Back to Journals » Drug Design, Development and Therapy » Volume 17
Synthesis and Biological Evaluation of Gentiopicroside Derivatives as Novel Cyclooxygenase-2 Inhibitors with Anti-Inflammatory Activity
Authors Ren G , Zhang Q, Xia P , Wang J , Fang P , Jin X , Peng X, Xu Y , Zhang J , Zhao L
Received 3 December 2022
Accepted for publication 7 March 2023
Published 23 March 2023 Volume 2023:17 Pages 919—935
DOI https://doi.org/10.2147/DDDT.S398861
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Guojin Ren,1,* Qili Zhang,1,2,* Pengfei Xia,1– 4 Jie Wang,1 Pengxia Fang,1 Xiaojie Jin,1 Xuejing Peng,1– 4 Yanli Xu,1,5 Jian Zhang,1– 4 Lei Zhao1– 5
1Gansu University of Chinese Medicine, Lanzhou, 730000, People’s Republic of China; 2Northwest Collaborative Innovation Center for Traditional Chinese Medicine Co-Constructed by Gansu Province & MOE of PRC, Lanzhou, 730000, People’s Republic of China; 3Key Laboratory of Chemistry and Quality of TCM of the College of Gansu Province, Lanzhou, 730000, People’s Republic of China; 4Gansu Province Engineering Laboratory for TCM Standardization Technology and Popularization, Lanzhou, 730000, People’s Republic of China; 5Lanzhou Institute for Food and Drug Control, Lanzhou, 730000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Zhao; Jian Zhang, Email [email protected]; [email protected]
Purpose: Nonsteroidal anti-inflammatory drugs cause a series of adverse reactions. Thus, the search for new cyclooxygenase-2 selective inhibitors have become the main direction of research on anti-inflammatory drugs. Gentiopicroside is a novel selective inhibitor of cyclooxygenase-2 from Chinese herbal medicine. However, it is highly hydrophilic owing to the presence of the sugar fragment in its structure that reduces its oral bioavailability and limits efficacy. This study aimed to design and synthesize novel cyclooxygenase-2 inhibitors by modifying gentiopicroside structure and reducing its polarity.
Materials and Methods: We introduced hydrophobic acyl chloride into the gentiopicroside structure to reduce its hydrophilicity and obtained some new derivatives. Their in vitro anti-inflammatory activities were evaluated against NO, TNF-α, PGE2, and IL-6 production in the mouse macrophage cell line RAW264.7 stimulated by lipopolysaccharide. The in vivo inhibitory activities were further tested against xylene-induced mouse ear swelling. Molecular docking predicted that whether new compounds could effectively bind to target protein cyclooxygenase-2. The inhibitory activity of new compounds to cyclooxygenase-2 enzyme were verified by the in vitro experiment.
Results: A total of 21 novel derivatives were synthesized, and exhibit lower polarities than the gentiopicroside. Most compounds have good in vitro anti-inflammatory activity. The in vivo activity results demonstrated that 8 compounds were more active than gentiopicroside. The inhibition rate of some compounds was higher than celecoxib. Molecular docking predicted that 6 compounds could bind to cyclooxygenase-2 and had high docking scores in accordance with their potency of the anti-inflammatory activity. The confirmatory experiment proved that these 6 compounds had significant inhibitory effect against cyclooxygenase-2 enzyme. Structure-activity relationship analysis presumed that the para-substitution with the electron-withdrawing groups may benefit the anti-inflammatory activity.
Conclusion: These gentiopicroside derivatives especially PL-2, PL-7 and PL-8 may represent a novel class of cyclooxygenase-2 inhibitors and could thus be developed as new anti-inflammatory agents.
Keywords: gentiopicroside derivatives, structural modification, anti-inflammatory, cyclooxygenase-2 inhibitors, molecular docking
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are well-known first-line drugs in the treatment of acute and chronic inflammation, including osteoarthritis (OA) and rheumatoid arthritis (RA) diseases. An example of an NSAID is celecoxib, which inhibits cyclooxygenase (COX) enzyme.1–3 COX is a kind of oxidoreductase enzyme and is the main mediator of inflammation by catalyzing the initial step of arachidonic acid metabolism and prostaglandin synthesis.4 It has three subtypes; among them, COX-2 is a major contributor enzyme in inflammatory processes whose expression is upregulated by inflammatory mediators.5 COX-2 is also the drug target enzyme of the main pharmacodynamic actions of NSAIDs. COX-1 and its metabolites play important roles in maintaining the physiological conditions of an organism, and the inhibition of the constitutive isoform COX-1 leads to gastrointestinal toxicity and affects renal functions.6,7 Thus, the inhibition of COX-1 is associated with the side effects of NSAIDs. COX-3 has similar structural and catalytic features to COX-1 and COX-2, but it exhibits 20% of the activity of COX-1 and COX-2 and has not been isolated in humans.4,7 The differential expression of the constitutive isoform COX-1 and the inducible isoform COX-2, as well as the finding that COX-1 is the major form expressed in the gastrointestinal tract, has led to the search for COX-2 selective inhibitors as anti-inflammatory agents that may diminish the gastrointestinal side effects of traditional NSAIDs. COX-2 selective inhibitors show equivalent efficacy with conventional NSAIDs, but they have reduced gastrointestinal side effects.4
First-generation COX inhibitors, ie aspirin, traditional NSAIDs, can cause gastrointestinal tract, liver, and kidney damage, among other toxic side effects, owing to the broad inhibition of COX-1 and COX-2 activities.8–10 In recent years, some COX-2 selective inhibitors have been found to cause serious adverse reactions in the cardiovascular system, thereby limiting their clinical application.11–13 Therefore, the search for safer and more effective COX-2 selective inhibitors with new structural types is necessary.14
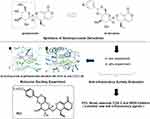
Our research groups have studied for nearly 10 years the effective anti-inflammatory substances and action mechanism of the Chinese herbal medicine Gentiana officinalis H. Smith and found that gentiopicroside is the main active anti-inflammatory ingredient. In vitro experiments have revealed its anti-inflammatory mechanism by inhibiting the phosphorylation of P38, ERK, and JNK in the MAPK pathway, reducing COX-2 expression and reducing PGE2 production.15 This finding was consistent with the COX-2/PGE2 anti-inflammatory pathway of COX-2 inhibitors in literature. Thus, gentiopicroside is a novel selective inhibitor of COX-2.16,17 Moreover, gentiopicroside is safe and nontoxic, but it is highly hydrophilic owing to the presence of the sugar fragment in its structure (Figure 1). This feature leads to reduced oral bioavailability, fast metabolism, short biological half-life, and limited efficacy. To identify a safe and effective novel COX-2 selective inhibitor, the structure of gentiopicroside requires modification. Therefore, we attempted to reduce its polarity while retaining its biological activity. Our research group have introduced hydrophobic cyclic acetals into the structure, synthesized a series of novel gentiopicroside derivatives and evaluated their anti-inflammatory activities in the previous study, as shown in Figure 2. Luckily, we got some novel selective COX-2 and iNOS inhibitors, such as compound P23.17 Inspired by previous research, In this study, we introduced hydrophobic active group into the gentiopicroside structure to reduce its hydrophilicity and enhance its lipophilicity, aiming to design and synthesize some new gentiopicroside derivatives by modifying its structure, and obtain a novel class of COX-2 inhibitors with excellent anti-inflammatory activity.
