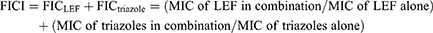Back to Journals » Infection and Drug Resistance » Volume 16
Synergy and Mechanism of Leflunomide Plus Fluconazole Against Resistant Candida albicans: An in vitro Study
Authors Li X, Zhang N, Zhang L, Liu C, Zheng S, Lou H
Received 20 April 2023
Accepted for publication 10 June 2023
Published 27 June 2023 Volume 2023:16 Pages 4147—4158
DOI https://doi.org/10.2147/IDR.S415229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiuyun Li,1,2 Ning Zhang,1 Liuping Zhang,3 Chang Liu,4 Shicun Zheng,1,* Hongxiang Lou2,*
1Maternal and Child Health Development Research Center, Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University, Jinan, Shandong Province, 250014, People’s Republic of China; 2Department of Natural Product Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, Shandong Province, 250012, People’s Republic of China; 3Pharmaceutical Department, Shanxian Central Hospital, Heze, Shandong Province, 274300, People’s Republic of China; 4Hospital for Reproductive Medicine Affiliated to Shandong University, Jinan, Shandong Province, 250021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shicun Zheng, Maternal and Child Health Development Research Center, Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University, Jinan, Shandong Province, 250014, People’s Republic of China, Tel/Fax +86 531 68795001, Email [email protected] Hongxiang Lou, Department of Natural Product Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, Shandong Province, 250012, People’s Republic of China, Tel/Fax +86 531 88382501, Email [email protected]
Objective: The global rise in the resistance of Candida albicans to conventional antifungals makes Candida albicans infections harder to treat. The main objective of this study was to investigate the antifungal effects and underlying mechanisms of leflunomide in combination with triazoles against resistant Candida albicans.
Methods: In this study, the microdilution method was used to determine the antifungal effects of leflunomide in combination with three triazoles on planktonic cells in vitro. The morphological transition from yeast to hyphae was observed under a microscope. The effects on ROS, metacaspase, efflux pumps, and intracellular calcium concentration were investigated, respectively.
Results: Our findings suggested that leflunomide + triazoles showed a synergistic effect against resistant Candida albicans in vitro. Further study concluded that the synergistic mechanisms were resulted from multiple factors, including the inhibited efflux of triazoles, the inhibition of yeast-to-hyphae transition, ROS increasing, metacaspase activation, and [Ca2+]i disturbance.
Discussion: Leflunomide appears to be a potential enhancer of current antifungal agents for treating candidiasis caused by resistant Candida albicans. This study can also serve as an example to inspire the exploration of new approaches to treating resistant Candida albicans.
Keywords: Candida albicans, triazoles, leflunomide, synergy, synergistic mechanism
Background
Increasing drug resistance in microorganisms is very common worldwide. Previous studies have mainly focused on multidrug-resistant bacteria. However, the global rise in antifungal resistance must also be emphasized. Indeed, mortality rates caused by invasive fungal infections are 40–60% or higher, particularly in settings with antifungal resistance.1–3 Even worse, the severe acute respiratory syndrome coronavirus (SARS-CoV-2) pandemic globally raises the risk of fungal infections because the SARS-CoV-2 infection alters patients’ immune and metabolic responses, which together creates an inflammatory environment that is highly conducive to fungal infections.4–6 In response to the rising threat of fungal infections, as well as the existing and emerging resistance and treatability issues, WHO developed the first fungal priority pathogens list.7 Among the list, Candida albicans (C. albicans) is assigned to the critical group, mainly given its antifungal resistance, mortality, evidence-based treatment, access to diagnostics, annual incidence and complications.
