Back to Journals » OncoTargets and Therapy » Volume 12
Systematic nutrition management for locally advanced nasopharyngeal carcinoma patients undergoing radiotherapy
Authors Huang JF , Sun RJ, Jiang WJ, Wu P, Zhang L, Xu MQ, Zhou LY, Pang QF , Wu YX, Yang B, Zhang FZ
Received 29 April 2019
Accepted for publication 4 July 2019
Published 10 October 2019 Volume 2019:12 Pages 8379—8386
DOI https://doi.org/10.2147/OTT.S213789
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Jian-Feng Huang,1,* Ren-Juan Sun,1,* Wen-Jun Jiang,1 Ping Wu,2 Li Zhang,1 Mei-Qin Xu,1 Le-Yuan Zhou,1 Qing-Feng Pang,3 Ya-Xian Wu,3 Bo Yang,1 Fu-Zheng Zhang1
1Department of Radiation Oncology, Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu, People’s Republic of China; 2Department of Nutriology, Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu, People’s Republic of China; 3Department of Physiopathology, Wuxi Medical School of Jiangnan University, Wuxi, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Yang; Fu-Zheng Zhang
Department of Radiation Oncology, Affiliated Hospital of Jiangnan University, 200 Huihe Road, Wuxi 214062, People’s Republic of China
Tel +86 510 8868 3246
; +86 510 8868 2111
Fax +86 510 8586 0321
Email [email protected]; [email protected]
Objective: To evaluate the impact of systematic nutrition management (SNM) on nutritional status, treatment-related toxicity, quality of life (QoL), response rates, and survival in patients with locally advanced nasopharyngeal carcinoma (LA-NPC) treated by radiotherapy (RT).
Methods: In this retrospective study, 56 patients with LA-NPC were selected as nutrition management group (NG) for SNM during RT till 1 month later. Another 56 patients with LA-NPC receiving RT without SNM as control group (CG) were identified from the hospital database and matched pairs with NG patients according to age, gender, stage, and body mass index (BMI) prior to RT.
Results: At 1 month after RT, the percentage of malnourished patients with BMI <18.5 kg/m2 was statistically significant reduced in NG as compared to the CG group (35.7% vs 58.9%, P=0.014). Nutritional indexes of body weight, hemoglobin, prealbumin, and lymphocyte in the NG were statistically significant higher than those in the CG group (P<0.05). NG patients had statistically significant less grade 3–4 oral mucositis during RT compared with the CG group (32.1% vs 51.8%, P=0.035). Furthermore, at 1 month after RT, an improved QoL was observed in NG patients with respect to physical, role and social functions, symptom scales of fatigue and pain, and the global health status as compared to the CG group (P<0.05). With a median follow-up of 24.8 months, there were no statistical differences between NG and CG (P>0.05) for the 2-year progression-free survival and overall survival (84.2% versus 79.5% and 94.7% versus 92.3%, respectively.).
Conclusion: SNM for LA-NPC patients treated by RT resulted in better nutritional status, reduced treatment-related toxicity and improved QoL.
Keywords: nasopharyngeal carcinoma, radiotherapy, nutrition management, clinical outcome
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most prevalent malignancies in the Southeast Asia populations, and most patients are diagnosed with locally advanced disease (LA-NPC).1 With intensity-modulated radiotherapy (IMRT) and systemic treatment, disease control and survival of LA-NPC patients have been substantially improved.2–4 Nevertheless, nutritional problems such as weight loss and reduced protein-calorie intake remain quite common during RT, which have been found to be associated with lower survival rate and worse quality of life (QoL).5–9 Systematic nutritional monitoring and intervention for patients with head and neck cancer could improve treatment outcomes.10–12 So far, however, there is little research focusing on nutrition management for LA-NPC patients, which differ greatly from non-NPC head and neck cancer patients in terms of epidemiology, etiology, pathology, clinical presentation, treatment method, and response to treatment.
