Back to Journals » Journal of Inflammation Research » Volume 16
Synergistic Enhancement of Isoforskolin and Dexamethasone Against Sepsis and Acute Lung Injury Mouse Models
Authors Fang Y, Xiao C, Wang L, Wang Y, Zeng J, Liang Y, Huang R, Shi Y, Wu S, Du X, Sun S, Li M, Zheng Y, Wu H, Guo Q, Yang W
Received 14 May 2023
Accepted for publication 9 October 2023
Published 7 December 2023 Volume 2023:16 Pages 5989—6001
DOI https://doi.org/10.2147/JIR.S421232
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Yan Fang,1,2,* Chuang Xiao,1,* Lueli Wang,1,* Youlan Wang,3 Jun Zeng,1 Yaping Liang,1 Rong Huang,1 Yunke Shi,2 Sha Wu,1 Xiaohua Du,2 Shibo Sun,2 Min Li,2 Yuanyuan Zheng,2 Hongxiang Wu,1 Qiuzhe Guo,4 Weimin Yang1
1School of Pharmaceutical Science and Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming, People’s Republic of China; 2The First Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 3Kunming Institute of Medical Sciences, Kunming, People’s Republic of China; 4Fuwai Yunnan Cardiovascular Hospital, Kunming, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiuzhe Guo; Weimin Yang, Email [email protected]; [email protected]
Background: Sepsis is initiated by the dysfunctional response of the host immune system to infection. Septic shock and acute lung injury (ALI) are the main etiology of death caused by sepsis. Glucocorticoids, which are commonly used in clinic to antagonize the inflammatory response of sepsis, may cause serious side effects. Isoforskolin (ISOF) from the plant Coleus forskohlii stimulates adenylyl cyclase, increases the cAMP level and inhibits inflammatory response. The aim of this study was to investigate the synergistic effect of ISOF with dexamethasone (DEX) to prevent and ameliorate septic inflammation.
Methods: Lipopolysaccharide (LPS) of 30 and 5 mg/kg (iv.) was used to induce sepsis and ALI mice model respectively in vivo. BEAS-2B cells stimulated by LPS were applied as cell model in vitro. The cumulative survival of mice with LPS-induced sepsis and the histopathological changes of lungs in mice with acute lung injury were observed, and the secretion of pro-inflammatory cytokines was analyzed by ELISA. The expression of RGS2 in BEAS-2B cells was detected by immunoblotting assay and PCR.
Results: In the sepsis mice model, ISOF (10 mg/kg) combined with DEX (10 mg/kg.) (ip.) pretreatment significantly increased mice survival rate from 33.3% to 58.3%, which was significantly higher than that of ISOF or DEX treated alone. In the ALI mice model, ISOF, DEX pretreatment alone and combined application attenuated pulmonary pathological changes in ALI mice. Furthermore, ISOF, DEX alone or combined administration decreased MPO, MDA, IL-6, and IL-8 levels, while significantly synergistic effects were observed in the combined treatment group compared with ISOF or DEX alone. In BEAS-2B cells, combined pretreatment with ISOF and DEX significantly decreased the expression of IL-8 and increased the expression of RGS2.
Conclusion: The results indicated that ISOF in combination with DEX synergistically improves survival rate and attenuates ALI in mice model through anti-inflammatory and antioxidant effects.