 |
Figure 1 Structure of gentiopicroside. |
Materials and Methods
Materials and Reagents
Gentiana officinalis H. Smith was collected from Dingxi, Gansu province of China and identified by Dr. Ling Jin from Gansu University of Chinese Medicine. Research and collection of plant material were carried out in accordance with guidelines of our institution and national regulations. The voucher specimen is deposited in the herbarium of Gansu University of Chinese Medicine and number is 621102190726031LY. Gentiopicroside was isolated from Gentiana officinalis H. Smith by our group.16 Paratoluensulfonyl chloride, benzoyl chloride, m-nitrobenzoyl chloride, 4-methylbenzoyl chloride, 3-chlorobenzoyl chloride, 4-bromobenzoyl chloride, 3,4,5-trimethoxybenzoyl chloride, and a total of 21 acyl chloride raw materials were purchased from Energy Chemical. Chloroform-d and methanol-d4 were purchased from Armar Marchemicals. Mouse macrophage RAW 264.7 was purchased from Procell Life Science and Technology. CCK-8, lipopolysaccharide (LPS), and other biological reagents were purchased from Sigma-Aldrich. Griess assays (using determined NO) were purchased from Beyotime Biotechnology. TNF-α, PGE2, IL-6, and COX-2 ELISA kits were purchased from Feiya Biotechnology.
Animals
KM mice [SPF; weighing (20 ± 2) g] were provided by the Laboratory Animal Center of Gansu University of Chinese Medicine (SCXK2011-0001, Lanzhou, China). The mice were housed under controlled conditions (relative humidity of 60% ± 5% at 25 ± 2 °C) on a 12/12 h day/night cycle. Standard laboratory food and water were provided. The mice were subjected to fasting for at least 12 h before the experiments and had free access to water.18 All animal experiments were approved by the Institutional Animal Care and Use Committee of Gansu University of Chinese Medicine Besides, the guide for the care and use of laboratory animals was followed for the welfare and treatment of the laboratory animals.
Synthesis of Gentiopicroside Derivatives
A total of 21 derivatives of gentiopicroside (Table 1) were synthesized according to the procedure shown in Figure 3. The specific reaction process was as follows. Gentiopicroside (1 mmol) was precisely weighed in a 50 mL two-neck bottle, added with a magnet, and sealed. The air was replaced with N2 three times. An appropriate amount of CH3CN solvent was added followed by stirring until gentiopicroside completely dissolved. Acyl chloride (1.1 equiv.), pyridine (2.0 equiv.), and CH3CN were then added to 25 mL, followed by heating and reflux stirring at 90 °C for 24 h. After slowly adding trimethylamine (0.5 equiv.) with a syringe and allowing all reactions to occur in a vacuum, the reaction process was detected by thin-layer chromatography (TLC) until it was completed. The compound were filtered, the solvent was evaporated, and the compounds were separated and purified by column chromatography (CH2Cl2-MeOH=20:1). The structures of the newly synthesized compounds were characterized by NMR, MS, and IR.
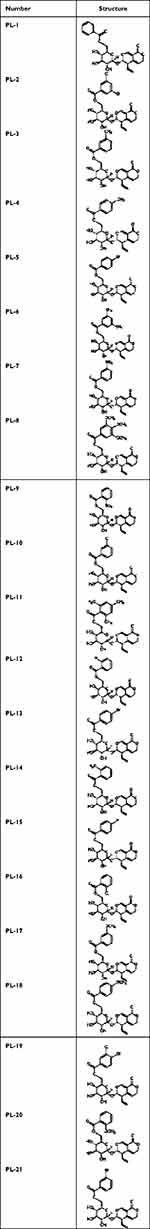 |
Table 1 Structures of Gentiopicroside Derivatives |
 |
Figure 3 Synthesis of gentiopicroside derivatives. |
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl benzoate (PL-1)
The product was obtained as colorless powder. Yield (67.7%), mp 111–113 °C. 1H NMR (400 MHz, CDCl3, δ in ppm, J in Hz): δH 8.01 (d, J = 8.0 Hz, 2H), 7.54–7.48 (m, 2H), 7.39 (t, J = 8.0 Hz, 2H), 5.58–5.49 (m, 1H), 5.42 (t, J = 4.0 Hz, 1H), 5.34 (d, J = 4.0 Hz, 1H), 5.15–5.09 (m, 3H), 5.02–4.85 (m, 2H), 4.71–4.62 (m, 3H), 4.52 (dd, J =12.0, 4.0 Hz, 1H), 4.38 (s, 1H), 3.68–3.63 (m, 1H), 3.60–3.58 (m, 1H), 3.53–3.47 (m, 1H), 3.39–3.34 (m, 1H), 3.23 (q, J = 8.0 Hz, 1H). 13C NMR (100 MHz, CDCl3, δ in ppm): δC 166.7, 164.2, 149.7, 133.2, 132.9, 129.8, 129.7, 128.4, 125.7, 118.7, 115.7, 103.7, 98.9, 97.5, 76.1, 74.2, 72.9, 70.2, 69.6, 63.9, 45.2. HR-MS (ESI) m/z: 483.1264 [M+Na]+, Calcd. 483.1262 for C23H24NaO10. IR (KBr, ν in cm−1): 3420.75, 2918.62, 1720.19, 1610.07, 1275.62, 1068.16.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 4-methylbenzoate (PL-2)
The product was obtained as colorless powder. Yield (47.9%), mp 107–109 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.89 (d, J = 8.0 Hz, 2H), 7.47 (s, 1H), 7.18 (d, J = 8.0 Hz, 2H), 5.60–5.47 (m, 1H), 5.42 (s, 1H), 5.33 (d, J = 4.0 Hz, 1H), 5.15 (d, J = 8.0 Hz, 1H), 5.12 (s, 1H), 5.05–4.82 (m, 3H), 4.69 (d, J = 8.0 Hz, 2H), 4.61 (d, J = 12.0 Hz, 2H), 4.54–4.44 (m, 1H), 3.69–3.55 (m, 2H), 3.54–3.43 (m, 2H), 3.36 (t, J = 8.0 Hz, 1H), 3.23 (q, J = 4.0 Hz, 1H), 2.36 (s, 3H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 166.8, 164.2, 149.7, 143.9, 133.0, 129.8, 129.1, 127.1, 125.7, 118.7, 115.6, 103.7, 98.8, 97.5, 77.3, 76.1, 74.3, 72.9, 70.2, 69.6, 45.2, 21.6. HR-MS (ESI) m/z: 497.1426 [M+Na]+, Calcd. 497.1418 for C24H26NaO10. IR (KBr, ν in cm−1): 3422.72, 2883.84, 1718.64, 1275.70, 1068.52.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3-nitrobenzoate (PL-3)