Notably, infections in the bloodstream are difficult to treat and sometimes deadly because of the drug resistance of C. albicans. Most available conventional antifungal drugs are either ineffective against triazole-resistant C. albicans or exhibit efficacy only when used at very high doses. To solve the problem of resistant C. albicans, studies on adjuvants to existing antifungal agents have received wide attention. Combination therapy with antifungals and non-antifungals may have strong beneficial effects against resistant C. albicans and has been a research focus in recent years. Numerous studies have found that several non-antifungal agents, such as antibacterials, calcium channel blockers, and immunomodulators, could enhance the sensitivity of resistant C. albicans to antifungal drugs.8–11
Since leflunomide (LEF), an immunomodulator, was approved by the US Food and Drug Administration in 1988 for rheumatoid arthritis, it has been increasingly used in clinical applications.12 Until now, LEF has been reported to exert multiple effects, including anti-inflammatory, antiproliferative, anti-chemotherapeutic resistance, and anti-angiogenic effects.13 Nevertheless, many questions remain to be answered about the potential clinical applications of LEF. Several immunomodulators have been studied with a view to repurpose them as antifungal chemotherapies. Immunomodulators, such as tacrolimus, cyclosporine A, and budesonide, have been proven to exert synergistic effects with triazoles against resistant C. albicans in vitro.10,14,15 Inspired by these findings, the present study aimed to evaluate whether LEF and triazoles can exert synergistic effects against resistant C. albicans in vitro, and the underlying synergistic mechanisms for these drug combinations.
Materials and Methods
Strains
Four C. albicans strains, and eight non-albicans Candida species (NAC) strains, including four Candida krusei (C. krusei) strains and four Candida glabrata (C. glabrata) strains, were used in this study. All these Candida strains were clinical isolates from previously established stocks and were kindly provided by Professor Shujuan Sun (Shandong Provincial Qianfoshan Hospital, Jinan, China). These strains were by Matrix-Assisted Laser Desorption Ionization Time-of-flight Mass Spectrometry (EXS3000, Zhongyuan Huiji Biotechnology Co., LTD), and their relevant information was shown in Table S1.
Antifungal Agents
All active pharmaceutical ingredients, including LEF, fluconazole (FLC), itraconazole (ITR), and voriconazole (VRC) were purchased from Dalian Meilun Biotech Co., Ltd., Dalian, China. Solutions of LEF, ITR and VRC were prepared with ethyl alcohol. FLC solution was dissolved in sterile water. Stock solutions were stored away from light at −20 °C.
Determination of Minimum Inhibitory Concentrations (MICs) of Planktonic Cells in vitro
The in vitro antifungal effects of LEF and triazoles against Candida spp. were determined using the broth microdilution method according to the M27-A3 guidelines of the Clinical and Laboratory Standards Institute (CLSI).16 MIC in this study was defined as the lowest drug concentration of the drug capable of inhibiting cell growth by 80% in comparison with the control by visual observation.16,17
All drug solutions were diluted twofold to achieve final concentrations ranging from 16–1024 μg/mL for LEF, 0.0625–128 μg/mL for ITR, and 0.0313–64 μg/mL for VRC. Next, 100 μL cell suspension of Candida spp. was added to 96-well microplates. Wells containing 100 μL of fungal suspension and 100 μL of RPMI 1640 served as the control. Susceptibility tests were performed in 96-well plates at 35 °C for 24 h (for FLC) or 48 h (for ITR and VRC), according to the CLSI M27-A3.16 All experiments were repeated thrice.
The fractional inhibitory concentration index (FICI) model was used to evaluate the synergistic effects of these drug combinations in vitro. Calculation of FICIs was carried out as shown below:
Test of the Inhibitory Effect on Yeast-to-Hypha Transition
Spider medium was used to induce the morphological transition from yeast to hypha in 96-well plates.19,20 Test plates were supplemented with a cell suspension (2 × 105 CFU/mL) of resistant C. albicans CA10 and drugs. Plates without drugs served as the control. C. albicans was allowed to grow for 4 h at 35 °C. Before imaging, the medium was gently removed and discarded to retain the adherent cells, and the wells in the plate were washed three times with sterilized PBS to remove the nonadherent cells. The hyphal growth in the control and drug-treated plates was visualized under a bright-field Olympus fluorescence microscope (Leica DMi8, Germany) using a 40× objective lens. The experiment was repeated three times.
Test of Rhodamine 6G (Rh6G) Efflux
Rh6G efflux was assessed to specifically detect the interference of LEF on the intracellular concentration of triazoles in resistant C. albicans CA10 cells, according to a previously described protocol with minor modifications.21 Briefly, C. albicans cells were incubated overnight at 35 °C in a liquid YPD medium. The cells were harvested and resuspended in sterilized PBS (without glucose) at 1×107 cells/mL and then starved for 1 h. After centrifugation and washing three times, the cells were stained with Rh6G at a final concentration of 10 µM for 50 min in the dark. Rh6G absorption was stopped by an ice-water bath (15 min). The equilibrated cells with Rh6G were washed to remove the extracellular Rh6G and then co-incubated with 64 µg/mL LEF. Equilibrated cells without LEF treatment were used as the control. The mean fluorescent intensity (MFI) of cells was recorded with a BD FACSAria II flow cytometer (Becton Dickinson, United States) at an excitation wavelength of 488 nm and an emission wavelength of 530 nm. The interval point was 50 min. Each assay was performed in triplicate.