Keywords: isoforskolin, sepsis, acute lung injury, pulmonary inflammation, glucocorticoids, regulator of G-protein signaling 2
Introduction
Sepsis is a dysregulated response of the host immune system to infection, resulting in life-threatening organ dysfunction.1 Sepsis has a high incidence and is one of the leading causes of death worldwide.2 Despite advances in intensive care medicine and related disciplines, the mortality rate for patients who develop septic shock remains high, creating a heavy economic burden.3 Lungs are more vulnerable to inflammatory cytokines than other organs and are the most susceptible target organ, especially in sepsis patients.4 Acute lung injury (ALI) and severe acute respiratory distress syndrome (ARDS) are serious respiratory diseases that are the main cause of sepsis and the manifestation of sepsis involving the lungs. Despite decades of clinical efforts, mortality in patients with ALI remains high (>50%), and sepsis-related ALI/ARDS has a higher mortality rate compared with ALI induced by other causes.5,6 Lipopolysaccharide (LPS) is an endotoxin, which can activate the inflammatory signal pathway via toll-like receptor 4 (TLR4), induce a large number of inflammatory cytokines and recruit extensive macrophages and neutrophils in the lung.7
To date, there are no effective therapeutic drugs for sepsis and ALI, and the mortality rate remains high. The guidelines recommend corticosteroids used in the presence of shock for some benefit in sepsis.8 Dexamethasone (DEX) is a glucocorticoid (GC) commonly used in clinical practice and exhibits anti-inflammatory properties in a variety of diseases, such as sepsis.9 Studies have shown that DEX has a protective effect on pulmonary dysfunction.10,11 But the use of large amounts of GCs can lead to slow clearance of viruses and bacteria in the body, and the adverse effects of GC have limited their use.12 Therefore, the systemic use of GCs for the treatment of sepsis and ALI is controversial, and new effective therapeutic agents and strategies to increase the efficacy of GCs are urgently needed.
In recent years, anti-inflammatory drugs regulating cAMP are gaining more and more attention. It has been found through numerous studies that cAMP acts as an anti-inflammatory agent by inducing the regulation of pro-catabolic mediators, apoptosis, bubble cell action and phagocytosis, non-phagocytic recruitment of macrophages, macrophage polarization, and restoration of aspects of tissue homeostasis.13 Isoforskolin (ISOF), the extract of medicinal plant Coleus forskohlii in Yunnan, is an adenylyl cyclase agonist which can increase cAMP levels in different tissues and cells, thus regulating many cell functions and has anti-inflammatory effect.14 Increasing cAMP has been shown to have a preventive effect on ALI, asthma and COPD.15–17 We previously found that ISOF can improve the survival rate of LPS-induced sepsis mice, and ISOF can reduce the lung injury of various models caused by LPS intravenous injection and in situ perfusion, and reduce the levels of neutrophil and inflammatory cytokines in the lung tissues.14 Moreover, ISOF showed inhibitory effect on LPS-induced release of inflammatory mediators in human mononuclear leukocytes.18
Regulator of G-protein signaling 2 (RGS2) is an important member of the G protein-binding receptor family, a GTPase-activating protein that attenuates Gq signaling. RGS2 is distributed in airway epithelial cells and airway smooth muscle, it inhibits leukocyte aggregation, has anti-inflammatory and bronchoprotective effects, and is a potential therapeutic target for asthma and COPD.19–25 GCs have two different modes of action, genomic and non-genomic mechanisms. Genomic mechanisms are those in which GCs bind to their nuclear receptors and act by regulating the expression of relevant genes in the nucleus. In contrast, GCs are also able to regulate intracellular cAMP and Ca2+ levels and their downstream signaling pathways in a rapid response, causing cellular and organismal changes, and there may be membrane receptors for GCs, also known as non-genomic effects of GC. Stephanie Greer et al found that adenosine A2B receptor agonists, when used in conjunction with glucocorticoids, may be useful in the treatment of inflammatory diseases that do not respond well to glucocorticoid monotherapy. The mechanism of action is through mediated gene expression associated with cAMP formation, which RGS2 is one of the genes found to be positively cooperative. The gene expression of RGS2 represents a genomic mechanism that may contribute to the therapeutic activity of GCs.19–25 Therefore, the aim of the present study was to investigate the synergistic effect of ISOF and DEX in combination to antagonize septic and ALI, and to explore the possible mechanisms of synergistic effect.
