D, Koyanagi A, Ward PB, et al. Chronic physical conditions, multimorbidity and physical activity across 46 low and middle income countries. Int J Behav Nutr Phys Act. 2017;14(1):6. doi:10.1186/s12966-017-0463-5
125. Koyanagi A, Stubbs B, Smith L, Gardner B, Vancampfort D. Correlates of physical activity among community-dwelling adults aged 50 or over in six low- and middle-income countries. PLoS One. 2017;12(10):e0186992. doi:10.1371/journal.pone.0186992
126. Vancampfort D, Stubbs B, Koyanagi A. Physical chronic conditions, multimorbidity and sedentary behavior amongst middle-aged and older adults in six low- and middle-income countries. Int J Behav Nutr Phys Act. 2017;14(1):147. doi:10.1186/s12966-017-0602-z
127. Vancampfort D, Lara E, Stubbs B, Swinnen N, Probst M, Koyanagi A. Physical activity correlates in people with mild cognitive impairment: findings from six low- and middle-income countries. Public Health. 2018;156:15–25. doi:10.1016/j.puhe.2017.12.002
128. Vancampfort D, Stubbs B, Veronese N, Mugisha J, Swinnen N, Koyanagi A. Correlates of physical activity among depressed older people in six low-income and middle-income countries: a community-based cross-sectional study. Int J Geriatr Psychiatry. 2018;33(2):e314–22. doi:10.1002/gps.4796
129. Vancampfort D, Stubbs B, Mugisha J, Firth J, Schuch FB, Koyanagi A. Correlates of sedentary behavior in 2375 people with depression from 6 low- and middle-income countries. J Affect Disord. 2018;234:97–104. doi:10.1016/j.jad.2018.02.088
 © 2022 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2022 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