Test of ROS Production
Intracellular ROS production in resistant C. albicans CA10 was determined by a BD FACSAria II flow cytometer (Becton Dickinson, United States) using 2, 7-dichlorodihydrofluorescein diacetate (DCFH-DA), according to our previous publication with minor modifications.21 DCFH-DA is a nonpolar, nonfluorescent compound that readily diffuses across membranes. It is hydrolyzed within the cell by esterases to the polar, nonfluorescent, membrane-impermeable derivative 2, 7-dichlorodihydrofluorescein (DCFH), which is rapidly oxidized by ROS to the highly fluorescent 2, 7-dichlorofluorescein (DCF).22 CA10 cell suspensions (5 × 105 CFU/mL) were treated with different drugs for 4 h, and cells treated without drugs served as the control group. Then, the cells were harvested and stained with 10 μM DCFH-DA (MedChem Express, NJ, USA) in the dark for 30 min at room temperature. The MFI of each group was detected by flow cytometry.
Test of Metacaspase Activity
CaspACETM FITC-VAD-FMK (Promega, Madison, Wisconsin, USA) is a fluorescent dye that binds specifically to the active site of metacaspases, which fluorescence intensity was measured to identify the metacaspase activation in resistant C. albicans CA10, as described previously with a few modifications.19 A suspension (5×106 CFU/mL) of CA10 cells was treated with drugs and incubated overnight (35 °C, 200 rpm). Cells treated without drugs served as the control. Subsequently, the cells were washed twice with sterilized PBS and collected. The collected cells were treated with a mixed liquid of 50 mmol/L K2HPO4, 5 mmol/L EDTA, and 50 mmol/L DTT for 30 min (35 °C, 100 rpm) to dissolve the cell wall. After washing twice with sterilized PBS and collection, the cells were incubated with 1.5% snailase for 45 min (35 °C, 100 rpm) to obtain CA10 cells without the cell wall. Finally, the cells were stained with 5 µM CaspACE FITC-VAD-FMK at 35 °C for 1 h in darkness. Then, 30 µL of cell suspension was placed on a glass slide and photographed using a 40× objective lens under an Olympus fluorescence microscope (Leica DMi8, Germany). The experiment was repeated three times.
Test of Intracellular Calcium Concentration ([Ca2+]i)
Fura-3AM is an indicator of [Ca2+]i. The effects of LEF in combination with FLC on [Ca2+]i were tested using the fluorescent probe Fluo-3AM.23 Briefly, resistant C. albicans CA10 cells were collected and washed twice with a HBSS buffer. Cells were resuspended in 1 mL of HBSS buffer treated with 0.1% pluronic F-127, and stained with Fluo-3AM (5 µM) for 40 min without light. Unabsorbed Fluo-3AM was removed by washing the cells. After the cells were treated with or without drugs, the MFIs of Fluo-3AM were immediately recorded using the flow cytometry as above described. [Ca2+]i calculation was performed using protocols as we previously described.21 All experiments were repeated thrice.
Statistical Analysis
Every experiment was independently carried out at least three times, and data are shown as the mean ± SD. The results were analyzed by the GraphPad Prism 8 software. Asterisks indicate statistically significant data (*P < 0.05, **P < 0.01, and ***P < 0.001).