The product was obtained as colorless powder. Yield (74.3%), mp 111–113 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 8.76 (s, 1H), 8.43–8.29 (m, 2H), 7.65 (t, J = 8.0 Hz, 1H), 7.45 (s, 1H), 5.64–5.52 (m, 1H), 5.48 (s, 1H), 5.40 (d, J = 4.0 Hz, 1H), 5.19–5.11 (m, 2H), 5.09–4.82 (m, 3H), 4.79–4.67 (m, 4H), 4.59–4.52 (m, 1H), 3.80–3.71 (m, 1H), 3.69–3.59 (m, 1H), 3.57–3.47 (m, 1H), 3.38 (t, J = 8.0 Hz, 1H), 3.26 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 164.5, 164.3, 149.6, 148.2, 135.5, 132.8, 131.6, 129.8, 127.6, 125.5, 124.5, 118.8, 115.9, 103.7, 98.9, 97.6, 76.1, 74.0, 72.9, 70.2, 69.7, 64.8, 45.2. HR-MS (ESI) m/z: 528.1109 [M+Na]+, Calcd. 528.1120 for C23H23NNaO12. IR (KBr, ν in cm−1): 3365.82, 1724.52, 1611.92, 1533.43, 1351.77, 1263.81, 1068.14.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3-chlorobenzoate (PL-4)
The product was obtained as colorless powder. Yield (60.2%), mp 127–129 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.96 (s, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.49 (d, J = 12.0 Hz, 2H), 7.34 (t, J = 8.0, 1H), 5.62–5.51 (m, 1H), 5.45 (s, 1H), 5.36 (d, J = 4.0 Hz, 1H), 5.21–5.11 (m, 2H), 5.08–4.83 (m, 3H), 4.77–4.61 (m, 3H), 4.56–4.37 (m, 2H), 3.68 (s, 1H), 3.64–3.56 (m, 1H), 3.55–3.42 (m, 1H), 3.36 (t, J = 8.0 Hz, 1H), 3.25 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 165.5, 164.2, 149.6, 134.5, 133.2, 132.9, 131.6, 129.8, 129.7, 127.9, 125.6, 118.8, 115.7, 103.7, 98.8, 97.6, 76.7, 76.1, 74.1, 72.9, 70.2, 69.9, 64.3, 45.2. HR-MS (ESI) m/z: 517.0876 [M+Na]+, Calcd. 517.0872 for C23H23ClNaO10. IR (KBr, ν in cm−1): 3410.30, 2916.33, 1722.62, 1426.37, 1258.63, 1070.28.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 4-bromobenzoate (PL-5)
The product was obtained as yellow powder. Yield (54.5%), mp 108–110 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.85 (d, J = 8.0 Hz, 2H), 7.53 (d, J = 8.0 Hz, 2H), 7.47 (s, 1H), 5.60–5.49 (m, 1H), 5.44 (s, 1H), 5.36–5.29 (m, 1H), 5.20–5.10 (m, 2H), 5.05–4.85 (m, 3H), 4.75–4.59 (m, 3H), 4.58–4.37 (m, 2H), 3.67 (s, 1H), 3.63–3.54 (m, 1H), 3.52–3.44 (m, 1H), 3.34 (t, J = 8.0 Hz, 1H), 3.23 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 166.0, 164.2, 149.6, 132.9, 131.8, 131.3, 128.7, 128.4, 125.5, 118.7, 115.8, 103.7, 98.9, 97.6, 76.0, 74.1, 72.9, 70.1, 69.6, 64.1, 45.2. HR-MS (ESI) m/z: 561.0363 [M+Na]+, Calcd. 561.0367 for C23H23BrNaO10. IR (KBr, ν in cm−1): 3389.95, 2882.87, 1719.60, 1399.05, 1272.75, 1068.77.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 2-chlorobenzoate (PL-6)
The product was obtained as colorless powder. Yield (66.3%), mp 114–116 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.83 (d, J = 8.0 Hz, 1H), 7.48 (s, 1H), 7.42–7.37 (m, 2H), 7.32–7.26 (m, 1H), 5.64–5.51 (m, 1H), 5.44 (s, 1H), 5.39 (d, J = 4.0 Hz, 1H), 5.20–5.11 (m, 2H), 5.09–4.82 (m, 3H), 4.74–4.63 (m, 2H), 4.60–4.45 (m, 3H), 3.69 (s, 1H), 3.65–3.56 (m, 1H), 3.56–3.47 (m, 1H), 3.37 (t, J = 8.0 Hz, 1H), 3.24 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 165.7, 164.3, 149.7, 133.6, 132.9, 132.8, 131.7, 131.1, 129.8, 126.7, 125.7, 118.8, 115.7, 103.7, 98.7, 97.4, 76.1, 74.1, 73.0, 70.2, 69.6, 64.4, 45.2. HR-MS (ESI) m/z: 517.0876 [M+Na]+, Calcd. 517.0872 for C23H23ClNaO10. IR (KBr, ν in cm−1): 3377.29, 1720.59, 1610.32, 1252.17, 1071.27.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3,4-dichlorobenzoate (PL-7)
The product was obtained as colorless powder. Yield (43.9%), mp 105–107 °C. 1H NMR(400 MHz, d6-DMSO, δ in ppm, J in Hz): δH 8.08 (d, J = 2.0 Hz, 1H), 7.95–7.88 (m, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.42 (s, 1H), 5.58–5.51 (m, 1H), 5.43–5.32 (m, 2H), 5.22–5.08 (m, 4H), 5.07–4.92 (m, 2H), 4.61 (t, J = 8.0 Hz, 2H), 4.42 (dd, J = 12.0, 8.0 Hz, 1H), 3.64–3.60 (m, 1H), 3.38 (s, 1H), 3.28–3.18 (m, 3H), 3.06–3.01 (m, 1H). 13C NMR(100 MHz, d6-DMSO, δ in ppm): δC 163.2, 162.1, 148.3, 135.8, 133.2, 131.2, 130.7, 130.2, 129.6, 128.6, 124.3, 117.3, 115.6, 102.7, 98.8, 96.7, 75.8, 73.2, 72.1, 69.4, 68.6, 64.1, 43.8. HR-MS (ESI) m/z: 551.0487 [M+Na]+, Calcd. 551.0482 for C23H22Cl2NaO10. IR (KBr, ν in cm−1): 3420.02, 2914.44, 1721.76, 1610.71, 1562.54, 1274.82, 1069.05.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3,5-dichlorobenzoate (PL-8)
The product was obtained as colorless powder. Yield (42.1%), mp 132–134 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.97–7.69 (m, 2H), 7.47 (s, 2H), 5.65–5.53 (m, 1H), 5.47 (s, 1H), 5.39 (s, 1H), 5.18 (d, J = 8.0 Hz, 2H), 5.09–4.82 (m, 3H), 4.81–4.64 (m, 3H), 4.48 (s, 2H), 3.71 (s, 1H), 3.65–3.55 (m, 1H), 3.51–3.41 (m, 1H), 3.37 (t, J = 8.0 Hz, 1H), 3.28–3.25 (m, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 164.2, 164.2, 149.6, 135.3, 132.9, 132.9, 132.6, 128.0, 125.6, 118.8, 115.8, 103.7, 98.8, 97.6, 76.0, 74.0, 72.9, 70.2, 69.7, 64.8, 45.20. HR-MS (ESI) m/z: 551.0487 [M+Na]+, Calcd. 551.0482 for C23H22Cl2NaO10. IR (KBr, ν in cm−1): 3309.93, 2883.14, 1726.47, 1610.65, 1570.84, 1430.92, 1271.17, 1070.99.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 4-chlorobenzoate (PL-9)
