Back to Journals » Cancer Management and Research » Volume 15
Synthesis of Gingerol-Metals Complex and in-vitro Cytotoxic Activity on Human Colon Cancer Cell Line
Authors Khdary NH , Alangari AA, Katubi KM, Alanazi M, Alhassan A, Alzahrani SD, Khan Z, Alanazi IO
Received 4 October 2022
Accepted for publication 4 January 2023
Published 27 January 2023 Volume 2023:15 Pages 87—98
DOI https://doi.org/10.2147/CMAR.S391546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Nezar H Khdary,1 Abdulaziz A Alangari,2 Khadijah M Katubi,3 Mohammad Alanazi,4 Ahmed Alhassan,1 Sami D Alzahrani,1 Zahid Khan,4 Ibrahim O Alanazi1
1Institute of Materials Science, King Abdulaziz City for Science and Technology, Riyadh, Kingdom of Saudi Arabia; 2College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia; 3Department of Chemistry, College of Science, Princess Nourah Bint Abdulrahman University, Riyadh, Kingdom of Saudi Arabia; 4Department of Biochemistry, College of Science, King Saud University, Riyadh, Kingdom of Saudi Arabia
Correspondence: Nezar H Khdary, Institute of Materials Science, King Abdulaziz City for Science and Technology (KACST), Riyadh, Kingdom of Saudi Arabia, Tel +966-114814236, Email [email protected] Ibrahim O Alanazi, Aging institute, King Abdulaziz City for Science and Technology (KACST), Riyadh, Kingdom of Saudi Arabia, Tel +966114813289, Email [email protected]
Introduction: Herbs are excellent sources of medicinal substances, and their curative abilities have been recognized to treat many ailments and are used for example as antioxidants, analgesics, anti-inflammatories, antipyretics, and many other medicinal uses. The properties of natural compounds and their health effects have been studied extensively, especially those that originate from plant sources such as ginger. The ginger plant contains many chemical compounds, such as 6-gingerol, which is characterized by containing active groups such as carbonyl and hydroxide, which can be attached to metal molecules. This is what was done in this study, where the formation of complexes with a group of metals was studied and their effect on cancer cells was investigated. These complexes will open new horizons for further study of medicinal uses.
Methods: The synthesis of gingerol-metal complexes was carried out by conjugating gingerol molecules with Ag, Au, Cd, Co, Cu, Ni, and Zn metal ions. The extracted gingerol was transferred to culture tubes and deionized water-DMSO were added followed by sonication. The tubes were incubated at 90°C for two days as well as the control sample. The samples were then filtered and the complex solutions were transferred into new tubes for further studies. Different characterization techniques such as FT-IR, UV-vis spectroscopy, FESEM, and EDX are used to confirm the formation of the complexes. The in vitro of the complexes was tested by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay against the human colorectal cancer cell lines HCT116 and HT29 which exhibited strong cytotoxicity.
Results: The gingerol-metal complexes showed an enhancement as an anticancer agent compared to the control. The in vitro anticancer activity showed that the Ag-gingerol complex showed the most activity among the other complexes.
Discussion: Gingerol-metal complexes can inhibit cancer cells, noting that the potency of the complex depends on the type of metal used.