Materials and Methods
Chemicals and Reagents
ISOF (purity >99.9%) was synthesized by Basilea Pharmaceutica (Jiangsu, China) according to the patent method of ISOF synthesis (ZL201610044817.8). LPS (E.coli serotype o111: B4) was obtained from Sigma (USA). DEX injection was supplied from Tiantaishan, Chengdu (China). Superoxide dismutase (SOD) assay kit, malondialdehyde (MDA) assay kit, myeloperoxidase (MPO) assay kit were purchased from Nanjing Jiancheng Bioengineering Institute (China). Anti-RGS2 antibody was obtained from Santa Cruz (USA). Mouse IL-8 and IL-6 ELISA kits were supplied from Proteintech (USA). Trizol reagent, primescript™RT reagent kit with gDNA Eraser, SYBRGreen were obtained from TaKaRa (Japan).
Animals
Male C57BL/6 mice (body weight: 18–22 g, age: 6–8 weeks) were purchased from Charles River Laboratories (Beijing, China). Animals were kept in specific non-pathogenic facilities, free access to food and water, and 12 hours in light and dark every day. Animal experiments were approved by the Animal Experimental Ethical Committee of Kunming Medical University (KMMU-2018001). Animals were received humane care in compliance with the National Institutes of Health guidelines.
The mice were sacrificed by cervical dislocation method at the end of the experiment.
Septic Model and Drug Administration
The sepsis model was established by injection of LPS through the tail vein. Mice in each group were given LPS (30 mg/kg dissolved in saline) at a dosage volume of 200 μL/mouse, with 12 animals in each group. To explore the effects of ISOF, DEX, ISOF combined with DEX on cumulative survival rate of LPS-induced sepsis mice, mice were divided into ISOF group (1.25, 2.5, 5, 10, 20 mg/kg), DEX group (1.25, 2.5, 5, 10, 20 mg/kg) and the combined administration group (ISOF2.5, DEX2.5, ISOF2.5 +DEX2.5, ISOF10, DEX10, ISOF10 +DEX10 mg/kg). Mice were pretreated by intraperitoneal injection of the corresponding drugs for 3 days and then injected with LPS 30 mg/kg via tail vein on the third day. The survival time of mice within 72 hours was observed.
Acute Lung Injury Mouse Model and Drug Administration
LPS intravenous injection to induce septicemic lung injury is the most widely used method for modeling ALI.26–28 All mice were randomly divided into five groups: control group, LPS group, LPS+ISOF (10 mg/kg) group, LPS+DEX (5 mg/kg) group, LPS+ISOF (10 mg/kg)+DEX (5 mg/kg) group. ISOF was dissolved in DMSO and diluted with saline and LPS dissolved in saline, with a volume of 200 μL for each mouse.
On the first day, the mice in the treatment group were given an intraperitoneal injection of the corresponding drug for pretreatment. The normal group and LPS group were given an equal volume of DMSO intraperitoneal injection. The drug was administered once a day for 3 consecutive days. Mice in the LPS group, LPS+ISOF group, LPS+DEX group and LPS+ISOF+DEX group were injected with LPS (5mg/kg) via caudal vein at 2 hours after administration on the third day. Mice underwent euthanasia 24 hours after injection of LPS.
The left lung lobes were placed in 4% paraformaldehyde for HE staining and immunohistochemistry. The middle lobe of the right lung was weighed to measure the wet weight, and the wet lungs were then dried in a petri dish at 60°C for 24 hours and reweighed (dry weight) to calculate the wet/dry weight ratio (W/D) of the lung. The remaining lung tissues were homogenized and the supernatants were used for the detection of MDA, MPO, SOD, IL-6, and IL-8.
Cell and Cellular Model of LPS-Induced Inflammatory Response
Human bronchial epithelial cells (BEAS-2B cell line) were purchased from American Type Culture Collection. BEAS-2B cells were cultured in DMEM containing 10% FBS (Biological Industries, Israel) with 1% penicillin-streptomycin solution at 37°C, 5% CO2 cell culture incubator. The experimental groups are as follows: control group, LPS group, LPS+DEX group, LPS+ISOF group, and LPS+DEX+ISOF group. The concentrations of LPS, DEX, and ISOF were 2.5μg/mL, 1μmoL and 10μmoL respectively. The cells were pretreated with corresponding drugs for 2 hours before LPS was added. Cells were collected at 6 h for detection.