The product was obtained as colorless powder. Yield (65.4%), mp 132–134 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.93 (d, J = 8.0 Hz, 2H), 7.47 (s, 1H), 7.36 (d, J = 8.0 Hz, 2H), 5.61–5.49 (m, 1H), 5.44 (s, 1H), 5.34 (d, J = 2.8 Hz, 1H), 5.17–5.12 (m, 2H), 5.06–4.81 (m, 3H), 4.76–4.60 (m, 3H), 4.55–4.40 (m, 2H), 3.67 (s, 1H), 3.63–3.55 (m, 1H), 3.54–3.45 (m, 1H), 3.35 (t, J = 8.0 Hz, 1H), 3.24 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 165.8, 164.2, 149.6, 139.7, 132.9, 131.1, 128.8, 128.2, 125.5, 118.7, 115.8, 103.7, 98.9, 97.6, 76.0, 74.1, 72.9, 70.1, 69.6, 64.1, 45.2. HR-MS (ESI) m/z: 517.0875 [M+Na]+, Calcd. 517.0872 for C23H23ClNaO10. IR (KBr, ν in cm−1): 3358.98, 1720.78, 1611.19, 1274.01, 1068.59.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3,4,5-trimethoxybenzoate (PL-10)
The product was obtained as colorless powder. Yield (52.7%), mp 145–147 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.48 (s, 1H), 7.28 (s, 2H), 5.61–5.49 (m, 1H), 5.46 (s, 1H), 5.38 (d, J = 3.2 Hz, 1H), 5.17 (d, J = 4.0 Hz, 1H), 5.14 (s, 1H), 5.09–4.88 (m, 3H), 4.80–4.59 (m, 3H), 4.58–4.43 (m, 2H), 3.89 (s, 3H), 3.87 (s, 6H), 3.73–3.65 (m, 1H), 3.60 (t, J = 8.0 Hz, 1H), 3.45 (t, J = 8.0 Hz, 1H), 3.36 (t, J = 8.0 Hz, 1H), 3.24 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 166.5, 164.1, 152.9, 149.4, 142.5, 132.9, 125.5, 124.6, 118.7, 115.9, 107.1, 103.8, 98.6, 97.3, 76.0, 74.3, 73.0, 70.3, 69.6, 64.2, 60.9, 56.3, 45.2. HR-MS (ESI) m/z: 573.1565 [M+Na]+, Calcd. 573.1686 for C26H30NaO13. IR (KBr, ν in cm−1): 3727.65, 2941.67, 1718.09, 1610.80, 1504.34, 1416.80, 1336.10, 1226.88, 1128.40.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 2,4,6-trimethylbenzoate (PL-11)
The product was obtained as colorless powder. Yield (41.8%), mp 120–122 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.47 (s, 1H), 6.80 (d, J = 12.0 Hz, 2H), 4.64–4.53 (m, 1H), 5.42 (s, 1H), 5.35 (d, J = 4.0 Hz, 1H), 5.23–5.14 (m, 2H), 5.04–4.80 (m, 3H), 4.74–4.55 (m, 3H), 4.48–4.31 (m, 2H), 3.62 (t, J = 8.0 Hz, 1H), 3.56 (t, J = 8.0 Hz, 1H), 3.45–3.37 (m, 1H), 3.31 (t, J = 8.0 Hz, 1H), 3.24 (q, J = 4.0 Hz, 1H), 2.29–2.22 (m, 9H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 170.1, 164.2, 149.6, 139.6, 135.4, 132.9, 130.4, 128.5, 125.6, 118.9, 115.7, 103.7, 98.6, 97.3, 76.1, 74.1, 73.0, 70.2, 69.6, 63.9, 53.48, 45.3, 21.1, 20.0. HR-MS (ESI) m/z: 525.1721 [M+Na]+, Calcd. 525.1731 for C26H30NaO10. IR (KBr, ν in cm−1): 3366.56, 1721.70, 1611.40, 1457.24, 1268.99, 1065.65.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 2-methylbenzoate (PL-12)
The product was obtained as colorless powder. Yield (41.9%), mp 132–134 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.92–7.85 (m, 1H), 7.48 (s, 1H), 7.41–7.32 (m, 1H), 7.24–7.12 (m, 2H), 5.62–5.49 (m, 1H), 5.42 (s, 1H), 5.36 (d, J = 4.0 Hz, 1H), 5.16 (d, J = 4.0 Hz, 1H), 5.13 (s, 1H), 5.05–4.80 (m, 3H), 4.75–4.58 (m, 3H), 4.51–4.34 (m, 2H),3.69–3.62 (m, 1H), 3.58 (d, J = 12.0 Hz, 1H), 3.53–3.44 (m, 1H), 3.36 (t, J = 8.0 Hz, 1H), 3.23 (q, J = 4.0 Hz, 1H), 2.54 (s, 3H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 167.7, 164.2, 149.7, 140.2, 132.9, 132.2, 131.7, 130.8, 129.3, 125.8, 125.7, 118.7, 115.7, 103.7, 98.8, 97.5, 76.1, 74.2, 73.0, 70.3, 69.6, 63.8, 45.2, 21.9. HR-MS (ESI) m/z: 497.1416 [M+Na]+, Calcd. 497.1418 for C24H26NaO10. IR (KBr, ν in cm−1): 3401.81, 2883.37, 1719.27, 1610.48, 1258.26, 1067.19.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3-methoxybenzoate (PL-13)
The product was obtained as colorless powder. Yield (61.6%), mp 127–129 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.60 (d, J = 8.0 Hz, 1H), 7.55–7.49 (m, 1H), 7.47 (s, 1H), 7.33–7.26 (m, 1H), 7.09–6.99 (m, 1H), 5.60–5.48 (m, 1H), 5.42 (s, 1H), 5.34 (d, J = 4.0 Hz, 1H), 5.23–5.08 (m, 3H), 5.06–4.79 (m, 3H), 4.76–4.59 (m, 3H), 4.54–4.45 (m, 1H), 3.80 (s, 3H), 3.69–3.63 (m, 1H), 3.59 (d, J = 8.0 Hz, 1H), 3.54–3.45 (m, 1H), 3.36 (t, J = 12.0 Hz, 1H), 3.22 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 166.6, 164.2, 159.5, 149.7, 132.9, 131.1, 129.5, 125.7, 122.1, 119.3, 118.7, 115.6, 114.6, 103.7, 98.8, 97.5, 76.1, 74.2, 72.9, 70.2, 69.6, 64.0, 55.4, 45.1. HR-MS (ESI) m/z: 513.1331 [M+Na]+, Calcd. 513.1367 for C24H26NaO11. IR (KBr, ν in cm−1): 3375.62, 1719.41, 1610.13, 1278.89, 1069.32.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 2-methoxybenzoate (PL-14)
The product was obtained as colorless powder. Yield (47.2%), mp 119–121 °C. HR-ESI-MS m/z: 513.1376 [M+Na]+, Calcd. 513.1376 for C24H26NaO11. IR (KBr, ν in cm−1): 3378.15, 1719.15, 1610.29, 1254.17, 1069.28. 1H NMR (400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.90–7.72 (m, 1H), 7.55–7.36 (m, 2H), 7.01–6.88 (m, 2H), 5.63–5.52 (m, 1H), 5.44 (s, 1H), 5.38 (d, J = 4.0 Hz, 1H), 5.26–5.07 (m, 3H), 5.06–4.80 (m, 3H), 4.68 (d, J = 8.0 Hz, 1H), 4.62–4.43 (m, 3H), 3.86 (s, 3H), 3.66–3.49 (m, 3H), 3.36 (t, J = 8.0 Hz, 1H), 3.23 (q, J = 4.0 Hz, 1H). 13C NMR (100 MHz, CDCl3, δ in ppm): δC 166.2, 164.0, 159.3, 149.6, 134.0, 132.9, 132.0, 125.7, 120.2, 119.4, 118.7, 115.7, 112.2, 103.7, 98.7, 97.4, 76.1, 74.1, 73.0, 70.3, 69.5, 63.8, 56.0, 45.2. HR-MS (ESI) m/z: 513.1376 [M+Na]+, Calcd. 513.1376 for C24H26NaO11. IR (KBr, ν in cm−1): 3378.15, 1719.15, 1610.29, 1254.17, 1069.28.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3-methylbenzoate (PL-15)