Results
Planktonic Cell Assay
The antifungal effects of LEF in combination with three triazoles (FLC, ITR and VRC) against twelve Candida spp. strains were evaluated as shown in Tables 1–3. The data in Tables 1–3 show that LEF alone has little obvious antifungal effects on twelve Candida spp. strains, with MIC >1024 µg/mL. However, when LEF (0.0313–0.5 µg/mL) was combined with each of the triazoles, the MICs of the triazoles against the two resistant C. albicans strains were significantly reduced: the MIC of FLC from > 512 µg/mL to 0.25–0.5 µg/mL, the MIC of ITR from > 64 µg/mL to 0.0625 µg/mL, and the MIC of VRC from >64 µg/mL to 0.0313–0.0625 µg/mL, indicating a strong synergistic effect. No synergistic effect was found for triazole-susceptible C. albicans and NAC strains.
 |
Table 1 Drug Interactions of LEF and FLC Against Candida spp. in vitro |
 |
Table 2 Drug Interactions of LEF and ITR Against Candida spp. in vitro |
 |
Table 3 Drug Interactions of LEF and VRC Against Candida spp. in vitro |
Treatment with FLC+LEF Inhibited Yeast-to-Hypha Transition
The inhibition of yeast-to-hypha transition in resistant C. albicans was shown using Spider medium. Microscopic images of the hyphae showed the effects against the hyphal growth of resistant C. albicans. The control and drug alone groups showed massive hyphal growth; however, the presence of FLC+LEF significantly inhibited hyphal growth by reducing the length and number of hyphae (Figure 1). The results from these experiments indicated that FLC+LEF inhibits the yeast-to-hypha transition of resistant C. albicans.
Rh6G Efflux Assay
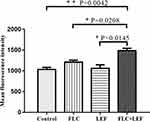
Both Rh6G and triazoles are substrates for efflux pumps. Here, Rh6G was used as the fluorescent alternative of triazoles to evaluate the efflux of triazoles. Previous works by our group indicated that inhibiting the efflux of triazoles plays a role in reversing antifungal resistance.15,24 The cells in the presence of LEF showed significantly lower MFI values than those obtained from the control group, suggesting that the efflux of triazoles is inhibited in the LEF-treated cells (Figure 2).
Treatment with FLC+LEF Increases ROS Level
ROS plays an important role as an initiator of early apoptosis in yeasts and other filamentous fungi. To determine whether the antifungal mechanism of FLC+LEF is conferred by apoptosis, we examined the ROS levels. DCFH-DA is the preferred dye to detect changes in ROS. The increased DCFH-DA fluorescence represents an increase in ROS. The intracellular ROS in the drug combination group was elevated as there were significant increases in DCFH-DA fluorescence (Figure 3) compared with that in the control and drug alone groups. These findings preliminarily indicated that FLC+LEF partially exerts synergistic antifungal effects on resistant C. albicans by triggering cell apoptosis.
Treatment with FLC+LEF Induces Metacaspase Activation
Metacaspases, which are caspase-like cysteine proteases in yeast, are significantly associated with the generation of ROS and mitochondrial dysfunction. Metacaspases play a central role in the early stages of apoptosis and can be detected by FITC-VAD-FMK staining. Cells with activated intracellular metacaspases show green fluorescence. In our study, obvious green fluorescence was observed in the FLC+LEF group, but was nearly undetectable in the other three groups was nearly undetectable (Figure 4). These results demonstrated that FLC+LEF could induce metacaspase activation in resistant C. albicans CA10 cells.
Treatment with FLC+LEF Increases [Ca2+]i
As one of the most important signaling messengers in cells, Ca2+ plays an important role in many organisms. Our previous work demonstrated that [Ca2+]i can be closely associated with drug resistance in fungi.21,25 Moreover, [Ca2+]i acts as an initiator of apoptosis.26 In this study, Fluo-3AM was used to measure [Ca2+]i levels in resistant C. albicans cells. [Ca2+]i was significantly increased in cells treated with the combination of LEF and FLC (Figure 5) at 20 min (P<0.001). Our results indicated that the synergistic mechanisms of this drug combination might be associated with the unexpected movement of [Ca2+]i.