Cell Viability Assay
BEAS-2B cells (1 × 104 cells/well) were seeded on 96- well culture plates in triplicate for 48 h and incubated with ISOF (5, 10, 15, 20 μmol/L), DEX (0.5, 1, 10 μmol/L), LPS (2.5, 5, 10 μg/mL) for 6 h. Cell viability was assessed using the MTS assay.
Lung Histopathology
The lower lobe of the right lung was routinely dehydrated, embedded, sectioned and HE stained, and the degree of inflammatory response in the sections was observed using an automated microscope. Lung histopathology was scored concerning the Smith scoring system.29 A semi-quantitative analysis of 0–4 points was performed for pulmonary edema, alveolar and interstitial hemorrhage, alveolar and interstitial inflammation, and solid lung formation, respectively.
Lung Tissue Homogenate
Lung tissues were weighed accurately, and double-distilled water was added in the ratio of tissue weight (g): solution volume (mL) = 1:19 and homogenized on ice. After centrifugation at 10,000 rpm for 10 min, the homogenate supernatants were transferred and stored at −80°C.
Determination of MDA, MPO and SOD in Lung Tissue Homogenate
MDA, MPO and SOD are the main indicators used to evaluate the oxidative damage in acute lung injury. The level of MDA, MPO and SOD was determined according to the manufacturer’s instructions.
Quantitative Real-Time PCR
Trizol was used to extract total RNA from cultured cells according to the manufacturer’s instructions and then reverse transcripted into cDNA. SYBRGreen-based general fluorescence detection was performed using the Applied Biosystems 7500 detection system for real-time RT-PCR detection. PCR conditions were pre-denaturation at 95°C for 30s, PCR reaction: 40 cycles, 95°C for 30s, 63°C for 30s. The relevant primer sequences were: RGS2 (Forward Primer AAAAGCTGTCCTCAAAAGCAAGG, Reverse primer TTTTCTGGGCAGTTGTAAAGCAG), IL-8 (Forward Primer ACTGAGAGTGATTGAGAGTGGAC, Reverse primer AACCCTCTGCACCCAGTTTTC), GAPDH (Forward Primer CATGTTCGTCATGGGTGTGAACCA, Reverse primer AGTGATGGCATGGACTGTGGTCAT).
Western Blot Analysis
Proteins were extracted from cultured cells by using RIPA lysis buffer. The protein concentrations were detected by the BCA test kit (Beyotime). Total protein was mixed with 5 × loading buffer, boiled, separated by 10% SDS-PAGE, and transferred to PVDF membranes, which were blocked with QuickBlock™ Western Blocking Solution (Beyotime) for 1 hour. PVDF membranes were incubated with the specific primary antibodies diluted in blocking solution overnight at 4°C. After being washed with TBST five times, the PVDF membranes were incubated with the secondary antibody at room temperature for 1.5 hours. Finally, bands were detected and quantified by using Amersham Imager 600 with an ECL reagent. The exposure times for RGS2 and GAPDH were 3.2 seconds and 0.4 seconds, respectively.
ELISA Assay
IL-6 and IL-8 levels in the supernatant of mouse lung homogenate and cell culture suspensions were measured with ELISA kits according to the instructions.