In this matched-pair retrospective study, LA-NPC patients who received definitive IMRT with or without SNM were analyzed. The aim of this work was to evaluate the impact of SNM on nutritional status, treatment-related toxicity, QoL, response rates, and survival in LA-NPC patients treated by RT.
Methods
Patient selection
Inclusion criteria were as follows: 1) newly diagnosed NPC with histological confirmation; 2) clinically staged T3–4N0–3M0 or T1–4N1–3M0; 3) Eastern Cooperative Oncology Group performance status 2 or lower; 4) no severe cardiopulmonary diseases; 5) treated by IMRT, and SNM was given in NG during RT till 1 month after treatment.
Between April 2014 and August 2017, 56 eligible patients with LA-NPC were selected as nutrition management group (NG) who treated at Affiliated Hospital of Jiangnan University and met the inclusion criteria; another 56 patients with LA-NPC receiving RT without SNM as control group (CG) were identified from the hospital database and matched pairs with NG according to age, gender, stage, and body mass index (BMI) prior to RT. Patients’ characteristics at baseline in both groups are detailed in Table 1. All patients were staged according to 2010 Union for International Cancer Control staging system. This study was conducted in accordance with the principles of the Declaration of Helsinki, and it was approved by the Institutional Ethics Committee of Affiliated Hospital of Jiangnan University. All patients with LA-NPC in the NG obtained written informed consent prior to RT and SNM. For those matched-pair patients with LA-NPC, an exemption of written informed consent for inclusion was granted by the Institutional Ethics Committee, since the retrospective data are already there and clinical follow-up should be always needed for all treated patients anyway. Non-parametric tests were used to ensure that there was no statistically significant difference in the concerned factors between the matched pairs.
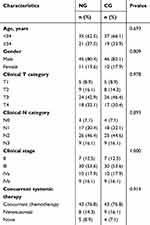 |
Table 1 Baseline characteristics of patients |
Treatment modality
IMRT was administered to all patients. The doses of RT were consistent with the recommendations of the National Comprehensive Cancer Network guidelines, with a total dose of 66–76 Gy to the primary tumor and involved cervical lymph nodes in 30–35 daily fractions. Dose constraints to organs at risk were in agreement with the Radiation Therapy Oncology Group (RTOG) 0225 protocol.
All enrolled patients received two cycles of neoadjuvant chemotherapy with the doublet regimen of docetaxel (75 mg/m2 on day 1) plus nedaplatin (80 mg/m2 on day 2). Concurrent systemic regimens during the course of IMRT are detailed in Table 1. Most patients (76.8%) received concurrent chemotherapy (CCT) with the doublet regimen of docetaxel (75 mg/m2 on day 1) plus nedaplatin (80 mg/m2 on day 2).
Nimotuzumab (200 mg, weekly) was administered in 17 patients (8 in NG and 9 in CG), and the other 9 patients (5 in NG and 4 in CG) received IMRT alone without any systemic therapy.
Nutrition management
NG patients received systematic nutrition management (SNM). Briefly, nutritional assessment was performed weekly from the beginning of RT till 1 month after RT by a registered dietician using Patient-Generated Subjective Global Assessment (PG-SGA), which has been accepted by the Oncology Nutrition Dietetic Practice Group of the American Dietetic Association as the standard for nutritional assessment for cancer patients. They were scored and classified in three degrees: normal-nutrition (0–3 scores, PG-SGA A), moderate malnutrition (4–8 scores, PG-SGA B), and severe malnutrition (≥9 scores, PG-SGA C). Individualized nutritional interventions were administrated according to the total PG-SGA score of each patient. Patients with PG-SGA A were provided with nutritional counseling by a personalized dietary prescription. In addition to nutritional counseling, the malnourished (PG-SGA B or C) patients received oral supplements (Nutrison, Nutricia Ltd, Milupa GmbH) consisting of an energy-dense, high-protein, ready-to-use formula (4.62 kcal/g; 16% proteins, 36% lipids, 48% carbohydrates). The daily energy requirements were estimated by Harris-Benedict equation with a correcting factor of 1.5, and the total daily protein requirements were calculated at 1.5 g/kg of body weight. If patients were unable to maintain an adequate oral intake (less than 50% of the estimated requirements for two consecutive weeks), enteral nutrition by a nasogastric tube was administrated.