Keywords: ginger, gingerol, gingerol-complexes, colon cancer, tumor cell line
Introduction
The discovery of new materials is a major challenge in the treatment of deadly diseases and is of particular interest to researchers. The number of fatalities which is attributed to cancer diseases increases on daily basis. Since the discovery of cisplatin, the first metal-based chemotherapy drug, metal complexes with metal-carbon bonds have been used as antitumor agents.1 Thousands of platinum analogs have been developed, and cisplatin or derivatives like carboplatin or oxaliplatin are used to treat around half of all cancer patients who get chemotherapy.2 Despite this, the high toxicity and resilience of tumor cells have motivated the evolution of innovative metal-based treatments currently being tested in clinical studies. In addition, several transition metals, such as copper or ruthenium, have sparked a lot of attention as possible substitutes for platinum-based anticancer drugs in recent years.3
The development of new lead compounds for cancer therapy depends on their capacity to target various pathways (eg, apoptosis induction, cell cycle arrest, or proteasome inhibition) which makes them very promising therapeutics. DNA damage repair pathways are one of the significant ways in cancer treatment. As the human cells are bound to continuous DNA damage which subsequently leads to change or damage in genome transcription or replication. Hence, DNA repairing materials trigger the affected spots to detect the damaged sites and activate pathways for treating the damaged cells. However, one of these agents’ most distinguishing characteristics is their adaptability.4 Toxicological prevention is a term used to describe the process of avoiding exposure to many of the compounds listed in the literature that have yet to be tested in animal models for toxicity. Herbs and natural products are excellent sources of medicinal substances, and their healing capabilities and value have long been recognized.5 Natural bioactive compounds’ characteristics and health effects, particularly those originating from plant sources such as oregano, have been extensively investigated. Phytochemicals are active compounds found in medicinal plants that are not required for normal human body function but have health or illness-relieving properties.6 Gingerol is one of the most prevalent and widely used chemicals in ginger.7 The fresh roots of ginger contain a significant amount of 6-gingerol [5-hydroxy-1-(4-hydroxy-3-methoxyphenyl) decan-3-one].8
Ginger’s strong and physiologically active compounds are 6-gingerol, 6-shogaol, zingerone, phenolics, and flavonoids. 6-gingerol is the most prevalent bioactive molecule in ginger with antioxidant, analgesic, anti-inflammatory, and antipyretic properties.9,10 For example, In vitro tests were conducted by Gwang Hun Park and others to assess the anti-cancer effects of ginger leaf and then clarify any probable underlying mechanisms. They revealed that GL exposure reduced the viability of human colorectal cancer cells (HCT116, SW480, and LoVo cells) and caused apoptosis in a dose-dependent manner.11 Sahdeo Prasad and Amit K Tyagi discussed the evidence for ginger extract’s chemopreventive and chemotherapeutic potential.12 The active ingredients of ginger have been evaluated using in vitro, animal models, and patients. The active ingredients in ginger, such as 6-gingerol and 6-shogaol, have anticancer properties that are effective against GI cancer. A number of signaling molecules, including STAT3, ERK1/2, Akt, NF-B, COX-2, PI3KTNF, MMP-9, and other cell growth regulating proteins, are modulated by ginger, which is thought to have anticancer properties.12 Additional research employing ginger’s crude ethanolic extract in vitro and in animals indicates promising anticancer efficacy against Cholangiocarcinoma (CCA) without any discernible toxicity.13 Wang et al reviewed the biological aspects of 6-gingerol in vitro and in vivo. They point out that 6-gingerol is the primary pharmacologically active component of ginger.14 Through its impact on several biological pathways related to apoptosis, cell cycle regulation, cytotoxicity, and angiogenesis suppression, 6-gingerol has been demonstrated to have anticancer properties. Thus, 6-gingerol has attracted a lot of interest as a possible therapeutic agent for the prevention and/or treatment of numerous diseases due to its effectiveness.14 In this work, for the first time, the gingerol-metals complex was synthesized based on the production of metal complexes between two molecules of gingerol. The interaction of the metal ion solution with the molecule gingerol in the suitable medium was synthesized using Ag, Au, Cd, Co, Cu, Ni, and Zn ions to produce metal complex compounds and investigate their effect on the inhibition of cancer cells
Experimental Work
Instruments
FT-IR (Fourier Transform Infrared Spectroscopy) measurements were recorded under vacuum optics, using a VERTEX 70v BRUKER spectrophotometer (Bruker, Ettlingen, Germany) with a resolution of 4 cm−1 and 64 scans. The absorbance for the samples was determined using UV-3600 (Shimadzu, Kyoto, Japan). High-resolution shape and morphology imaging and elemental analysis were studied using Field Emission SEM (JSM-7800F, Jeol) and the metal microanalysis was determined using Energy Dispersive X-ray Spectroscopy (OXFORD Instrument, X-MaxN).
Chemicals
Silver sulfate was obtained from Scharlau Chemie (Spain). Nickel nitrate, copper(II) sulfate, Cadmium sulfate-hydrate, and Cobalt chloride hexahydrate were procured from Merck (UK.). Gold(III) chloride hydrate, Zinc sulfate heptahydrate, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (UK). Double DI water was utilized throughout the experiment which was obtained using a Milli-Q water filtration system.