Statistical Analysis
Statistical Analysis Kaplan-Meier test and Log rank test were used to compare survival curves. Cox proportional hazards regression model was used to analyze the effect of the combined use of ISOF and DEX on survival curves. The experimental data were expressed as mean ± SEM and analyzed by IBM SPSS statistics 23.0 software. The data were first tested for normality and ANOVA, and if they conformed to the normal distribution and chi-square, one-way ANOVA was used; if the variance was not chi-square, the rank-sum test was performed, and P-values < 0.05 were considered statistically significant. SigmaPlot 12.5 graphics software was used for plotting. The synergistic effect of the two drugs was calculated according to the Bliss Independence formula. Bliss Independence is described by the probability independence equation: EA + EB - EA ×EB, 0 ≤ EA/ EB≤1, EA and EB are the respective effects of ISOF and DEX. The resultant Composite Index (CI) can be calculated as 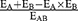 . EAB is the combined effect of ISOF and DEX. The CI is accepted as the standard measure of a combination effect to indicate an antagonistic >1, additive = 1, or synergistic<1.30,31
. EAB is the combined effect of ISOF and DEX. The CI is accepted as the standard measure of a combination effect to indicate an antagonistic >1, additive = 1, or synergistic<1.30,31
Results
Synergistic Protective Effect of DEX and ISOF on the Cumulative Survival in LPS-Induced Sepsis Mice
The results showed that the median survival time of mice in the LPS group was 20 hours. The cumulative survival rates of mice in the ISOF (1.25, 2.5 mg/kg) and DEX (1.25, 2.5 mg/kg) groups were not significantly different from those in the LPS group, suggesting that the above dose groups had no antagonistic effect on LPS-induced sepsis death in mice. The cumulative survival rates of ISOF at 5, 10 mg/kg and DEX at 5, 10, 20 mg/kg were 25.0%, 33.3%, 25%, 33.3% and 33.3% respectively, and the median survival times were 32 hours, 48 hours, 32 hours, 42 hours and 44 hours, respectively, which were significantly higher than those of other groups (Figure 1A and B).
To further explore whether ISOF combined with DEX had antagonistic effects on LPS-induced sepsis in mice, the ineffective dose of ISOF 2.5 mg/kg and DEX 2.5 mg/kg were used. The results showed that mice in both the ISOF and DEX groups died within 24 hours, but the cumulative survival rate increased to 25% for mice administered in the combined group, and the median survival time of mice increased to 32 hours (Figure 1C). Therefore, ISOF (2.5 mg/kg) combined with DEX (2.5 mg/kg) could significantly improve the cumulative survival rate of LPS-induced sepsis mice compared with ISOF and DEX alone. The effective dose of ISOF 10 mg/kg and DEX 10 mg/kg were further selected for combined use. The results are shown in Figure 1D. Compared with the LPS group, ISOF, DEX, and ISOF+DEX all significantly improved the cumulative survival rate of sepsis mice, and the highest cumulative survival rate of 58.3% was observed in the ISOF+DEX group, which was significantly higher than that of ISOF and DEX alone (Figure 1D). Cox proportional risk regression model was used for statistical analysis, and the results showed that there was a significant interaction between ISOF and DEX (Figure 1E). The Bliss Independence model was used to calculate whether the two drugs had a synergistic effect, and the results of different doses showed that the Combination Index (CI) was <1, suggesting a synergistic effect of ISOF and DEX (Table 1).
 |
Table 1 Bliss Independence Model to Calculate Drug Interactions |
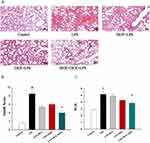
ISOF Combined with DEX Alleviated the Pathological Changes in ALI Mice
We further investigated the therapeutic effects of ISOF, DEX, and ISOF combined with DEX on LPS-induced ALI in mice. The results of HE staining of mouse lung tissues are shown in Figure 2A. LPS group showed obvious manifestations of alveolitis, with a large number of inflammatory cells and erythrocytes infiltrating the alveolar cavity and interstitium, and the alveolar wall was edematous, with the significant widening of the septum and structural destruction of the alveolar wall. Pretreatment alone and combined application attenuated pathological changes such as decreased neutrophil infiltration, mucus and red blood cells in the alveolar cavity. The lung injury index (Smith score) and wet/dry weight ratio were significantly increased in the LPS group compared with control group, and were lower in the LPS+ISOF+DEX group than in the LPS group (Figure 2B and C). These data indicated that ISOF combined with DEX reduced the inflammatory lung injury, and the effect was significantly enhanced compared with ISOF and DEX alone.