The product was obtained as colorless powder. Yield (48.9%), mp 128–130 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.81 (d, J = 8.0 Hz, 2H), 7.48 (s, 1H), 7.40–7.22 (m, 2H), 5.62–5.47 (m, 1H), 5.43 (s, 1H), 5.35 (d, J = 4.0 Hz, 1H), 5.20–5.08 (m, 2H), 5.07–4.79 (m, 3H), 4.78–4.57 (m, 3H), 4.56–4.29 (m, 2H), 3.73–3.64 (m, 1H), 3.63–3.55 (m, 1H), 3.54–3.44 (m, 1H), 3.37 (t, J = 8.0 Hz, 1H), 3.23 (q, J = 4.0 Hz, 1H), 2.35 (s, 3H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 166.9, 164.2, 149.7, 138.2, 134.0, 133.0, 130.2, 129.7, 128.3, 126.9, 125.7, 118.7, 115.7, 103.7, 98.8, 97.5, 76.1, 74.2, 72.9, 70.2, 69.6, 63.9, 45.2. HR-MS (ESI) m/z: 497.1438 [M+Na]+, Calcd. 497.1418 for C24H26NaO10. IR (KBr, ν in cm−1): 3419.82, 2917.35, 1719.43, 1610.12, 1279.14, 1203.85, 1069.31.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3,5-dimethylbenzoate (PL-16)
The product was obtained as colorless powder. Yield (44.8%), mp 132–134 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.61 (s, 2H), 7.48 (s, 1H), 7.14 (s, 1H), 5.62–5.49 (m, 1H), 5.44 (s, 1H), 5.37 (d, J = 4.0 Hz, 1H), 5.20–5.06 (m, 2H), 5.05–4.84 (m, 3H), 4.74–4.59 (m, 3H), 4.56–4.39 (m, 2H), 3.71–3.63 (m, 1H), 3.62–3.54 (m, 1H), 3.54–3.42 (m, 1H), 3.37 (t, J = 8.0 Hz, 1H), 3.24 (q, J = 4.0 Hz, 1H), 2.31 (s, 6H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 167.1, 164.1, 149.6, 138.0, 134.8, 133.0, 129.7, 127.4, 125.7, 118.6, 115.7, 103.7, 98.8, 97.4, 76.1, 74.3, 73.0, 70.3, 69.6, 63.9, 45.2, 21.1. HR-MS (ESI) m/z: 515.1565 [M+Na]+, Calcd. 515.1575 for C25H28NaO10. IR (KBr, ν in cm−1): 3424.37, 2917.69, 1719.15, 1610.17, 1217.86, 1072.56.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 2-nitrobenzoate (PL-17)
The product was obtained as colorless powder. Yield (49.7%), mp 120–122 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.88–7.73 (m, 2H), 7.71–7.56 (m, 2H), 7.48 (s, 1H), 5.72–5.60 (m, 1H), 5.54 (d, J = 4.0 Hz, 1H), 5.47 (s, 1H), 5.25–5.10 (m, 2H), 5.07–4.85 (m, 2H), 4.79–4.58 (m, 5H), 4.49–4.37 (m, 1H), 3.77–3.64 (m, 1H), 3.64–3.54 (m, 1H), 3.42 (d, J = 8.0 Hz, 1H), 3.40–3.32 (m, 1H), 3.29 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 165.1, 164.4, 149.9, 148.5, 133.1, 132.9, 132.1, 130.4, 127.0, 125.7, 123.8, 118.6, 115.6, 103.6, 98.8, 97.6, 76.1, 73.8, 72.9, 70.1, 69.6, 65.5, 45.2. HR-MS (ESI) m/z: 528.1126 [M+Na]+, Calcd. 528.1112 for C23H23NNaO12. IR (KBr, ν in cm−1): 3359.45, 1723.30, 1610.71, 1534.57, 1362.79, 1291.59, 1066.76.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 2-fluorobenzoate (PL-18)
The product was obtained as colorless powder. Yield (57.7%), mp 132–134 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.90 (t, J = 8.0 Hz, 1H), 7.53–7.42 (m, 2H), 7.17 (t, J = 4.0 Hz, 1H), 7.08 (t, J = 8.0 Hz, 1H), 5.63–5.50 (m, 1H), 5.44 (s, 1H), 5.38 (d, J = 4.0 Hz, 1H), 5.19–5.10 (m, 2H), 5.06–4.84 (m, 3H), 4.78–4.64 (m, 3H), 4.55–4.38 (m, 2H), 3.73–3.65 (m, 1H), 3.64–3.57 (m, 1H), 3.56–3.48 (m, 1H), 3.37 (t, J = 8.0 Hz, 1H), 3.24 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 164.4, 164.4, 164.4, 163.2, 160.6, 149.8, 134.7, 134.7, 132.9, 132.2, 125.7, 124.1, 118.7, 118.5, 118.4, 117.1, 116.9, 115.6, 103.6, 98.8, 97.5, 76.2, 74.0, 72.9, 70.2, 69.6, 64.2, 45.2. HR-MS (ESI) m/z: 525.1353 [M+Na]+, Calcd. 525.1367 for C23H23FNaO10. IR (KBr, ν in cm−1): 3418.40, 2885.07, 1719.69, 1611.80, 1489.66, 1299.62, 1069.11.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 4-fluorobenzoate (PL-19)
The product was obtained as colorless powder. Yield (49.6%), mp 109–111 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 8.11–7.94 (m, 2H), 7.48 (s, 1H), 7.07 (t, J = 8.0 Hz, 2H), 5.62–5.50 (m, 2H), 5.45 (s, 1H), 5.35 (d, J = 4.0 Hz, 1H), 5.18–5.10 (m, 2H), 5.07–4.83 (m, 3H), 4.77–4.58 (m, 3H), 4.57–4.32 (m, 2H), 3.73–3.64 (m, 1H), 3.64–3.55 (m, 1H), 3.54–3.45 (m, 1H), 3.34 (t, J = 12.0 Hz, 1H), 3.24 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 167.1, 165.7, 164.6, 164.2, 149.6, 133.0, 132.4, 132.3, 126.0, 126.0, 125.5, 118.6, 115.8, 115.7, 115.5, 103.7, 98.9, 97.5, 76.0, 74.1, 72.9, 70.1, 69.6, 64.0, 45.2. HR-MS (ESI) m/z: 501.1162 [M+Na]+, Calcd. 501.1167 for C23H23FNaO10. IR (KBr, ν in cm−1): 3419.95, 2885.99, 1721.08, 1606.68, 1508.59, 1275.40, 1068.76.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 4-(trifluoromethoxy)benzoate (PL-20)