Extraction and Synthesis
The extraction process was applied according to Ghasemzadeh et al15 method with some modifications to improve the separation process. 200g of fresh ginger was transferred to a 500 mL round-bottomed flask connected with a condenser which was followed by further addition of 200 mL ethanol and the temperature was maintained at 90°C for 24 H. After that, the mixture was filtered using microfilter paper. The light brown ethanolic extract was taken into a 500 mL flask and left to concentrate (evaporate) at room temperature under a vacuum. The purification and separation were carried out according to the Tao et al technique16 with some modifications as the silica gel column chromatography used was a 30 cm (Height),10 cm i.d. (Rotaflo, England) column packed with activated silica gel at 130°C (40–75 µm) and the final eluent for extraction was an acidic mixture of acetic acid in methanol (5:95). The final product was kept at 4°C until it is used.
Synthesis of Gingerol-Metals Complexes Using Gingerol
Gingerol-metals were synthesized as follows: Initially, 0.139822 g of the extracted product was transferred to culture tubes Soda-lime glass with DIN thread and P.P. screw cap 22 mL, deionized water, and DMSO were added (4:1) and sonicated for 45 min. The pH of the solution in each tube was adjusted to pH7. Then, 5 ml of a specific metal solution was gradually added (Table 1). The tubes were incubated at 90°C for two days as well as the control sample. Afterward, the tubes were taken from the incubator and cooled down to ambient temperature. The samples were then filtered using (FL091, Filter paper, 110 mm) and the gingerol-metals complexes solutions were transferred into new tubes and coded as (CT, S1, S2, S3, S4, S5, S6, and S7). The tubes were cooled at 4°C and stored.
 |
Table 1 The Metals Solution Utilized in Complexes |
Cell Culture and Cell Lines
The human colon cancer cell lines HTC116 and HT29 were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 50 units/mL penicillin G, and 50 μg/mL streptomycin (GIBCO) at 37 °C in a humidified atmosphere with 5% CO2.
Cytotoxicity Assay
HTC116 and HT29 colon cancer cell lines, which were purchased from ATCC (Manassas VA, USA), were plated at a density of 6000 cells/well in a 96-well plate was incubated at 37°C overnight and treated in triplicate with various concentrations of complex Materials (S1-S2-S3-S4-S5-S6-S7). After 48 h of treatment with the complex except for s1, it was incubated for 24 h to measure the cell viability based on the ability of viable cells to convert a soluble tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, MTS) to a formazan product by using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA) [34]. Soluble MTS formazan product was estimated using an ELISA microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490 nm wavelength. The 50% inhibitory concentration (IC50) values for the complexes exhibiting antiproliferative activity were calculated using publicly available software at AAT Bioquest website (https://www.aatbio.com/tools/ic50-calculator).
Results and Discussion
Fourier-Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of CTR (control sample, gingerol) and gingerol-metals complexes are illustrated in Figure 1, and the obtained bands are in Table 2. The gingerol shows bands at 3419 cm−1 which is attributed to hydroxy group stretching for phenol.17,18
 |
Table 2 The I.R. Bands for the Control Sample and Gingerol-Metals Complexes |
 |
Figure 1 The I.R. spectra of the control sample and gingerol-metals complex ; CT, S1, S2, S3, S4, S5, S6, S7. |
Stretching of the C-H atoms in symmetric and asymmetric is responsible for the absorption bands at about 2940 and 2875 cm−1, respectively.19 The hydrogen-bonded carbonyl appeared at 1719 cm−1 and the band at 1628 cm−1 corresponds to C=O stretching20 and the methyl group band appeared at 1424 cm−1. The C-O stretching vibration21 is attributed to the distinctive peak at 1387 cm−1. The hydroxy group showed a band at 1156 cm−1, whereas the stretched hydroxy group appeared at 1118 cm−1. The characteristic peak at 712 cm−1 is ascribed to the C=C in the benzene ring.22–24 Metal complexes in the instance of gingerol-associated metals caused the appearance of new bands and the displacement of existing bands from their initial positions to lower values. For example, the band at 2940 was shifted to 2928 cm−1 and the characteristic peak at 2875 was shifted to 2866 cm−1 and the 1714 cm−1 band shifted to 1726 cm−1. A new band has emerged at 2366 cm−1 and 2328 cm−1, which is attributed to the hydroxy-metal bond (O-M). C-H aromatic band occurred at 1663 cm−1 and the 1628 cm−1 band shifted to 1624 cm−1. The new band at 1516 cm−1 could be attributed to C-O-M and the band at 1461 cm−1 is ascribed to C-H bending.10 The band at 1424 cm−1 shifted to 1430 cm−1 and the band at 1277 cm−1 was in the same position which is attributed to C-H aromatic. The C-H bending is accountable for the band at 1461 cm−1. The 1424 cm−1 band shifted to 1430 cm−1 and the C-H aromatic corresponds to the band at 1277 cm−1. The band at 1256 cm−1 is ascribed to C-O stretching and the band at 1118 cm−1 was relocated to 1116 cm−1 and is consigned to the vibration of C-O.25 The new band at 1516 and bands at around 567, 574, and 549 cm−1 are attributed to the C-O-M band and the band at 1232 cm−1 is indicated to the formation of (M-O) bond26 which confirms the formation of organometal complexes.