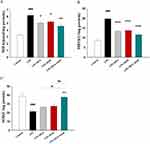
ISOF Combined with DEX Ameliorated the Levels of MDA, MPO, and SOD in ALI Mice
The lung tissues were homogenized to determine the MDA, MPO and SOD levels, and the results in Figure 3A–C showed that MDA and MPO were significantly higher and SOD was significantly lower in the LPS group compared with the control group, while MDA and MPO were significantly lower and SOD was higher in the ISOF combined with DEX group compared with the LPS group. The results suggest that ISOF combined with DEX could antagonize the oxidative stress response in the lungs of ALI mice.
ISOF Combined with DEX Reduced IL-6, IL-8 Levels in ALI Mice
In this study, the IL-6 and IL-8 levels in the lung tissues of mice in each group were measured by ELISA, and the results showed that ISOF and DEX both reduced the levels of IL-6 and IL-8 in the lung tissues of ALI mice, especially the ISOF+DEX group had a significant effect (Figure 4A and B)
Effects of ISOF, DEX and the Combination of Both on LPS-Induced BEAS-2B Cell Model
RGS2 is an important anti-inflammatory protein, and many studies have found that RGS2 has bronchoprotective effects and anti-inflammatory activity.19,25 Bronchial epithelial cells play an important role in amplifying and regulating the inflammatory process caused by immune cells.32 Therefore, we speculated that the synergistic effect of ISOF combined with DEX might related to the upregulation of RGS2 expression in bronchial epithelial cell, which could enhance the efficacy of DEX. Thus, cellular experiments were performed to verify this.
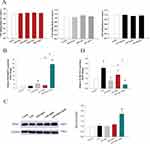
The results showed that no significant cell proliferation and inhibition were observed in the detected concentration range of ISOF, DEX, or LPS in BEAS-2B cells (Figure 5A). To explore the regulatory effects of ISOF, DEX and their combination on RGS2 in the LPS-induced BEAS-2B inflammatory cell model, the expression level of RGS2 in each group was measured. The results were shown in Figure 5B, LPS had no effect on the expression of RGS2 in BEAS-2B cells compared to the control group. ISOF and DEX alone mildly upregulated RGS2 expression, while the combination of ISOF and DEX resulted in a significant upregulation of RGS2 expression. The results indicated that the combination of ISOF and DEX could significantly upregulate LPS-induced RGS2 mRNA expression in BEAS-2B cells. We further verified the protein expression level in each experimental group. The results showed that LPS slightly inhibited the expression of RGS2 in BEAS-2B cells, and the combined use of ISOF and DEX had a significant effect on RGS2 expression, and its upregulation level was significantly enhanced compared with ISOF and DEX use alone (Figure 5C). To further confirm the antagonistic effect of the combination on the LPS-induced inflammatory response, we examined the expression of IL-8 in BEAS-2B cells. The results were shown in Figure 5D, the expression of IL-8 mRNA was significantly upregulated in LPS group. ISOF, DEX and ISOF+DEX could inhibit the LPS-induced IL-8 mRNA expression, and the combination of ISOF and DEX could enhance the effect of ISOF and DEX alone.
Discussion
So far, there are no effective therapeutic agents for sepsis and ALI. Although there is still no complete consensus on the use of GCs in sepsis and ALI, current studies still show that GCs can suppress the inflammatory response and improve survival in animal models of ALI and clinical patients33 and that GCs can suppress the inflammatory response in a variety of pulmonary inflammatory diseases, and existing studies have shown that drugs that increase cAMP in combination with GCs can improve anti-inflammatory effects.34 The mechanism of GC action is mainly mediated through genomic and non-genomic mechanisms by binding to the glucocorticoid receptor, directly inhibiting the transcription of inflammatory cytokines such as nuclear factor-kappa B (NF-κB) and activate protein-1 (AP-1) or activating anti-inflammatory genes such as RGS2.35,36
Oxidative damage is one of the important pathogenesis of ALI.37,38 LPS activates enzymatic and non-enzymatic systems to produce large amounts of ROS, leading to the oxidation of cellular proteins, lipids and DNA. Lipid oxidation can lead to cellular and mitochondrial membrane damage, causing cell necrosis, and DNA damage due to ROS can cause apoptosis in lung tissue, further aggravating lung injury. Our study suggests that the combined use of ISOF and DEX is superior to ISOF or DEX alone in terms of antioxidant damage capacity.