Patients in CG received general nutrition counseling and a booklet with nutrition advice for radiation-induced toxicity. Referral to Nutrition Department for nutritional intervention usually occurred when symptoms or weight loss were manifest but patients with less severe side effects generally did not receive a specialized evaluation.
Patient evaluation and follow-up
Response rates were evaluated by the Response Evaluation Criteria in Solid Tumors at 3 months after RT. All patients were followed-up at intervals of 3 months after RT for 3 years, biannually for the next 2 years and annually thereafter.
Treatment-related toxicity during RT was assessed and recorded weekly by the radiotherapists using the Acute and Late Radiation Morbidity Scoring Criteria of RTOG. Radiation treatment breaks were defined as the interruption time of radiation >7 days.
Nutritional indexes, including body weight, BMI, hemoglobin, albumin, prealbumin, and lymphocyte were retrospectively collected. Changes of these indexes between pre-RT and 1 month after RT were analyzed. Patients with BMI <18.5 kg/m2 were defined as malnutrition.
Patients’ QoL was assessed with the European Organization for Research and Treatment for Cancer Quality of Life Questionnaire core 30 (EORTC QLQ-C30) by the dietician before RT and 1 month after RT. In the EORTC QLQ-C30, there were five functional scales, three symptom scales, six single items, and a global health status scale. Higher scores indicated better QoL on global health status and functional scales and worse QoL on symptom scales and single items. Changes of these scales or items between pre-RT and 1 month after RT were analyzed.
Statistical analysis
The data were entered into an Excel spreadsheet and analyzed using the STAT software, version 12.0. The chi-squared test was used to analyze the enumeration data. Differences in continuous variables between groups were assessed using the Student t-test. Progression-free survival (PFS) was defined as the time between pathological diagnosis and the first occurrence of locoregional or distant recurrence or the last follow-up date. Overall survival (OS) was measured from the time of pathological diagnosis to the date of death or the last follow-up date. The estimated PFS and OS were calculated by the Kaplan–Meier method. The PFS or OS was compared between the two groups with log-rank test. A two-tailed P<0.05 was accepted as statistically significant.
Results
Response rates and survival
Objective response rate was 100% in both groups at 3 months after RT, with complete response and partial response rates of 82.1% vs 80.4% (P>0.05) and 17.9% vs 19.6% (P>0.05) in NG and CG.
At a median follow-up of 24.8 months [range, 8.7–54.2 months], a total of 19 patients failed with distant metastasis: 9 in NG and 10 in CG, and 11 of them (5 in NG and 6 in CG) died of disease progression. Another patient in the CG developed locoregional failure. The 2-year OS of NG and CG patients was 94.7% (95% confidence interval [CI]: 82.2–99.3%) and 92.3% (95% CI: 79.1–98.3%), respectively (P>0.05, Figure 1). The 2-year PFS of NG and CG patients was 84.2% (95% CI: 68.7–93.9%) and 79.5% (95% CI: 63.5–90.7%), respectively (P>0.05, Figure 2).