The product was obtained as colorless powder. Yield (45.8%), mp 132–134 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 7.96 (d, J = 8.0 Hz, 1H), 7.85 (s, 1H), 7.55–7.32 (m, 3H), 5.64–5.49 (m, 1H), 5.46 (s, 1H), 5.36 (d, J = 4.0 Hz, 1H), 5.19–5.10 (m, 2H), 5.09–4.83 (m, 3H), 4.82–4.64 (m, 3H), 4.60–4.39 (m, 2H), 3.74–3.65 (m, 1H), 3.64–3.56 (m, 1H), 3.53–3.41 (m, 1H), 3.36 (t, J = 8.0 Hz, 1H), 3.25 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 165.3, 164.3, 149.6, 149.2, 149.1, 132.8, 131.9, 130.0, 128.1, 125.5, 122.1, 121.6, 119.1, 118.6, 115.8, 103.8, 98.8, 97.5, 76.1, 74.1, 72.9, 70.2, 69.6, 64.4, 45.2. HR-MS (ESI) m/z: 5567.1099 [M+Na]+, Calcd. 567.1085 for C24H23F3NaO10. IR (KBr, ν in cm−1): 3417.50, 2888.69, 1725.47, 1611.48, 1259.58.
((2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-((3S,4R)-8-oxo-4-vinyl-3,4,6,8-tetrahydropyrano[3,4-c]pyran-3-yloxy)-tetrahydro-2H-pyran-2-yl)methyl 3-bromobenzoate (PL-21)
The product was obtained as colorless powder. Yield (47.3%), mp 132–134 °C. 1H NMR(400 MHz, CDCl3, δ in ppm, J in Hz): δH 8.11 (s, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.47 (s, 1H), 7.33–7.24 (m, 1H), 5.62–5.51 (m, 1H), 5.45 (s, 1H), 5.36 (d, J = 4.0 Hz, 1H), 5.21–5.11 (m, 2H), 5.08–4.82 (m, 3H), 4.76–4.62 (m, 3H), 4.59–4.40 (m, 2H), 3.73–3.65 (m, 1H), 3.64–3.56 (m, 1H), 3.53–3.45 (m, 1H), 3.37 (t, J = 8.0 Hz, 1H), 3.25 (q, J = 4.0 Hz, 1H). 13C NMR(100 MHz, CDCl3, δ in ppm): δC 165.3, 164.2, 149.7, 136.1, 132.9, 132.6, 131.8, 130.1, 128.3, 125.6, 122.5, 118.8, 115.7, 103.7, 98.9, 97.6, 76.1, 74.1, 72.9, 70.2, 69.6, 64.3, 45.2. HR-MS (ESI) m/z: 561.0350 [M+Na]+, Calcd. 561.0367 for C23H23BrNaO10. IR (KBr, ν in cm−1): 3389.01, 1721.49, 1610.48, 1424.17, 1256.82, 1067.62.
In vitro Biological Evaluation
In vitro experiments were divided into 25 groups in total, namely, normal group, model group, celecoxib group, gentiopicroside group, and 21 dose groups. The effect of gentiopicroside derivatives on cell viability was investigated by CCK-8 cell-viability assay, and results showed that all newly synthesized compounds had no cytotoxicity within 100 μg/mL (viability > 90%). Their in vitro inflammatory activity was evaluated against the release of the inflammatory factors prostaglandin E2 (PGE2), nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) by the LPS-stimulated mouse macrophage cell line RAW264.7.19,20 The cells were pretreated with compounds (at 100, 50, 25, and 12.5 μg/mL) for 2 h except the normal and model groups, respectively, and then stimulated with LPS (1 μg/mL) for 24 h except the normal group. The culture medium was then collected. The NO levels were determined by the Griess assay, and the PGE2, TNF-α, and IL-6 levels were determined with ELISA kits.21–24 The IC50 of each group (excluded the normal and model group) were calculated based determined data, the final experimental results were represented by IC50.
In vivo Biological Evaluation
The KM mice were randomly divided into 24 groups, namely, control, celecoxib, gentiopicroside, and 21 dose groups, with 10 mice per group. Mice in each group were administered with a dose of 150 mg/kg for 3 days. On the last day, each animal received 0.1 mL of xylene on the anterior and posterior surfaces of the right ear. The left ear was considered as a control. After 30 min, the animals were killed by cervical dislocation, and both ears were sampled. Circular sections were collected using a cork borer with a diameter of 8 mm and weighed. The degree of ear swelling was calculated based on the weight of the left ear without receiving xylene.25,26
Molecular Docking
Crystal structures of COX-2 (PDB ID: 5ikr) were retrieved from the PDB database. The docking operation was performed with Schrödinger software. The 3D structures of compounds with in vitro and in vivo inflammatory activity were generated using the Quantum Mechanics module and docked into the structures of COX-2 with the Induced Fit Docking method.
Data and Statistical Analysis
The data of the in vivo anti-inflammatory activities were expressed as mean ± standard error of the mean (SEM). The QQ plots were used to assess data distribution. One-way analysis of variance (ANOVA) was applied to determine the significant differences between groups. Differences were considered to be significant at P<0.05. The statistical analysis was conducted using SPSS-software (version 21.0). GraphPad prism (version 8.0.2) was used for drawing graphs.27
Results and Discussion
Chemistry
21 novel gentiopicroside derivatives were synthesized by selective esterification of the primary hydroxyl group in gentiopicroside. According to the experimental, the optimum reaction conditions are that the addition of acyl chloride and pyridine are 1.1 and 2.0 equivalents of the substrate. The addition of triethylamine can accelerate the reaction rate and increase the yield. The structure of the gentiopicroside derivatives were determined by IR, MS and NMR, and the spectral data agreed with the proposed structures. Their hydrophilicities were preliminarily evaluated by their TLC relative shift values. All derivatives were found to exhibit lower polarities than the parent compound gentiopicroside.