UV-Visible Spectroscopy Study
The production of complexation between metal ions and gingerol to form an gingerol-metals complex has been identified using UV-visible spectra. The wavelengths measured ranged from 230 nm to 800 nm. Figure 2 depicts the spectra of samples S1 to S7 including the control sample. All investigated samples had similar absorption for bands (I) and (II) in the interspecific study, which could be attributed to absorption overlap in this region. However, as compared to the control (gingerol) Figure 3 shows considerably different absorption values at max 243 nm in the band (II) region. The spectra of the S1 sample indicated a shift in the maximum absorption peak wavelength. The spectral examination of S1-S7 revealed absorption peaks at 230 and 242 nm, with a bit of shift in the band depending on the metal ion which is typical of complex formation.27,28
 |
Figure 3 The UV-Vis spectra of the gingerol-metals complex separately with the control, where it is noted that there is a shift on the peaks due to the formation of the complexation. |
High-Resolution Field Emission SEM and Metal Microanalysis
The morphology of the dried complexes and the metal microanalysis results are shown in Figure 4. Several peaks have been identified for the metals and organic compounds. The initial peak at Kα = 0.277 keV corresponds to carbon and the secondary peak at Kα = 0.52 keV represents oxygen. The peak at Kα = 2.984 keV characterizes Ag. The peaks at Kα 7.47 keV and Lα 0.851 keV are assigned for Ni. Cu showed two peaks at 8.04 keV for Kα and 0.93 keV for Lα. The peak at 3.133 keV is attributed to Cd and the peak at 6.92 keV and 0.76 keV is ascribed to Co Kα and Lα, respectively. The Au showed only a clear peak at 2.210 keV and Zn showed a peak for Kα at 8.630 and Lα at 1.012 keV. These results confirm the presence of metal in the structure of the complex.
 |
Figure 4 (A) SEM, EDX for Control sample (CT), S1, S2, S3. (B) SEM, EDX for S4, S5, S6 and S7. |
The previous results confirm the formation of the metal complexes and the suggested structure is shown in Figure 5, which is consistent with other studies of the metal complexes.29–31
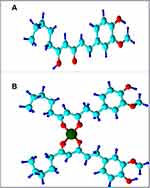 |
Figure 5 Suggested structure of the complex formation between gingerol and metal ions; (A) gingerol structure, and (B) the structure of the gingerol-metals complex. |
In-vitro Antitumorigenic Effect on Colon Cancer Cell Lines
The antineoplastic activity of the gingerol-metals complexes was experimented with by examining its cytotoxic effect on colorectal cancer cell lines HCT116 and HT-29 using MTS assay. A wide range from 2 μM to 20 μM concentrations of test compounds were used on these colorectal cancer cells. The compound S1 reveals a considerable decrease in cell viability with the increase in the concentration of the drug after 24 h treatment period in both the cell lines as shown in Figure 6. While S2 and S4 show a significant decrease in cell viability with increasing concentrations of the drugs after 48 h and the S6 complex showed cytotoxic activity at a concentration above 10 μM as shown in Figure 7. The highest tested concentration of 20 μM shows a decrease of 23% in the viability in both the cell lines. The IC50 for the metal complexes on HCT116 and HT-29 cell lines are displayed in Table 3. Compared to HCT116, the HT-29 cells showed higher sensitivity to the complex. Pranzini et al indicated that 5-Fluorouracil (5-FU) is a primary standard chemotherapy drug for the management of multiple solid malignancies, comprising advanced and metastatic colorectal cancer (CRC). 5-FU shows its toxicity on cancer cells by interfering with nucleotide biosynthesis and nucleotide pool composition, primarily by inhibiting thymidylate synthase. Their data show that after 48 hours, the 5-FU IC50 for HCT-116 and HT-29 is around 3.2 µM and 1.6 µM, respectively.32 While our results for S1, after 24 hours show 5.24 µM for HCT-116 and 3.185 µM for HT-29, respectively, for the half-maximal inhibitory concentration (IC50). The effect of complexing N- carbene ligand with silver(I) was studied by Huque et al revealed that the Ag-complex increase the efficiency against the HCT 116 and HT29 cell lines.33 In another study by Mohamed et al, silver(I)-NHC complexes show far less effect compared to our data against HCT 116 and HT29 cell lines.34 The S3, S5 as well as S7 gingerol-metals complexes in our data did not demonstrate cytotoxic activity in the colorectal cancer cells.