Discussion
Triazoles have been applied in the clinic for over 50 years. Over the years, the application of triazoles has been limited by the resistance of C. albicans. The general resistance of C. albicans to the available antifungal drugs and the emergence of multidrug-resistant strains make urgent the need to develop novel effective antifungal approaches. Seeking out new compounds with antifungal activity from a wide range of natural products, including essential oils, can be a means to combat resistant C. albicans.27–29 On the other hand, drug repurposing is also an anticipative chemotherapeutic strategy that serves to combat the resistance of C. albicans. Non-antifungal drugs, as sensitizers of existing antifungal drugs, can combined with existing antifungal drugs to act synergically against drug-resistant fungi, which has attracted wide attention as one of the forms of drug repurposing. Synergy implies a rapid increase in the antifungal effects of two agents acting together. The synergistic effects of combining agents have been highlighted as a novel antifungal approach. The combination strategy allows each individual drug to exert its ideal efficacy with very low MIC. Much effort has been expended to identify adjuvants of antifungal drugs from pharmacologically distinct families. To date, combinations of a range of commonly used antifungal drugs with various non-antifungal drugs or compounds have been found to show synergistic effects in combating resistant C. albicans. Inspired by the synergistic antifungal effects of immunosuppressants plus antifungal drugs, this study was primarily conducted to investigate the effects of LEF, an immunomodulator, in combination with triazoles and to determine their mode of action against Candida strains. No synergistic effect was found for these drug combinations against susceptible C. albicans strains, susceptible NAC strains, or resistant NAC strains. However, we were surprised that LEF acts synergistically with each triazole against two tested resistant C. albicans strains in vitro, as interpreted by the FICI model. LEF decreased the MIC of triazoles approximately 1, 024–2, 048 times in this study, indicating its great potential as an adjuvant for triazoles to reverse the resistance of C. albicans. Many experiments are needed to explain this phenomenon. When studying the synergistic mechanisms, FLC is selected as the representative of triazole because of its advantages of low price and wide application. Besides, we randomly selected CA10 from these two resistant C. albicans strains for further exploration of the underlying mechanisms of the drug combination FLC+LEF, considering that the MIC of CA10 and CA16 was the same when exposed to these two drugs.
In this study, we explored several synergistic mechanisms, including traditional mechanisms (eg efflux pumps, morphological transition) and noncanonical mechanisms (eg apoptosis, [Ca2+]i). C. albicans, as a polymorphic fungus, resides in host niches in both yeast and hypha forms. C. albicans yeasts can form hyphae both in planktonic cultures and during the maturation step of biofilm formation.30 The formation of C. albicans biofilm can lead to strong resistance to conventional antifungal agents.31,32 As the most closely related virulence factor of biofilm, hyphae formed by C. albicans undoubtedly play a central role in the resistance of C. albicans.33,34 Defining the effect of FLC+LEF on the morphogenesis of resistant C. albicans is critical for developing targeted fungal therapeutics to prevent resistant C. albicans colonization and pathogenesis. Accordingly, the effect of FLC+LEF on the growth of C. albicans hyphae was determined in this study. As expected, the results in our study indirectly verified the role of morphogenesis in fungal resistance, and demonstrated that FLC+LEF could obviously inhibit the yeast-to-hypha transition of resistant C. albicans.
The overexpression of efflux pumps, one of the canonical mechanisms in C. albicans resistance, is among the culprits of antifungal agent resistance in recent decades. C. albicans with enhanced efflux pumps shows resistance to triazoles, and inhibitory strategies on the activity of efflux pumps are being explored. The activity of efflux pumps in triazole-resistant C. albicans, especially Cdr 1, can be intuitively reflected by the intracellular accumulation of Rh6G, a fluorescent alternative to triazoles.35 This study indicated that the addition of LEF could significantly suppress the efflux of triazoles, which can be indicated by the fluorescence intensity of Rh6G (Figure 2). These findings demonstrated that the synergism between LEF and triazoles may be related to the elevated intracellular triazoles in resistant C. albicans.