Inflammatory response is the core mechanism of ALI. IL-6 and IL-8 are important pro-inflammatory factors produced by activated neutrophils, alveolar macrophages and lung structural cells.39 Elevated IL-6 and IL-8 levels are strongly associated with morbidity and mortality in patients with ALI.40 Therefore, IL-6 and IL-8 levels are currently considered as markers of the degree of physiological damage and persistent cellular damage in ALI.41 The results in this study suggest that the combination of ISOF and DEX has a stronger effect on antagonizing LPS-induced lung pathology and inflammation than ISOF or DEX alone.
We further explored the molecular mechanism of the anti-inflammatory response of ISOF combined with DEX in BEAS-2B inflammatory cell model. The results showed that ISOF and DEX combined pre-incubation significantly increased the expression of RGS2, while downregulated IL-8 expression; synergistic enhanced effects were observed in ISOF and DEX combined group. RGS2 is widely present in the cell membrane, cytoplasm and nucleus of human and animal tissues. It can inhibit airway smooth muscle contraction and leukocyte aggregation function by blocking GPCRs from receiving signals via Gq.42 RGS2 can reduce calcium inward flow induced by agonists of GPCRs, a mechanism that is extremely important in the pathogenesis of COPD and asthma. RGS2 has been reported to have bronchoprotective effects and anti-inflammatory activity against LPS-induced acute neutrophilic inflammation in mice. Inhaled corticosteroids and long-acting β2-adrenoceptor agonists can induce up-regulation of RGS2, which is beneficial for ameliorating acute neutrophilic aggravation of airway disease.25 RGS2 deficiency promoted airway hyperresponsiveness, mucin expression and airway remodeling in mice. In human airway epithelial cells and airway smooth muscle, LABAs and other cAMP-enhancing drugs and GCs increased RGS2 mRNA and protein expression, and LABA combined with GCs had a synergistic effect on RGS2 expression upregulation. In BEAS-2B cells, the induction of RGS2 expression by LABA was enhanced and prolonged by GC, and its upregulation was much greater than the simple sum of the effects alone and the increased dosage of GCs alone.19,24 In this study, ISOF combined with DEX significantly upregulated RGS2 expression, which was consistent with previous reports on the upregulation of RGS2 by LABA or forskolin combined with GC. Furthermore, ISOF combined with DEX significantly downregulated LPS-induced inflammatory factor IL-8 mRNA expression and upregulated RGS2 mRNA expression levels, consistent with the report that RGS2 overexpression inhibited acetylcholine-induced IL-8 release.19 The above evidence suggested that RGS2 might play a role in the antagonism of DEX against LPS induced inflammatory factor release, while additional studies are still needed to clarify the molecular mechanisms of synergetic effect. Furthermore, systematic preclinical research is also required to evaluate the potential benefit of combined treatment with ISOF and DEX in animal models.
Conclusion
In conclusion, this study reported for the first time that ISOF (an adenylyl cyclase agonist) combined with DEX improve septic and ALI in mice, and found that ISOF combined with DEX had a synergistic effect, which could improve the survival rate of the LPS-induced sepsis in mice, and reduce lung injury and pulmonary inflammation. Mechanistically, the synergistic effect of ISOF and DEX may be associated with enhanced anti-inflammatory and antioxidant. The synergistic effect of DEX and ISOF may provide new therapeutic strategy for the treatment of ALI and sepsis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81870037, 82160013, 82160007, 81760869), Yunnan Provincial Science and Technology Department in China (Nos. 202001AY070001-044, 202105AF150015, 202102AA310030), and Chuncheng Talent Program (202202).