 |
Table 3 IC50, the Measure of the Effectiveness of a Substance in Inhibiting a Specific Biological or Biochemical Function |
 |
Figure 6 Continued. |
 |
Figure 7 Continued. |
The antitumor properties of Ag(I) ions are continuously explored in various types of human cancer cells including pancreatic cancer cell PANC-1, lung cancer cells H1299 and breast cancer. It has been noticed that the antitumor characteristics of Ag(I) ions are implicated in a wide range of mechanisms including DNA damage35 stimulation of cell apoptosis, necrosis, and autophagy36,37 and the generation of reactive oxygen species (ROS)38 and in the inhibition of cell migration and invasion.39 Several studies indicated that silver(I) complexes show better cytotoxicity against tumor cells including myeloid leukemia cells K562, MCF-7 breast cancer, colorectal Adenocarcinoma Cells Caco-2 cells, and human Hepatoma HepG2.40,41 Our study suggested that gingerol-metal complex-Ag has the potential to be developed as chemotherapeutic agent against colorectal cancers.
Conclusions
Developing an efficient material with a valid target for the cancer cells is critical. In the recent past, gingerol-metals complexes have been synthesized with natural products which possess great potency in the treatment of cancer treatments. In the current study, a novel series of gingerol-metals complexes were investigated and the complexation is confirmed by applying different techniques. The results demonstrated that gingerols’ efficiency was improved when it integrated with metal ions. The activity depends on the metal type (Ag, Au, Cd, Co, Cu, Ni, and Zn). For example, the gingerol-metals complex-Ag showed the most activity among the other complexes. The in-vitro anticancer activity was evaluated using HCT116 and HT29 human colorectal cancer cell lines. The gingerol-metals complexes (S1) showed significant inhibition in cell viability after 24 h of the treatment period in HT-29 cells and HCT116 cell lines with half-maximal inhibitory focus (IC50). The effect of the prepared materials is based on unique chemical composition background with an environment friendly for cancer therapy. The results suggest that S1 has the potential to be applied as a chemotherapeutic synthetic drug for colorectal cancer. Finally, the prepared materials can be progressively developed for improvement in cancer treatment.