Anti-Inflammatory Activity
The in vivo and in vitro anti-inflammatory activities of all newly synthesized compounds were investigated using literature methods. In vitro results showed that NO production strongly decreased after intervention with PL-2, PL-3, PL-4, PL-5, PL-6, PL-7, PL-8, PL-9, PL-12, PL-20, and PL-21, and their IC50 value was lower than that of the parent compound gentiopicroside (59.97 μg/mL). The other compounds showed poor inhibition (IC50 > 62 μg/mL) (Figure 4).
 |
Figure 4 Effect of gentiopicroside derivatives on LPS-induced NO production in RAW 264.7 macrophages. Abbreviations: C, Celecoxib; GPS, Gentiopicroside. |
By examining the changes in TNF-α, the production was found to be strongly reduced after intervention with PL-2, PL-3, PL-7, PL-8, PL-9, PL-12, PL-16, PL-17, and PL-21. Their IC50 value was lower than that of the parent compound gentiopicroside (36.56 μg/mL) and the positive control celecoxib (34.30 μg/mL). The other compounds did not show significant effects (IC50 > 45 μg/mL) (Figure 5).
 |
Figure 5 Effect of gentiopicroside derivatives on LPS-induced TNF-α production in RAW 264.7 macrophages. Abbreviations: C, Celecoxib; GPS, Gentiopicroside. |
The changes in IL-6 showed that production was strongly reduced after intervention with PL-2, PL-4, PL-5, PL-8, PL-9, PL-20, and PL-21. Their IC50 value was lower than that of the parent compound gentiopicroside (43 μg/mL). In particular, PL-2 showed a more obvious inhibitory effect with an IC50 value of 5.70 μg/mL. The other compounds did not show significant effects (IC50 > 47 μg/mL) (Figure 6).
 |
Figure 6 Effect of gentiopicroside derivatives on LPS-induced IL-6 production in RAW 264.7 macrophages. Abbreviations: C, Celecoxib; GPS, Gentiopicroside. |
By examining the changes in PGE2, the production was found to be strongly reduced after intervention with PL-3, PL-5, PL-7, PL-8, PL-9, PL-19, and PL-20. Their IC50 value was lower than that of the parent compound gentiopicroside (24.31 μg/mL). The other compounds did not show significant effects (IC50 > 40 μg/mL). Together, several compounds displayed stronger inhibitory effects on the secretion of inflammatory cytokines than the parent compound gentiopicroside (Figure 7).
 |
Figure 7 Effect of gentiopicroside derivatives on LPS-induced PGE2 production in RAW 264.7 macrophages. Abbreviations: C, Celecoxib; GPS, Gentiopicroside. |
The in vivo activity of all compounds was further tested against mouse ear swelling. Results showed that derivatives PL-2, PL-4, PL-7, PL-8, PL-12, PL-13, PL-16, and PL-18 had higher inhibition rates (48.22%, 36.19%, 55.01%, 43.51%, 35.84%, 51.65%, 40.95%, and 40.73%, respectively) than the parent compound gentiopicroside (35.34%) (Table 2).
 |
Table 2 Effects of Gentiopicroside Derivatives on Xylene Swelling in Mice ( |
Molecular Docking
The 3D structures of compounds with in vitro and in vivo inflammatory activity were docked into the structure of COX-2, and the binding free energy and docking score were calculated. Results showed that compounds PL-2, PL-3, PL-4, PL-5, PL-7, and PL-8 had high absolute values of docking score and binding energy than the parent compound gentiopicroside (Table 3). Their compounds could be well docked into the pockets and bind to the inflammation target COX-2 by making hydrogen bonds. For example, the hydroxyl group on the compound PL-5 pyran ring formed hydrogen bonding with the target protein HIS386, GLN454, THR212, ASN382 of COX-2, the compound PL-4 formed hydrogen bonding with the target protein THR212, HIS214, ASN382 of COX-2, etc. The residues ASN382 and THR212 of COX-2 could form hydrogen bond with multiple compounds. Hydrogen bonding played an important role in the molecular docking between gentiopicroside derivatives and inflammatory target COX-2. The docking models of some gentiopicroside derivatives with COX-2 in Figures 8 and 9. This finding was consistent with their potencies of anti-inflammatory activities.
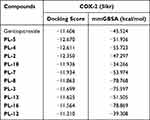 |
Table 3 Docking Scores and Binding Energy of Gentiopicroside Derivatives with COX-2 |
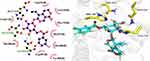 |
Figure 8 2D and 3D docking model of gentiopicroside derivatives PL-4 with COX-2. |
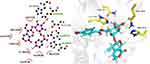 |
Figure 9 2D and 3D docking model of gentiopicroside derivatives PL-5 with COX-2. |
Inhibitory Activity of Gentiopicroside Derivatives Against COX-2
The inhibitory activity of gentiopicroside derivatives with significant in vitro and in vivo inflammatory activity against COX-2 enzyme were further investigated. Results demonstrated that compounds PL-2, PL-3, PL-4, PL-5, PL-7, and PL-8 had obvious inhibitory effect against COX-2. These results were consistent with those of the molecular docking. Therefore, these compounds were novel COX-2 inhibitors (Table 4).
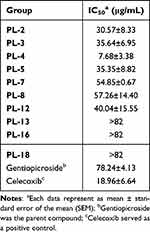 |
Table 4 Effect of Gentiopicroside Derivatives on LPS-Induced COX-2 Production in RAW 264.7 Macrophages |
Structure-Activity Relationship Analysis
Some novel COX-2 inhibitors were synthesized by introducing hydrophobic acyl chloride into the gentiopicroside structure. Their hydrophilicity decreased and their lipophilicity was enhanced. The change in polarity played a key role in the anti-inflammatory activities from gentiopicroside to its derivatives. The effects of acyl chloride cannot be ignored, and the types and positions of substituents on benzoyl chloride groups determined the difference in anti-inflammatory activity. The derivatives with the groups CH3, Cl, NO2, F, Br, and OCH3 also showed good activity. Para-substitution with electron-withdrawing groups may have benefited the anti-inflammatory activity.
