Back to Journals » Journal of Multidisciplinary Healthcare » Volume 9
Systematic, early identification of dementia and dementia care management are highly appreciated by general physicians in primary care – results within a cluster-randomized-controlled trial (DelpHi)
Authors Thyrian JR , Eichler T, Pooch A, Albuerne K, Dreier A, Michalowsky B , Wucherer D, Hoffmann W
Received 9 September 2015
Accepted for publication 13 November 2015
Published 19 April 2016 Volume 2016:9 Pages 183—190
DOI https://doi.org/10.2147/JMDH.S96055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jochen René Thyrian,1,* Tilly Eichler,1,* Andrea Pooch,1 Kerstin Albuerne,1 Adina Dreier,2 Bernhard Michalowsky,1 Diana Wucherer,1 Wolfgang Hoffmann1,2
1German Center for Neurodegenerative Diseases (DZNE), Site Greifswald, WG Interventional Health Care Research, Greifswald, 2Department of Epidemiology of Health Care and Community Health, Institute for Community Medicine, University of Greifswald, Greifswald, Germany.
*These authors contributed equally to this work
Background: There is evidence about the benefits of early detection of dementia and subsequent provision of adequate treatment and care. However, there is a lack of knowledge about the acceptance of detection and intervention procedures. These analyses describe the attitudes of general physicians [GPs] toward 1) dementia in general, 2) systematic detection of people with dementia, and 3) an intervention approach after they have experienced both. Comparisons are made based on experience with systematic screening and dementia-specific intervention.
Methods: Postal, cross-sectional survey to all n=1,252 GPs in the Mecklenburg-Western Pomerania, Germany. A subsample was drawn based on participation in the randomized, controlled, prospective intervention DelpHi-MV trial (Dementia: life- and person-centered help in Mecklenburg-Western Pomerania). In this trial, systematic screening is implemented and an intervention group receives support through dementia care management (DCM). GPs were categorized into either GPs with DCM and systematic screening (DCM-GP), GPs with systematic screening only (DelpHi-GP), or GPs not participating in the trial. Data from n=257 GPs were available. Attitudes toward dementia were assessed using a validated questionnaire.
Results: There was strong agreement toward the helpfulness of implementing a brief cognitive screening test (89.9% agreed). Approximately two-thirds of the respondents indicated that they had identified at least some patients as being cognitively impaired for the first time. The majority of the respondents indicated agreement toward DCM. It was described as supportive and helpful. The qualified nurses were perceived as competent in dementia care and 79.3% would like to be supported with DCM. Attitudes toward dementia are positive and do not differ between groups.
Conclusion: The results indicate that early recognition and DCM is highly appreciated by GPs and is considered feasible or wanted to be implemented in routine care.
Keywords: dementia care management, screening, general physician, primary care, implementation
Background
In Germany, there are approximately 1.5 million people living with dementia. Adequate treatment and support of these people is a major challenge for the health care system. Among the specific challenges, the early detection of symptomatic dementia as well as the integration of the patients into the existing structures are provided by the health care system. The majority of people with dementia live at home1 and more than 99% of patients with dementia (PWD) living at home consult their primary care physician at least once a year.2 Thus, the appropriate setting for the early detection of dementia and the integration into the dementia-specific treatment seems to be the general physician (GP).3 There is empirical evidence that GPs consider dementia care as a relevant topic and attitudes toward caring for people with dementia are positive.4 GPs have been described to be dedicated to and concerned with caring for their PWD.5 However, surveys indicate that there is a need to improve the detection process, the inclusion of caregivers, with a very few participants agreeing that this is already happening to the appropriate degree.