Acknowledgments
The Authors express their great thanks to King Abdulaziz City for Science and Technology for technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Heras BL, Amesty Á, Estévez-Braun A, Hortelano S. Metal complexes of natural product like-compounds with antitumor activity. Anticancer Agents Med Chem. 2018;19:48–65. doi:10.2174/1871520618666180420165821
2. Frezza M, Hindo S, Chen D, et al. Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des. 2010;16(16):1813–1825. doi:10.2174/138161210791209009
3. Gałczyńska K, Drulis-Kawa Z, Arabski M. Antitumor activity of Pt(II), Ru(III) and Cu(II) complexes. Molecules. 2020;25:1–14. doi:10.3390/molecules25153492
4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
5. Atanasov AG, Zotchev SB, Dirsch VM, et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–216. doi:10.1038/s41573-020-00114-z
6. Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42. doi:10.3390/plants6040042
7. Foudah AI, Shakeel F, Yusufoglu HS, Ross SA, Alam P. Simultaneous determination of 6-shogaol and 6-gingerol in various ginger (Zingiber officinale Roscoe) extracts and commercial formulations using a green RP-HPTLC-densitometry method. Foods. 2020;9(8):1136. doi:10.3390/foods9081136
8. Antoniewicz (Kałduńska) J, Jakubczyk K, Gutowska I, Janda K. Ginger (Zingiber officinale) – a spice with therapeutic properties. Med Ogólna Nauki Zdr. 2021;27:40–44. doi:10.26444/monz/134013
9. Shirin Adel PR, Prakash J. Chemical composition and antioxidant properties of ginger root (Zingiber officinale). J Med Plants Res. 2010;4:2674–2679. doi:10.5897/JMPR09.464
10. Abu-Dief AM, Mohamed IMA. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ J Basic Appl Sci. 2015;4:119–133. doi:10.1016/j.bjbas.2015.05.004
11. Park GH, Park JH, Song HM, et al. Anti-cancer activity of Ginger (Zingiber officinale) leaf through the expression of activating transcription factor 3 in human colorectal cancer cells. BMC Complement Altern Med. 2014;14:408. doi:10.1186/1472-6882-14-408
12. Prasad S, Tyagi AK. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract. 2015;2015. doi:10.1155/2015/142979
13. Plengsuriyakarn T, Viyanant V, Eursitthichai V, et al. Cytotoxicity, toxicity, and anticancer activity of Zingiber officinale Roscoe against cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13(9):4597–4606. doi:10.7314/apjcp.2012.13.9.4597
14. Wang S, Zhang C, Yang G, Yang Y. Biological properties of 6-gingerol: a brief review. Nat Prod Commun. 2014;9:1027–1030.
15. Ghasemzadeh A, Jaafar HZE, Rahmat A. Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology. BMC Complement Altern Med. 2015;15:1–10. doi:10.1186/s12906-015-0718-0
16. Tao YI, Li W, Liang W, Van Breemen RB. Identification and quantification of gingerols and related compounds in ginger dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2009;57:10014–10021. doi:10.1021/jf9020224
17. Žagar E, Grdadolnik J. An infrared spectroscopic study of H-bond network in hyperbranched polyester polyol. J Mol Struct. 2003;658:143–152. doi:10.1016/S0022-2860(03)00286-2
18. Solcà N, Dopfer O. Infrared spectra of the phenol-Ar and phenol-N 2 cations: proton-bound versus π -bound structures. Chem Phys Lett. 2000;325:354–359. doi:10.1016/S0009-2614(00)00675-8
19. Queiroz MF, Melo KRT, Sabry DA, Sassaki GL, Rocha HAO. Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs. 2015;13:141–158. doi:10.3390/md13010141
20. Mezzetti A, Spezia R. Time-resolved step scan FTIR spectroscopy and DFT investigation on triplet formation in peridinin-chlorophyll-a-protein from amphidinium carterae at low temperature. Spectroscopy. 2008;22:235–250. doi:10.1155/2008/682046
21. Asyana V, Haryanto F, Fitri LA, et al. Analysis of urinary stone based on a spectrum absorption FTIR-ATR. J Phys Conf Ser. 2016;694:012051. doi:10.1088/1742-6596/694/1/012051
22. Torrisi L, Venuti V, Crupi V, et al. RBS, PIXE, ion-microbeam and SR-FTIR analyses of pottery fragments from Azerbaijan. Heritage. 2019;2(3):1852–1873. doi:10.3390/heritage2030113
23. Gonultas O, Candan Z. Chemical characterization and ftir spectroscopy of thermally compressed eucalyptus wood panels. Maderas Cienc Tecnol. 2018;20:431–442.
24. Ramos JM, T. de M. Cruz MT, Costa AC, Versiane O, Soto CAT. Fourier transform infrared spectrum: vibrational assignments using density functional theory and natural bond orbital analysis of the bis(guanidoacetate) nickel(II) complex. Sci Asia. 2011;37(3):247–255. doi:10.2306/scienceasia1513-1874.2011.37.247
25. Tavacoli BJW, Bauër P, Fermigier M, Bartolo D. Electronic Supplementary Material (ESI) for soft matter; 2013:1–5.
26. Chukanov NV, Chervonnyi AD. IR Spectra of Minerals and Related Compounds, and Reference Samples’ Data. Springer; 2016. doi:10.1007/978-3-319-25349-7_2
27. Khedr AM, Issa RM, El-Kamary MA, Hassan RB. Rapid and simple spectrophotometric determination of Mn(II), Fe(III), Co(II), Ni(II) and Cu(II) ions in natural samples using 2-(2-hydroxynaphth-1- ylazo)-pyridine. Egypt J Chem. 2010;53:885–902.