 |
Figure 1 Kaplan–Meier survival curves of OS for NG and CG patients. Abbreviations: OS, overall survival; NG, nutrition management group; CG, control group. |
 |
Figure 2 Kaplan–Meier survival curves of PFS for NG and CG patients. Abbreviations: PFS, progression-free survival; NG, nutrition management group; CG, control group. |
Nutritional status
There were no statistical differences between NG and CG patients (P>0.05) for nutritional status prior to induction chemotherapy. Table 2 illustrates the changes of nutritional status between pre-RT and 1 month after RT in the two groups. The results showed that NG and CG patients had comparable nutritional status before RT in terms of body weight, BMI, levels of hemoglobin, albumin, prealbumin, and lymphocyte. At 1 month after RT, however, patients’ nutritional status has deteriorated dramatically in both groups for almost all of the indexes, except levels of albumin, where there is a trend of deterioration (P>0.05). The results also indicated that the nutritional indexes of body weight, levels of hemoglobin, prealbumin, and lymphocyte in NG were statistically significant higher than those in CG at 1 month after RT (P<0.05). In addition, 35.7% of NG patients and 58.9% of CG patients experienced malnutrition (BMI<18.5 kg/m2) at 1 month after RT, and the difference was statistically significant (P=0.014). Nasogastric tubes were administrated in four NG patients and three CG patients, respectively.
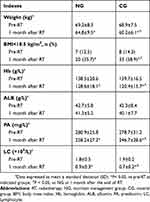 |
Table 2 Changes of nutritional status between pre-RT and 1 month after RT in both groups |
Treatment-related toxicity
Table 3 shows the comparison of treatment-related toxicity during RT in both groups. The results demonstrated that NG patients had statistically significant less grade 3–4 oral mucositis during RT as compared to the CG (32.1% vs 51.8%, P=0.035). Instead, no statistically significant differences were found between the two groups in the percentage of patients who suffered from grade 3–4 neutropenia, thrombocytopenia, anemia or radiodermatitis, as well as radiation treatment breaks for more than 7 days due to toxicity (P>0.05).
 |
Table 3 Treatment-related toxicities during radiotherapy in two groups |
Quality of life
As detailed in Table 4, QoL of patients has worsened significantly in both groups for most of the scales or items between measurements before RT and 1 month after completion of RT, except the emotional function, the symptom scales of nausea and vomiting, and the single item of diarrhea (P>0.05).
 |
Table 4 Changes of QoL between pre-RT and 1 month after RT in both groups |
It is noteworthy, however, that NG patients showed statistically significant improvements in physical, role and social functions comparing with the CG at 1 month after RT (P<0.05). Statistically significant improvements conferred by nutrition management were also observed for symptoms dimension of fatigue, pain, and the global health status. Instead, no statistically significant difference was found between the two groups in terms of financial conditions.
Discussion
Malnutrition is a frequent comorbidity in cancer patients and the incidence ranges from 39% to 71%.13–15 In patients with LA-NPC who received RT, malnutrition is further worsened by radiation-induced oral mucositis. Even worse, although a significant survival benefit has been achieved, the addition of concurrent chemotherapy results in increased acute toxicity and a higher incidence of malnutrition as compared to RT alone, which in turn compromise treatment tolerance and efficacy.16–18 Consequently, as an important aspect for the management of LA-NPC patients, appropriate nutritional support is imperative. In the present study, the effect of SNM on clinical outcomes of LA-NPC patients who received definitive IMRT was retrospectively evaluated.
Our study showed that the implementation of SNM was able to effectively mitigate treatment-related reductions of body weight, hemoglobin, prealbumin, and lymphocyte, which have been widely used to serve as nutrition indicators.19 The percentage of malnourished patients with BMI <18.5 kg/m2 at 1 month after RT was significantly reduced in the NG as compared to the CG (P=0.014). In fact, similar results of nutritional status improvements were also observed by cervical esophagostomy or prophylactic placement of percutaneous endoscopic gastrostomy tubes.19,20 Most recently, a randomized Phase II trial of nutritional counseling with or without oral nutritional supplements (ONS) for head and neck cancer patients undergoing RT conducted by Cereda et al, demonstrated that the additional provision of ONS from the beginning of RT and continuing for up to 3 months after the end of RT resulted in better weight maintenance, increased protein-calorie intake and improved anti-cancer treatment tolerance.21 These results strongly suggested that intensive nutrition support was crucial for nutritional status maintenance in cancer patients.