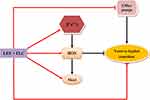
Recently, inducing apoptosis has been regarded as a vital approach to combatting C. albicans. For example, plagiochin E induces apoptosis in C. albicans cells through a ROS/metacaspase-dependent pathway.36 Amentoflavone triggers apoptosis in C. albicans cells through a •OH-induced mitochondria-dependent pathway.37 Researchers found that fungi do not completely recapitulate the mammalian apoptotic system.38 The possible architectural differences between the apoptotic mechanisms of fungi and mammalian cells may open the door for designing new antifungal agents to solve the problem of antifungal resistance. ROS is a major mediating factor of cell apoptosis in fungi. ROS can oxidize intracellular biological macromolecules, cause cell damage, and activate metacaspase/Mca1 and cause cell apoptosis in C. albicans.39,40 Metacaspase/Mca1 is located in downstream of the apoptosis pathway, and most of the apoptotic processes induce apoptosis by activating metacaspase/Mca1. Metacaspase/Mca1 has also been described as a key protease for apoptosis in C. albicans.41 In addition, Ca2+ is a key regulator not only of antifungal resistance but also of cell apoptosis. Studies have proven that increased [Ca2+]i can promote ROS generation and then activate the apoptotic pathway.42 Besides, the growth of hyphae is also closely connected to ROS production. It is proved that increased intracellular ROS can lead to filamentation defects of C. albicans.43,44 Pasrija et al showed that erg1 mutant C. albicans strains could not produce hyphae, and this mutation reduced the drug efflux activity of C. albicans, which indirectly indicates the relationship between yeast-to-hypha transition and efflux pumps.45 Considering our current results and the previous studies mentioned above, we have reason to suspect that the ROS-mediated apoptosis pathway plays an important role in antifungal drug resistance, and the ROS-mediated apoptosis pathway is likely to be the central part for LEF + FLC reversing C. albicans resistance (Figure 6). One of the shortcomings of this study is that it does not apply more resistant C. albicans strains to expand the scope of the study, which is what we want to continue to do in the future.
Conclusion
In conclusion, we found a novel drug combination, LEF+triazoles, against resistant C. albicans. In summary, this study first confirmed the synergistic antifungal effects of LEF in combination with triazoles against planktonic cells of resistant C. albicans in vitro. Further study concluded that the synergistic mechanisms resulted from multiple factors, mainly apoptosis. With a better understanding of the underlying synergistic mechanisms, this study provides a theoretical basis for the antifungal application of these drug combinations.
It also provides a reference for the study of antifungal drugs. Nevertheless, further explorations are still required to shed light on the molecular mechanisms of these drug combinations against resistant C. albicans. This study can also be an encouraging example of exploring new approaches against resistant C. albicans.
Abbreviations
LEF, Leflunomide; FLC, Fluconazole; ITR, Itraconazole; VRC, Voriconazole; MIC, Minimum inhibitory concentration; FICI, Fractional inhibitory concentration index; SMIC, Sessile minimum inhibitory concentration; CA, Candida albicans; CG, Candida glabrata; CK, Candida krusei.
Acknowledgments
We are very grateful to Dr. Yueling Wang (Department of Clinical Laboratory, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, 250014, Shandong Province, P.R. China) for his assistance in the identification of strains and the determination of drug susceptibility.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the [National Natural Science Foundation of China] under Grant [82173703], [Key Research and Development Plan of Shandong Province] under Grant [GG201809270073] and [Shandong Provincial Natural Science Foundation] under Grant [ZR2020QH365]. These funding organizations had no role in the design of the study and the collection, analysis and interpretation of the data.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gow NAR, Yadav B. Microbe profile: Candida albicans: a shape-changing, opportunistic pathogenic fungus of humans. Microbiology. 2017;163:1145–1147. doi:10.1099/mic.0.000499
2. Robbins N, Caplan T, Cowen LE. Molecular evolution of antifungal drug resistance. Annu Rev Microbiol. 2017;71(1):753–775. doi:10.1146/annurev-micro-030117-020345
3. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–163. doi:10.1128/CMR.00029-06
4. Naveen KV, Saravanakumar K, Sathiyaseelan A, et al. Human fungal infection, immune response, and clinical challenge-a perspective during COVID-19 pandemic. Appl Biochem Biotechnol. 2022;194(9):4244–4257. doi:10.1007/s12010-022-03979-5
5. Chavda VP, Mishra T, Kamaraj S, et al. Post-COVID-19 fungal infection in the aged population. Vaccines. 2023:11. doi:10.3390/vaccines11030555
6. Hoenigl M, Seidel D, Sprute R, et al. COVID-19-associated fungal infections. Nat Microbiol. 2022;7(8):1127–1140. doi:10.1038/s41564-022-01172-2
7. World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. World Health Organization; 2022.
8. Liu S, Yue L, Gu W, et al. Synergistic effect of fluconazole and calcium channel blockers against resistant Candida albicans. PLoS One. 2016;11(3):e0150859. doi:10.1371/journal.pone.0150859
9. Gao Y, Zhang C, Lu C, et al. Synergistic effect of doxycycline and fluconazole against Candida albicans biofilms and the impact of calcium channel blockers. FEMS Yeast Res. 2013;13(5):453–462. doi:10.1111/1567-1364.12048
10. Sun S, Li Y, Guo Q, et al. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob Agents Chemother. 2008;52(2):409–417. doi:10.1128/AAC.01070-07
11. Wibawa T, Baly I, Daeli PR, et al. Cyclosporine A decreases the fluconazole minimum inhibitory concentration of Candida albicans clinical isolates but not biofilm formation and cell growth. Trop Biomed. 2015;32:176–182.