Conclusion
Natural products play an important role in discovering safer, more economic, more effective, and less toxic anti-inflammatory drug candidates. Gentiopicroside is extracted from the traditional Chinese medicine, its anti-inflammatory effect attracted more attention. Moreover, gentiopicroside is a safe and nontoxic selective inhibitor of COX-2. However, it is highly hydrophilic owing to the presence of the sugar fragment in its structure, leading to reduced oral bioavailability, fast metabolism, short biological half-life, and limited efficacy. To identify a safe and effective novel COX-2 inhibitor, the structure of gentiopicroside needs to be modified. In this study, 21 novel derivatives were synthesized by introducing the hydrophobic active group into the gentiopicroside structure, thereby causing their hydrophilicities to be lower than that of the parent compound gentiopicroside. The in vivo and in vitro anti-inflammatory activities of some gentiopicroside derivatives were retained or improved. In particular, the activity of compounds PL-2, PL-7 and PL-8 was significantly higher than gentiopicroside. Molecular docking predicted that some anti-inflammatory compounds could be well docked into the pockets and bind to the inflammation targets COX-2. Structure-activity relationship analysis indicated that para-substitution with electron-withdrawing groups may have benefited the anti-inflammatory activity. The inhibitory activity of these compounds against COX-2 enzyme confirmed that these gentiopicroside derivatives represented a novel class of COX-2 inhibitors and could thus be developed as new anti-inflammatory agents.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 82160457, no. 81660577) and the Natural Science Foundation of Gansu Province (no. 21JR7RA564). We sincerely thank Dr. Jun Zeng of Lanzhou University for helping us with the chemistry experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alfayomy AM, Abdel-Aziz SA, Marzouk AA, et al. Design and synthesis of pyrimidine-5-carbonitrile hybrids as COX-2 inhibitors: anti-inflammatory activity, ulcerogenic liability, histopathological and docking studies. Bioorg Chem. 2021;108:104555. doi:10.1016/j.bioorg.2020.104555
2. Schjerning AM, McGettigan P, Gislason G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat Rev Cardiol. 2020;17(9):574–584. doi:10.1038/s41569-020-0366-z
3. Peng Y, Ao M, Dong B, et al. Anti-inflammatory effects of curcumin in the inflammatory diseases: status, limitations and countermeasures. Drug Des Devel Ther. 2021;15:4503–4525. doi:10.2147/DDDT.S327378
4. Ferrer MD, Busquets-Cortés C, Capó X, et al. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr Med Chem. 2019;26(18):3225–3241. doi:10.2174/0929867325666180514112124
5. Arora M, Choudhary S, Singh PK, et al. Structural investigation on the selective COX-2 inhibitors mediated cardiotoxicity: a review. Life Sci. 2020;251:117631. doi:10.1016/j.lfs.2020.117631
6. Sağlık BN, Osmaniye D, Levent S, et al. Design, synthesis and biological assessment of new selective COX-2 inhibitors including methyl sulfonyl moiety. Eur J Med Chem. 2021;209:112918. doi:10.1016/j.ejmech.2020.112918
7. Cui J, Jia J. Natural COX-2 inhibitors as promising anti-inflammatory agents: an update. Curr Med Chem. 2021;28(18):3622–3646. doi:10.2174/0929867327999200917150939
8. Grosser T, Ricciotti E, FitzGerald GA. The cardiovascular pharmacology of nonsteroidal anti-inflammatory drugs. Trends Pharmacol Sci. 2017;38(8):733–748. doi:10.1016/j.tips.2017.05.008
9. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147. doi:10.1016/j.bcp.2020.114147
10. Huang Z, Ma X, Jia X, et al. Prevention of severe acute pancreatitis with cyclooxygenase-2 inhibitors: a randomized controlled clinical trial. Am J Gastroenterol. 2020;115(3):473–480. doi:10.14309/ajg.0000000000000529
11. Ahmed EM, Hassan MSA, El-Malah AA, et al. New pyridazine derivatives as selective COX-2 inhibitors and potential anti-inflammatory agents; design, synthesis and biological evaluation. Bioorg Chem. 2020;95:103497.
12. Abdellatif KRA, Abdelall EKA, Labib MB, et al. Synthesis of novel halogenated triarylpyrazoles as selective COX-2 inhibitors: anti-inflammatory activity, histopatholgical profile and in-silico studies. Bioorg Chem. 2020;105:104418. doi:10.1016/j.bioorg.2020.104418
13. Ahmed EM, Kassab AE, El-Malah AA, et al. Synthesis and biological evaluation of pyridazinone derivatives as selective COX-2 inhibitors and potential anti-inflammatory agents. Eur J Med Chem. 2019;171:25–37. doi:10.1016/j.ejmech.2019.03.036
14. Mahboubi Rabbani SMI, Zarghi A. Selective COX-2 inhibitors as anticancer agents: a patent review (2014–2018). Expert Opin Ther Pat. 2019;29(6):407–427. doi:10.1080/13543776.2019.1623880
15. Zhao L, Ye J, Wu G-T, et al. Gentiopicroside prevents interleukin-1 beta induced inflammation response in rat articular chondrocyte. J Ethnopharmacol. 2015;172:100–107. doi:10.1016/j.jep.2015.06.031
16. Zhang QL, Zhang J, Xia PF, et al. Anti-inflammatory activities of gentiopicroside against iNOS and COX-2 targets. Chin Her Med. 2019;11(1):108–112.
17. Zhang QL, Xia PF, Peng XJ, et al. Synthesis, and anti-inflammatory activities of gentiopicroside derivatives. Chin J Nat Med. 2022;20(4):309–320. doi:10.1016/S1875-5364(22)60187-0
18. Fu R, Chen F, Guo Y. Anti-inflammatory mechanism and active ingredients of the Chinese tallow tree. J Ethnopharmacol. 2020;250:112497. doi:10.1016/j.jep.2019.112497
19. Wang XF, Li H, Jiang K, et al. Anti-inflammatory constituents from Perilla frutescens on lipopolysaccharide-stimulated RAW264.7 cells. Fitoterapia. 2018;130:61–65. doi:10.1016/j.fitote.2018.08.006
20. Labib MB, Fayez AM, El-Nahass E-S, et al. Novel tetrazole-based selective COX-2 inhibitors: design, synthesis, anti-inflammatory activity, evaluation of PGE2, TNF-α, IL-6 and histopathological study. Bioorg Chem. 2020;104:104308. doi:10.1016/j.bioorg.2020.104308
21. Singsai K, Charoongchit P, Chaikaew W, et al. Antilipoxygenase and anti-inflammatory activities of streblus asper leaf extract on xylene-induced ear edema in mice. Adv Pharmacol Pharm Sci. 2020;2020:3176391. doi:10.1155/2020/3176391
22. Oh YC, Jeong YH, Cho WK, Ha JH, Gu MJ, Ma JY. Anti-inflammatory and analgesic effects of pyeongwisan on LPS-stimulated murine macrophages and mouse models of acetic acid-induced writhing response and xylene-induced ear edema. Int J Mol Sci. 2015;16(1):1232–1251. doi:10.3390/ijms16011232
23. Liu Q, Mu Y, An Q, et al. Total synthesis and anti-inflammatory evaluation of violacin A and its analogues. Bioorg Chem. 2020;94:10342. doi:10.1016/j.bioorg.2019.103420
24. Halim PA, El-Nassan HB, El-Dash YS. Design and synthesis of novel ibuprofen derivatives as selective COX-2 inhibitors and potential anti-inflammatory agents: evaluation of PGE2, TNF-α, IL-6 and histopathological study. Med Chem. 2022;18(4):427–443. doi:10.2174/1573406417666210809162636
25. Xu J, Zhou L, Sun L, et al. 3α-angeloyloxy-ent-kaur-16-en-19-oic acid isolated from wedelia trilobata L. Alleviates xylene-induced mouse ear edema and inhibits NF-κB and MAPK pathway in LPS-stimulated macrophages. J Nat Prod. 2020;83(12):3726–3735. doi:10.1021/acs.jnatprod.0c00990
26. Gong LL, Yang S, Liu H, et al. Anti-nociceptive and anti-inflammatory potentials of Akebia saponin D. Eur J Pharmacol. 2019;845:85–90. doi:10.1016/j.ejphar.2018.11.038
27. Welday KS, Hailu GS, Taye DK. Design, synthesis, characterization and in vivo antidiabetic activity evaluation of some chalcone derivatives. Drug Des Devel Ther. 2021;15:3119–3129. doi:10.2147/DDDT.S316185
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.