Interestingly, our study also showed that the implementation of SNM was associated with reduced treatment-related side effects and improved QoL. As with other studies of RT for LA-NPC, oral mucositis was the most common acute toxicity. In our cohort, comparing with CG, the incorporation of SNM in NG significantly reduced the percentage of patients who suffered from grade 3–4 mucositis (51.8% vs 32.1%, P=0.035). We also noted that, at 1 month after RT, patients’ QoL has worsened in both groups for most of the scales or items. However, an improved QoL was observed in NG patients with respect to physical, role and social functions, symptom scales of fatigue and pain, and the global health status as compared to the CG (P<0.05). We attribute this to the nutritional status improvement of NG patients. Likewise, treatment toxicity and QoL improvements conferred by nutritional intervention have also been found in head and neck cancer patients.22,23
It is noteworthy that, although survival benefit of nutritional intervention for cancer patients has been reported in several series,10,24–26 in our study, however, at a median follow-up of 24.8 months, there was no significant difference between the two groups in terms of OS and disease-free survival. These results should be regarded as preliminary because of the relatively short follow-up and small sample size, as well as the selection bias in patient population. Further prospective studies are needed to determine the survival benefit of SNM.
The study here dose has some limitations. The main limitation aroused from the retrospective data which obtained through the past records. Another drawback was the potential bias introduced by patient selection. Further, the nutritional status and patients’ QoL were not assessed for a longer period more than 1 month post-RT, so the effects of nutritional support on long-term QoL improvement could not be analyzed in the present study. Additionally, some of the patients had financial difficulties to afford the costs of the nutritional interventions, so other cheaper and easier ways should be explored.
Conclusion
SNM for LA-NPC patients treated by RT resulted in better nutritional status, reduced treatment-related toxicity and improved QoL. Since the limitations of this retrospective study, the results should be interpreted cautiously and further clinical trials are needed to confirm these findings.
Acknowledgment
This work was supported by grants from program of Wuxi Young Medical Talents (No QNRC059) and Wuxi Medical Development Discipline (No FZXK004).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. doi:10.1016/S0140-6736(15)00055-0
2. Lee AWM, Tung SY, Ng WT, et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer. 2017;123(21):4147–4157. doi:10.1002/cncr.30850
3. Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi:10.1016/j.radonc.2013.10.020
4. You R, Sun R, Hua YJ, et al. Cetuximab or nimotuzumab plus intensity-modulated radiotherapy versus cisplatin plus intensity-modulated radiotherapy for stage II–IVb nasopharyngeal carcinoma. Int J Cancer. 2017;141(6):1265–1276. doi:10.1002/ijc.30819
5. Li G, Gao J, Liu ZG, et al. Influence of pretreatment ideal body weight percentile and albumin on prognosis of nasopharyngeal carcinoma: long-term outcomes of 512 patients from a single institution. Head Neck. 2014;36(5):660–666. doi:10.1002/hed.23357
6. Irungu CW, Oburra HO, Ochola B. Prevalence and predictors of malnutrition in nasopharyngeal carcinoma. Clin Med Insights Ear Nose Throat. 2015;8:19–22. doi:10.4137/CMENT.S12119
7. Miao J, Xiao W, Wang L, et al. The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol. 2017;143(7):1263–1273. doi:10.1007/s00432-017-2360-3
8. He Y, Chen L, Hu W, et al. Relationship between the comprehensive nutritional index and the EORTC QLQ-H&N35 in nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Nutr Cancer. 2017;69(3):436–443. doi:10.1080/01635581.2017.1283422