Early detection of symptomatic dementia is one of the most important determinants for the therapy of the disease.6 The World Alzheimer report 2011 states that the earlier a diagnosis is known for that the patients can be treated better medically, patients and their family members can adapt to the disease and learn to deal with it and its sequela.7 This concept is adapted in current guidelines which highlight the necessity of a formal dementia diagnosis for appropriate syndromic and etiological treatment.8 Early diagnosis also leads to higher cost-effectivity, which will further improve, if treatments and social care interventions become more effective in the future.9 In a primary care setting in Germany, only approximately 40% of patients screened positive for dementia in primary care had been formally diagnosed with dementia.10 Clearly, detection of symptomatic dementia in primary care needs to be improved. However, the implementation of routine screening for dementia is discussed controversially;11–13 and not recommended in current dementia guidelines, because there is still a lack of evidence that patients benefit from it.14–17
Conducting systematic screenings increases dementia diagnoses in routine care.18,19 One screening study has been conducted to analyze the diagnostic accuracy of brief screening instruments to detect dementia in community-dwelling people or primary care patients.14 While the diagnosis of dementia has often been associated with fears and stigma in an anecdotal study,7 approximately 70% of older German people agree to be screened for dementia.20,21 In USA, even more than 80% are interested in screenings.22 However, there are important disadvantages of systematic screenings. First, screenings are limited in their use, since most of the screenings neither provide norms for age, sex, and education nor can they provide clues toward differential diagnoses. Second, their limited specificity leads to a high rate of false positives.23,24 False-positive diagnoses can give rise to unnecessary medical interventions and costs for the health care system and may cause negative personal consequences for the patients and their relatives, including fears and social stigma.
While there is evidence about the benefits of early detection and subsequent provision of adequate treatment and care,25 there is a lack of knowledge about the acceptance of detection and intervention procedures among people applying those or being affected by those, ie, the care provider. Most studies assess attitudes toward screening without indicating whether or not there has been any previous experience with it. Furthermore, studies relate early detection with improved outcomes without giving details about the means how these were achieved. This information however, is crucial to the implementation process of new concepts.
An innovative concept to encounter these challenges is the provision of dementia care management (DCM).26–28 DCM aims to provide “optimum care” by integrating multi-professional and multimodal strategies to individualize and optimize treatment of dementia within the framework of the established health care and social service system. The intervention is conducted by Dementia Care Managers – nurses with dementia-specific training – at the people’s homes after having been systematically detected by GPs in their routine care. Based on German guidelines for evidence-based diagnoses and the treatment of dementia, in a first step, a comprehensive assessment of the care situation, needs and resources is conducted. After this, the DCM develops and implements an intervention plan tailored to the individual conditions and unmet needs in close cooperation with the GP and monitors its implementation. This concept is being evaluated in primary care close to routine since 2012 involving more than 130 GPs who gained experience regarding systematic detection and approximately half of them also in providing integrated care based on DCM.
Therefore, the aims of the current analyses are: 1) to describe attitudes toward systematic detection of people with dementia by GPs with experience in conducting screening in routine care, 2) to describe attitudes toward an intervention approach (DCM) to integrate people with dementia into the care system by GPs with experience with this approach in routine care, and 3) to compare general attitudes toward dementia care between GPs with specific experience in systematic detection/integrated care and GPs without this specific experience.
Methods
Sample
This analysis is part of the on-going, randomized, controlled, prospective intervention DelpHi-MV trial (Dementia: life- and person-centered help in Mecklenburg-Western Pomerania [MV]28) involving more than 130 primary care physician practices26–29 (Trial registration: NCT01401582; approved by the Ethical Committee of the Chamber of Physicians of Mecklenburg-Western Pomerania, registry number BB 20/11). Using the address lists of the Association of Statutory Health Insurance Physicians (Kassenärztlichen Vereinigung; KV) and the Medical Association (Ärztekammer MV; ÄK) all n=1,252 general physicians (GPs) in private practice were identified, contacted, and invited to participate in a written survey in the whole federal state of MV in February 2015. GP practices who had not responded after 4 weeks were reminded of their opportunity to participate by mail. Until termination of the survey in April 2015, n=257 GPs had participated, yielding a response rate of 20.5%.
A subsample of n=134 GPs was defined upon their participation into the DelpHi-trial as “DelpHi-GP”. DelpHi-GP received additional questions regarding attitudes toward systematic detection of people with dementia. These GPs had applied a systematic screening test in routine care according to the study protocol of the trial. This screening test was the DemTect, a brief cognitive screening test, validated and often used in primary care in Germany.30 Until the termination of the survey in April 2015, n=70 DelpHi-GP had participated, yielding a response rate of 52.2%.