28. Guidote AJM, Reyes RL, Kashihara R, Kurusu Y, Masuyama Y. Electronic effects in oxidation reactions utilizing dinuclear copper complexes with the Bis[3-(2-hydroxybenzylideneamino)phenyl] sulfone ligand. Kimika. 2014;25(2):11–22. doi:10.26534/kimika.v25i2.11-22
29. Scimeca M, Bischetti S, Lamsira HK, Bonfiglio R, Bonanno E. Energy dispersive X-ray (EDX) microanalysis: a powerful tool in biomedical research and diagnosis. Eur J Histochem. 2018;62:89–99.
30. Naseem S, Gevers B, Boldt R, Labuschagné FJWJ, Leuteritz A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019;9:3030–3040. doi:10.1039/C8RA10165E
31. Starlin T, Ragavendran P, Raj CA, Perumal PC, Gopalakrishnan VK. Element and functional group analysis of Ichnocarpus frutescens R.Br. (Apocynaceae). Int J Pharm Pharm Sci. 2012;4:343–345.
32. Pranzini E, Pardella E, Muccillo L, et al. SHMT2-mediated mitochondrial serine metabolism drives 5-FU resistance by fueling nucleotide biosynthesis. Cell Rep. 2022;40(7):111233. doi:10.1016/j.celrep.2022.111233
33. Haque RA, Choo SY, Budagumpi S, Iqbal MA, Al-Ashraf Abdullah A. Silver(I) complexes of mono-and bidentate N-heterocyclic carbene ligands: synthesis, crystal structures, and in vitro antibacterial and anticancer studies. Eur J Med Chem. 2015;90:82–92. doi:10.1016/j.ejmech.2014.11.005
34. Mohamed HA, Lake BRM, Laing T, Phillips RM, Willans CE. Synthesis and anticancer activity of silver(I)-N-heterocyclic carbene complexes derived from the natural xanthine products caffeine, theophylline and theobromine. Dalton Trans. 2015;44:7563–7569. doi:10.1039/C4DT03679D
35. Żyro D, Śliwińska A, Szymczak-Pajor I, Stręk M, Ochocki J. Light stability, pro-apoptotic and genotoxic properties of silver (I) complexes of metronidazole and 4-hydroxymethylpyridine against pancreatic cancer cells in vitro. Cancers. 2020;12:1–17.
36. Yuan YG, Zhang S, Hwang JY, Kong IK. Silver nanoparticles potentiates cytotoxicity and apoptotic potential of camptothecin in human cervical cancer cells. Oxid Med Cell Longev. 2018;2018:1–21. doi:10.1155/2018/6121328
37. Mani S, Balasubramanian MG, Ponnusamy P, Vijayan P. Antineoplastic effect of PAC capped silver nanoparticles promote apoptosis in HT-29 human colon cancer cells. J Clust Sci. 2019;30:483–493. doi:10.1007/s10876-019-01510-1
38. Miyayama T, Arai Y, Suzuki N, Hirano S. Mitochondrial electron transport is inhibited by disappearance of metallothionein in human bronchial epithelial cells following exposure to silver nitrate. Toxicology. 2013;305:20–29. doi:10.1016/j.tox.2013.01.004
39. Buttacavoli M, Albanese NN, Di Cara G, et al. Anticancer activity of biogenerated silver nanoparticles: an integrated proteomic investigation. Oncotarget. 2018;9(11):9685–9705. doi:10.18632/oncotarget.23859
40. Ota A, Tajima M, Mori K, et al. The selective cytotoxicity of silver thiosulfate, a silver complex, on MCF-7 breast cancer cells through ROS-induced cell death. Pharmacol Rep. 2021;73(3):847–857. doi:10.1007/s43440-021-00260-0
41. Żyro D, Radko L, Śliwińska A, et al. Multifunctional Silver(I) complexes with metronidazole drug reveal antimicrobial properties and antitumor activity against human hepatoma and colorectal adenocarcinoma cells. Cancers. 2022;14(4):900. doi:10.3390/cancers14040900
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.