9. Hong JS, Hua YJ, Su L, et al. Modified-nutrition index is a significant prognostic factor for the overall survival of the nasopharyngeal carcinoma patients who undergo intensity-modulated radiotherapy. Nutr Cancer. 2017;69(7):1011–1018. doi:10.1080/01635581.2017.1359311
10. Muller-Richter U, Betz C, Hartmann S, Brands RC. Nutrition management for head and neck cancer patients improves clinical outcome and survival. Nutr Res. 2017;48:1–8. doi:10.1016/j.nutres.2017.08.007
11. Brown TE, Banks MD, Hughes BG, Lin CY, Kenny LM, Bauer JD. Comparison of nutritional and clinical outcomes in patients with head and neck cancer undergoing chemoradiotherapy utilizing prophylactic versus reactive nutrition support approaches. J Acad Nutr Diet. 2018;118(4):627–636. doi:10.1152/ajplegacy.1975.229.6.1510
12. Platek ME. The role of dietary counseling and nutrition support in head and neck cancer patients. Curr Opin Support Palliat Care. 2012;6(4):438–445. doi:10.1097/SPC.0b013e32835999d5
13. Silva FR, de Oliveira MG, Souza AS, Figueroa JN, Santos CS. Factors associated with malnutrition in hospitalized cancer patients: a croos-sectional study. Nutr J. 2015;14:123. doi:10.1186/s12937-015-0113-1
14. Arends J. [Nutrition in cancer: effective in prevention and treatment?]. Dtsch Med Wochenschr. 2017;142(12):889–895. doi:10.1055/s-0042-111746
15. Gyan E, Raynard B, Durand JP, et al. Malnutrition in patients with cancer: comparison of perceptions by patients, relatives, and physicians-results of the nutri cancer 2012 Study. JPEN J Parenter Enteral Nutr. 2018;42(1):255–260. doi:10.1177/0148607116688881
16. Du CR, Ying HM, Kong FF, Zhai RP, Hu CS. Concurrent chemoradiotherapy was associated with a higher severe late toxicity rate in nasopharyngeal carcinoma patients compared with radiotherapy alone: a meta-analysis based on randomized controlled trials. Radiat Oncol. 2015;10:70. doi:10.1186/s13014-015-0377-9
17. Xu C, Zhang LH, Chen YP, et al. Chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma: a systemic review and meta-analysis of 2138 patients. J Cancer. 2017;8(2):287–297. doi:10.7150/jca.17317
18. Jin T, Li KX, Li PJ, et al. An evaluation of nutrition intervention during radiation therapy in patients with locoregionally advanced nasopharyngeal carcinoma. Oncotarget. 2017;8(48):83723–83733. doi:10.18632/oncotarget.19381
19. Xu Y, Guo Q, Lin J, et al. Benefit of percutaneous endoscopic gastrostomy in patients undergoing definitive chemoradiotherapy for locally advanced nasopharyngeal carcinoma. Onco Targets Ther. 2016;9:6835–6841. doi:10.2147/OTT.S117676
20. Chen W, Wang K, Tang J, et al. [Cervical esophagostomy improves the life quality of patients with dysphagia induced by radiotherapy for nasopharyngeal carcinoma]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51(3):179–182. doi:10.3760/cma.j.issn.1673-0860.2016.03.005
21. Cereda E, Cappello S, Colombo S, et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol. 2018;126(1):81–88. doi:10.1016/j.radonc.2017.10.015
22. Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 2005;27(8):659–668. doi:10.1002/hed.20221
23. Paccagnella A, Morello M, Da Mosto MC, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. 2010;18(7):837–845. doi:10.1007/s00520-009-0717-0
24. Lundholm K, Daneryd P, Bosaeus I, Korner U, Lindholm E. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: effects on survival, metabolism, and function. Cancer. 2004;100(9):1967–1977. doi:10.1002/cncr.20160
25. Klek S, Scislo L, Walewska E, Choruz R, Galas A. Enriched enteral nutrition may improve short-term survival in stage IV gastric cancer patients: A randomized, controlled trial. Nutrition. 2017;36:46–53. doi:10.1016/j.nut.2016.03.016
26. Capuano G, Grosso A, Gentile PC, et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck. 2008;30(4):503–508. doi:10.1002/hed.20737
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