A further subsample of 79 GPs was defined upon their participation into the intervention arm of DelpHi-trial into “DCM-GP”. DCM-GP received additional questions regarding attitudes toward DCM. These GPs had participated in the intervention arm of the DelpHi-study, which means they were supplied or had access to a nurse specifically trained in DCM. Until termination of the survey in April 2015, n=40 DCM-GP had participated, yielding a response rate of 50.6%.
No ethical approval for this postal survey was obtained. In addition to the questionnaire, a cover letter was provided explaining on what grounds the participant was chosen (publicly available address list of GPs, or having been participant of the DelpHi-study in which the informed consent explicitly allows recontacting for further studies/analyses). Participation in this survey was voluntary. GPs were informed that they could actively decline to participate (full address details were given in the cover letter) or decline by not responding. GP practices who did not respond in any way until the time of analysis were not contacted again. There are no consequences (neither positive nor negative) associated with participation or non participation in this survey. Subsequent to the assignment to either subgroup, all questionnaires were pseudonymized.
The respondents of this survey were on average 54 years of age and 56% of them were females. The majority of them (70%) were treating patients in single residency, GP care on average for 1,241 patients per quarter year. On average, 60 PWD were treated per quarter. They were divided equally between patients living at home and patients living in nursing homes. There were only slight differences between the “GPs in MV”, the “DelpHi-GP” and the “DCM-GP” that did not reach statistical significance. The detailed description of the total sample and the subsamples is given in Table 1.
Data assessment and analyses
The survey included data about 1) sociodemographics; 2) early identification of PWD; 3) DCM; and 4) personal views, attitudes, and competences regarding dementia (used by Thyrian and Hoffmann,4 modified according to Pentzek et al31,32 and Kaduszkiewicz et al5).
- We assessed age, sex, time since having been GPs in residency, type of practice (single, multiple GPs), geriatric qualification (yes/no), dementia-specific qualification (yes/no), average number of PWD treated per quarter, number of patients treated per quarter in total, number of PWD treated in nursing homes, number of PWD treated at home.
- Participants were asked for their agreement toward five statements regarding early detection of PWD on a 5-point Likert scale ranging from 1 “absolutely disagree” to 5 “absolutely agree”. The statements are provided in full text in Table 2. The instrument provided a field for commentaries/notes provided after each statement. These questions were only asked in participants of the DelpHi-trial (DelpHi-GP and DCM-GP).
- Participants were asked for their agreement with each of 14 statements regarding DCM on a 5-point Likert scale ranging from 0 “absolutely disagree” to 5 “absolutely agree”. The statements are provided in full text in Table 3. These questions were only asked in participants of the DelpHi-trial that were supported by DCM (DCM-GP). There was a field for commentaries/notes provided after each statement. Furthermore, the respondent was asked three questions to state advantages and disadvantages of specific parts of DCM and one open question for additional tasks the participant wanted DCM to take on. We excluded n=21 participants from this survey who did not include any PWD during the study period since the purpose of our assessment was to evaluate actual experiences with DCM.
- The questionnaire about personal views and attitudes consisted of 25 items yielding eight subscales according to a version used by Thyrian and Hoffmann.4 Eight items pertain to GPs personal views with the two factors “attitude” (four items) and “confidence” (four items).32 A total of 16 items were chosen to reflect general attitude (four items), the detection process (four items), caregivers (three items), self-help (two items), guidelines and continued education (two items), and competence (one item).5 All items are formulated like statements the respondent can agree or disagree (eg, Early recognition of dementia is beneficial to the health and well-being of the patient; It is a rewarding task for me as a GP to take care of the situation of my PWD). Statements were rated on a 5-point Likert scale from 1 “strongly disagree” to 5 “strongly agree” to reduce confusion in the participants. For each scale assessed, we calculated the mean of agreement with the items with 1 indicating a low agreement and 5 being the highest possible agreement.
The data were analyzed using descriptive statistics. Bivariate comparisons between groups were calculated using t-test or χ2 test depending on the level of measurement. Comparisons between all groups were calculated using χ2 test or η2 test depending on the level of measurement (categorial vs interval). Only statistically significant differences between groups are provided in this analysis and presented in the text and tables. We also analyzed the association between the attitudes examined and the characteristics of the participants in a multivariate analysis of variance with age/years in residency, sex, specific qualification, type of practice, number of patients/PWD treated per quarter as independent variable and respective attitude as dependent variable.