12. Zhang M, Qi C, Zha Y, et al. Leflunomide versus cyclophosphamide in the induction treatment of proliferative lupus nephritis in Chinese patients: a randomized trial. Clin Rheumatol. 2019;38(3):859–867. doi:10.1007/s10067-018-4348-z
13. Zhang C, Chu M. Leflunomide: a promising drug with good antitumor potential. Biochem Biophys Res Commun. 2018;496(2):726–730. doi:10.1016/j.bbrc.2018.01.107
14. Li Y, Sun S, Guo Q, et al. In vitro interaction between azoles and cyclosporin A against clinical isolates of Candida albicans determined by the chequerboard method and time-kill curves. J Antimicrob Chemother. 2008;61(3):577–585. doi:10.1093/jac/dkm493
15. Li X, Yu C, Huang X, et al. Synergistic effects and mechanisms of budesonide in combination with fluconazole against resistant Candida albicans. PLoS One. 2016;11(12):e0168936. doi:10.1371/journal.pone.0168936
16. Rex JH, Alexander BD, Andes D, et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition. Vol. 28. CLSI; 2008.
17. Savage KA, Parquet MC, Allan DS, et al. Iron restriction to clinical isolates of Candida albicans by the novel chelator DIBI inhibits growth and increases sensitivity to azoles in vitro and in vivo in a murine model of experimental vaginitis. Antimicrob Agents Chemother. 2018;62. doi:10.1128/AAC.02576-17
18. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. doi:10.1093/jac/dkg301
19. Chen X, Shi Y, Li Y, et al. Antifungal effects and potential mechanisms of lonidamine in combination with fluconazole against Candida albicans. Expert Rev Anti Infect Ther. 2021;19(1):109–115. doi:10.1080/14787210.2020.1811684
20. Chang W, Li Y, Zhang L, et al. Retigeric acid B attenuates the virulence of Candida albicans via inhibiting adenylyl cyclase activity targeted by enhanced farnesol production. PLoS One. 2012;7(7):e41624. doi:10.1371/journal.pone.0041624
21. Wang T, Flint S, Palmer J. Magnesium and calcium ions: roles in bacterial cell attachment and biofilm structure maturation. Biofouling. 2019;35(9):959–974. doi:10.1080/08927014.2019.1674811
22. Weissman L, Garty J, Hochman A. Rehydration of the Lichen Ramalina lacera results in production of reactive oxygen species and nitric oxide and a decrease in antioxidants. Appl Environ Microbiol. 2005;71(4):2121–2129. doi:10.1128/AEM.71.4.2121-2129.2005
23. Li Y, Jiao P, Li Y, et al. The synergistic antifungal effect and potential mechanism of D-penicillamine combined with fluconazole against Candida albicans. Front Microbiol. 2019;10:2853. doi:10.3389/fmicb.2019.02853
24. Zhang M, Yan H, Lu M, et al. Antifungal activity of ribavirin used alone or in combination with fluconazole against Candida albicans is mediated by reduced virulence. Int J Antimicrob Agents. 2020;55(1):105804. doi:10.1016/j.ijantimicag.2019.09.008
25. Li X, Sun S. Targeting the fungal calcium-calcineurin signaling network in overcoming drug resistance. Future Med Chem. 2016;8:1379–1381. doi:10.4155/fmc-2016-0094
26. Tian J, Lu Z, Wang Y, et al. Nerol triggers mitochondrial dysfunction and disruption via elevation of Ca(2+) and ROS in Candida albicans. Int J Biochem Cell Biol. 2017;85:114–122. doi:10.1016/j.biocel.2017.02.006
27. Spengler G, Gajdacs M, Donadu MG, et al. Evaluation of the antimicrobial and antivirulent potential of essential oils isolated from Juniperus oxycedrus L. ssp. macrocarpa aerial parts. Microorganisms. 2022;10(4):758. doi:10.3390/microorganisms10040758