Results
The majority of the respondents agreed with the various statements of early detection in primary care. There was strong agreement toward the helpfulness of implementing a brief cognitive screening test (M=4.4, 89.9% agreed). The agreement toward the feasibility of the screening test used in the study was high (m=3.9) with 85.6%. When asked whether a systematic screening for all elderly patients is useful, agreement was similarly high (m=3.8) with disagreement of approximately 13%. There was a difference between DCM-GP and DelpHi-GP in this item with 70% of the DCM-GP agreeing while only 55.2% of the DelpHi-GP are agreeing. However, the difference did not reach statistical significance. The majority of respondents indicated that they would keep on using brief screening tests for early identification (m=4.3) even after the DelpHi-trial, again with higher agreement in the group of the DCM-GP (85%) compared to the DelpHi-GP (72.4%). However, the difference did not reach statistical significance. Approximately two-thirds of the respondents indicated that they had identified at least some patients as being cognitively impaired for the first time by using the screening test. The detailed results are given in Table 2.
The majority of the respondents indicated agreement toward statements supporting DCM. The mean agreement ranged from the lowest 3.3 to the highest 4.4. The percentage of respondents agreeing to these statements ranged from 53.6% to 92.5%. The highest proportion of disagreement was stated regarding the usefulness of pharmaceutical recommendations given by pharmacists (32.2%). The detailed results are given in Table 3.
In general, the attitude toward dementia was slightly positive (ranging between 2.9 and 3.7) and differed only slightly between the groups under analysis. The attitude toward dementia detection and treatment, as defined by Pentzek et al,32 is higher in the DCM-GP than in the DelpHi-GP (3.7 vs 3.4) but does not reach a statistically significant difference (T=1.55, df =67, P=0.13). The same was true for the attitude toward the detection process, as measured according to Kaduszkiewicz et al5 (3.8 vs 3.3, T=1.46, df =66, P=0.15). In both cases, the GPs in MV showed an attitude similar to the DCM-GP, seeing dementia detection and treatment and the detection process more positive (3.6 and 3.7, respectively). There were only marginal differences toward the sample of GPs in Mecklenburg-Western Pomerania analyzed in 2011. The detailed results are given in Table 4. There is no statistically significant association between characteristics of the respondents and any attitudes under examination. The regression analyses did not reach significant results.
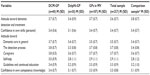  | Table 4 Attitudes toward dementia detection, treatment and care of general physicians (GPs) in Germany |
Conclusion
To our knowledge, this was the first study to analyze attitudes toward dementia detection and treatment in primary care in Germany with GPs having conducted systematic screening/identification and experienced subsequent, systematic, comprehensive DCM. The results indicated that a procedure for early recognition was highly appreciated by GPs and was considered feasible to be implemented in routine care. The GPs indicated that systematic screening helps to identify people with cognitive impairments the GPs did not identify before. There was high agreement to extend the systematic identification procedure even after recruitment for the trial has stopped. Agreement toward the implementation of a systematic identification tool, however, seemed to be higher in GPs who were offered DCM. This is an important evidence to be considered in the discussion about pros and cons of the implementation of early identification or screening for dementia. Our results implied that the attitude toward early detection is more positive in GPs who have a systematic access to adequate subsequent treatment and care as provided in the intervention group of the DelpHi-study. It made sense to the GPs to systematically identify PWD in routine care, but the consequences have to be taken care of in a similar systematic way.
DCM as defined and conducted in this study (DCM26–28) has been supporting GPs and was considered as a relieve in routine care by participating GPs. The integral parts of the DCM such as home visits, systematically identifying care needs, systematic and written feedback to the GP, evidence-based recommendations, etc were well accepted and appreciated. The GP thought that the patients and caregivers benefit from DCM likewise. The specifically qualified nurses were regarded as competent; time spent with them was considered as a useful investment. A continuation of the cooperation and even the introduction of DCM as a basic service in routine care are requested by the majority of the respondents.
However in interpreting our results, some methodological limitations have to be considered. First, we cannot rule out that the agreement toward study procedures was distorted by social desirability. Even though participation in the survey was voluntary and the data analysis was pseudonymized, respondents might have tended to more positive statements since they knew personally the authors of the survey as well as the study staff whose work was to be rated. Second, a selection bias in our sample under analysis might be a threat to the validity of our results. One selection bias could be linked to the recruitment procedure of the original trial. We cannot rule out that GPs who were already more supportive of early recognition or DCM participated in our trial since participation was voluntary. However, such a selection bias should have caused rather more and bigger differences in measuring attitudes and comparing the total sample of GPs and the GPs in our trial. Another selection bias could be due to the fact that not all of the GPs participating in the DelpHi-trial answered the survey. It might be that GPs disagreeing more with the early recognition or DCM did rather not answer the questionnaire. While this possibility cannot be ruled out, a response rate of over 50% is considerably high for a postal survey in primary care. Furthermore, the questionnaire gave the opportunity to express disagreement as much as agreement, so that we do not think that our results are skewed to any major extent. However, in the light of that, we carefully draw our conclusions, eg, by referring to the respondents more than referring to the GP in general. The second limitation of our study is that we draw conclusions from personal statements that are not backed by objective data. While this is the nature of surveys, it still has to be considered in drawing conclusions and implications for routine care. Even if the GP thinks that, eg, the patient benefits from DCM, our analysis does not provide proof of actual benefits. However, since this survey is part of a bigger trial, we will be able to evaluate the procedures of early recognition and DCM with objective data, once the study is finished. For implementation purposes, both are important–an effective and efficient intervention as well as the positive attitude by the GP toward this intervention as explained in this article.
Author contributions
JRT conceived and designed the study, conducted the analyses, and drafted the manuscript, TE designed the study, developed the survey, and was involved in drafting the manuscript, AP and KA made substantial contribution to data acquisition and data analyses, and critically revised the manuscript. AD DW, and BM made substantial contributions to the conception of the study and were involved in drafting the manuscript. WH made substantial contributions to interpretation of data and was involved in critically revising the manuscript. All authors have given final approval of the version to be published.
Acknowledgments
The authors like to thank the participating GPs for their support. The study was funded by the German Center for Neurodegenerative Diseases (DZNE).
Disclosure
The authors report no conflicts of interest in this work.
References
Bickel H. Epidemiologie und Gesundheitsökonomie [Epidemiology and health economy]. Stuttgart: Thieme; 2012:18–35. German. | |
Schwarzkopf L, Menn P, Leidl R, et al. Excess costs of dementia disorders and the role of age and gender – an analysis of German health and long-term care insurance claims data. BMC Health Serv Res. 2012;12:165. | |
Wagner G, Abholz H. Diagnose und Therapiemanagement der Demenz in der Hausarztpraxis [Diagnosis and therapy management in GP-practice]. Z Allgemeinmed. 2002;78(5):239–244. German. | |
Thyrian JR, Hoffmann W. Dementia care and general physicians-a survey on prevalence, means, attitudes and recommendations. Cent Eur J Public Health. 2012;20(4):270–275. | |
Kaduszkiewicz H, Wiese B, van den Bussche H. Self-reported competence, attitude and approach of physicians towards patients with dementia in ambulatory care: results of a postal survey. BMC Health Serv Res. 2008;8:54. | |
Solomon PR, Murphy CA. Should we screen for Alzheimer’s disease? a review of the evidence for and against screening Alzheimer’s disease in primary care practice. Geriatrics. 2005;60(11):26–31. | |
Prince M, Bryce R, Ferri C. World Alzheimer Report 2011 – The Benefits of Early Diagnosis and Intervention. London: Alzheimer’s Disease International (ADI); 2011. | |