28. Hamdy R, Fayed B, Hamoda AM, et al. Essential oil-based design and development of novel anti-Candida azoles formulation. Molecules. 2020;25(6):1463. doi:10.3390/molecules25061463
29. Cid-Chevecich C, Muller-Sepulveda A, Jara JA, et al. Origanum vulgare L. essential oil inhibits virulence patterns of Candida spp. and potentiates the effects of fluconazole and nystatin in vitro. BMC Complement Med Ther. 2022;22(1):39. doi:10.1186/s12906-022-03518-z
30. Lohse MB, Gulati M, Johnson AD, et al. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. 2018;16(1):19–31. doi:10.1038/nrmicro.2017.107
31. Desai JV, Mitchell AP, Ghannoum M, Parsek M, Whiteley M, Mukherjee P. Candida albicans biofilm development and its genetic control. Microbiol Spectr. 2015;3(3). doi:10.1128/microbiolspec.MB-0005-2014
32. Oppenheimer-Shaanan Y, Steinberg N, Kolodkin-Gal I. Small molecules are natural triggers for the disassembly of biofilms. Trends Microbiol. 2013;21(11):594–601. doi:10.1016/j.tim.2013.08.005
33. Gow NA, van de Veerdonk FL, Brown AJ, et al. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2011;10:112–122. doi:10.1038/nrmicro2711
34. Meng L, Zhao H, Zhao S, et al. Inhibition of yeast-to-hypha transition and virulence of Candida albicans by 2-alkylaminoquinoline derivatives. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.01891-18
35. Maesaki S, Marichal P, Vanden Bossche H, et al. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J Antimicrob Chemother. 1999;44(1):27–31. doi:10.1093/jac/44.1.27
36. Wu XZ, Cheng AX, Sun LM, et al. Plagiochin E, an antifungal bis(bibenzyl), exerts its antifungal activity through mitochondrial dysfunction-induced reactive oxygen species accumulation in Candida albicans. Biochim Biophys Acta. 2009;1790:770–777. doi:10.1016/j.bbagen.2009.05.002
37. Hwang IS, Lee J, Jin HG, et al. Amentoflavone stimulates mitochondrial dysfunction and induces apoptotic cell death in Candida albicans. Mycopathologia. 2012;173:207–218. doi:10.1007/s11046-011-9503-x
38. Frohlich KU, Fussi H, Ruckenstuhl C. Yeast apoptosis--from genes to pathways. Semin Cancer Biol. 2007;17:112–121. doi:10.1016/j.semcancer.2006.11.006
39. Tian H, Qu S, Wang Y, et al. Calcium and oxidative stress mediate perillaldehyde-induced apoptosis in Candida albicans. Appl Microbiol Biotechnol. 2017;101(8):3335–3345. doi:10.1007/s00253-017-8146-3
40. Hwang JH, Hwang IS, Liu QH, et al. (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie. 2012;94:1784–1793. doi:10.1016/j.biochi.2012.04.010
41. Chen Y, Zeng H, Tian J, et al. Dill (Anethum graveolens L.) seed essential oil induces Candida albicans apoptosis in a metacaspase-dependent manner. Fungal Biol. 2014;118(4):394–401. doi:10.1016/j.funbio.2014.02.004
42. Peng L, Yu Q, Zhu H, et al. The V-ATPase regulates localization of the TRP Ca(2+) channel Yvc1 in response to oxidative stress in Candida albicans. Int J Med Microbiol. 2020;310:151466. doi:10.1016/j.ijmm.2020.151466
43. Sharma M, Manoharlal R, Puri N, et al. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci Rep. 2010;30(6):391–404. doi:10.1042/BSR20090151
44. Bi S, Lv QZ, Wang TT, et al. SDH2 is involved in proper hypha formation and virulence in Candida albicans. Future Microbiol. 2018;13:1141–1156. doi:10.2217/fmb-2018-0033
45. Pasrija R, Krishnamurthy S, Prasad T, et al. Squalene epoxidase encoded by ERG1 affects morphogenesis and drug susceptibilities of Candida albicans. J Antimicrob Chemother. 2005;55(6):905–913. doi:10.1093/jac/dki112
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.