Deutsche Gesellschaft für Psychiatrie PuND, Deutsche Gesellschaft für Neurologie (DGN) [S-3 guideline dementia]. S-3 Leitlinie “Demenzen”. Available from: http://www.dgppn.de/fileadmin/user_upload/_medien/download/pdf/kurzversion-leitlinien/s3-leitlinie-demenz-lf.pdf; 2009. | |
Dixon J, Ferdinand M, D’Amico F, Knapp M. Exploring the cost-effectiveness of a one-off screen for dementia (for people aged 75 years in England and Wales). Int J Geriatr Psychiatry. 2015;30(5):446–452. | |
Eichler T, Thyrian JR, Hertel J, et al. Rates of formal diagnosis in people screened positive for dementia in primary care: results of the DelpHi-Trial. J Alzheimers Dis. 2014;42(2):451–458. | |
Borson S, Frank L, Bayley PJ, et al. Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement. 2013;9(2):151–159. | |
Le Couteur DG, Doust J, Creasey H, Brayne C. Political drive to screen for pre-dementia: not evidence based and ignores the harms of diagnosis. BMJ. 2013;347:f5125. | |
Brunet MD, McCartney M, Heath I, et al. There is no evidence base for proposed dementia screening. BMJ. 2012;345:e8588. | |
Lin JS, O’Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;159(9):601–612. | |
UK National Screening Committee (UK NSC). The UK NSC policy on Alzheimer’s Disease screening in adults. Available from: http://www.Legacy.Screening.Nhs.uk/dementia; 2010. Accessed September 1, 2015. | |
US Preventive Services Task Force. Screening for cognitive impairment in older adults. Available from: http://www.uspreventiveservicestaskforce.org/page/document/updatedsummaryfinal/congnitive-impairment-in-older-adults-screening; 2014. Accessed September 1, 2015. | |
Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin eV (DEGAM). DEGAM-Leitlinie Nr 12: Demenz. Düsseldorf: Omikron Publishing; 2008. | |
Borson S, Scanlan J, Hummel J, Gibbs K, Lessig M, Zuhr E. Implementing routine cognitive screening of older adults in primary care: process and impact on physician behavior. J Gen Intern Med. 2006;22(6):811–817. | |
Eichler T, Thyrian JR, Hertel J, et al. Rates of formal diagnosis of dementia in primary care: the effect of screening. Cogn Behav Assess. 2015;1(1):87–93. | |
Braun SR, Reiner K, Tegeler C, Bucholtz N, Boustani MA, Steinhagen-Thiessen E. Acceptance of and attitudes towards Alzheimer’s disease screening in elderly German adults. Int Psychogeriatr. 2014;26(3):425–434. | |
Holsinger T, Boustani M, Abbot D, Williams JW. Acceptability of dementia screening in primary care patients. Int J Geriatr Psychiatry. 2011;26(4):373–379. | |
Fowler NR, Boustani MA, Frame A, et al. Effect of patient perceptions on dementia screening in primary care. J Am Geriatr Soc. 2012;60(6):1037–1043. | |
Mitchell AJ, Meader N, Pentzek M. Clinical recognition of dementia and cognitive impairment in primary care: a meta-analysis of physician accuracy. Acta Psychiatr Scand. 2011;124(3):165–183. | |
Boustani M, Callahan CM, Unverzagt FW, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20(7):572–577. | |
Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer Report 2014 – Dementia and Risk Reduction, An Analysis of Protective and Modifiable Risk Factors. London: Alzheimer’s Disease International (ADI); 2014. | |
Eichler T, Thyrian JR, Dreier A, et al. Dementia care management: going new ways in ambulant dementia care within a GP-based randomized controlled intervention trial. Int Psychogeriatr. 2014;26(2):247–256. | |
Fiss T, Thyrian JR, Wucherer D, et al. Medication management for people with dementia in primary care: description of implementation in the DelpHi study. BMC Geriatr. 2013;13(1):121. | |
Thyrian JR, Fiss T, Dreier A, et al. Life- and person-centred help in Mecklenburg-Western Pomerania, Germany (DelpHi): study protocol for a randomised controlled trial. Trials. 2012;13:56. | |
Eichler T, Thyrian JR, Dreier A, et al. Dementia Care Management: Neue Wege in der ambulanten Demenzversorgung – ein Fallbeispiel. ZFA. 2015;1(91):31–37. | |
Kalbe E, Kessler J, Calabrese P, et al. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry. 2004;19(2):136–143. | |
Pentzeck M, Wagner G, Abholz HH. Die Entwicklung eines Wissenstests für Hausärzte zum Thema Demenz [The development of a dementia knowledge test for general practitioners]. Z Ärztl Fortbild Qualitätssich. 2006;100(4):283–289. German. | |
Pentzek M, Abholz HH, Ostapczuk M, Altiner A, Wollny A, Fuchs A. Dementia knowledge among general practitioners: first results and psychometric properties of a new instrument. Int Psychogeriatr. 2009;21(6):1105–1115. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