Disclosure
The authors declare no competing interests in this work.
References
1. Singer M, Deutschman CS, Seymour CW., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi:10.1164/rccm.201504-0781OC
3. Lehman KD. Evidence-based updates to the 2016 surviving sepsis guidelines and clinical implications. Nur Pract. 2019;44(2):26–33. doi:10.1097/01.NPR.0000552679.69145.80
4. Fiorentino V, Martini M, Dell’Aquila M, et al. Histopathological ratios to predict Gleason score agreement between biopsy and radical prostatectomy. Diagnostics. 2020;11(1):10. doi:10.3390/diagnostics11010010
5. Zhang M, Vrolijk M, Haenen GRMM. The Screening of Anticholinergic accumulation by Traditional Chinese Medicine. Int J Mol Sci. 2017;19(1):18. doi:10.3390/ijms19010018
6. Li L, Zhang Y-G, Tan Y-F, Zhao -J-J, Zhang H-R, Zhao B. Tanshinone II is a potent candidate for treatment of lipopolysaccharide-induced acute lung injury in rat model. Oncol Lett. 2018;15(2):2550–2554. doi:10.3892/ol.2017.7581
7. Milara J, Peiro T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68(5):410–420. doi:10.1136/thoraxjnl-2012-201761
8. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi:10.1016/S0140-6736(18)30696-2
9. Zhang J, Wang Z, Wang Y, Zhou G, Li H. The effect of dexmedetomidine on inflammatory response of septic rats. BMC Anesthesiology. 2015;15(1):68. doi:10.1186/s12871-015-0042-8
10. Heil LB, Santos CL, Santos RS, et al. The effects of short-term propofol and dexmedetomidine on lung mechanics, histology, and biological markers in experimental obesity. Anesth Analg. 2016;122(4):1015–1023. doi:10.1213/ANE.0000000000001114
11. Hanci V, Yurdakan G, Yurtlu S, Turan IÖ, Sipahi EY. Protective effect of dexmedetomidine in a rat model of α-naphthylthiourea–induced acute lung injury. J Surg Res. 2012;178(1):424–430. doi:10.1016/j.jss.2012.02.027
12. Gan H, Wang G, Hao Q, Wang QJ, Tang H. Protein kinase D promotes airway epithelial barrier dysfunction and permeability through down-regulation of claudin-1. J Biol Chem. 2013;288(52):37343–37354. doi:10.1074/jbc.M113.511527
13. Tavares LP, Negreiros-Lima GL, Lima KM, et al. Blame the signaling: role of cAMP for the resolution of inflammation. Pharmacol Res. 2020;159:105030. doi:10.1016/j.phrs.2020.105030
14. Yang W, Qiang D, Zhang M, et al. Isoforskolin pretreatment attenuates lipopolysaccharide-induced acute lung injury in animal models. Int Immunopharmacol. 2011;11(6):683–692. doi:10.1016/j.intimp.2011.01.011
15. Potter LR. Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol Ther. 2011;130(1):71–82. doi:10.1016/j.pharmthera.2010.12.005
16. Sciuto AM, Strickland PT, Kennedy TP, Guo YL, Gurtner GH. Intratracheal administration of DBcAMP attenuates edema formation in phosgene-induced acute lung injury. J Appl Physiol. 1996;80(1):149–157. doi:10.1152/jappl.1996.80.1.149
17. Hoffmann H, Hatherill JR, Crowley J, et al. Early post-treatment with pentoxifylline or dibutyryl cAMP attenuates Escherichia coli -induced acute lung injury in guinea pigs. Am Rev Respir Dis. 1991;143(2):289–293. doi:10.1164/ajrccm/143.2.289
18. Du X, Shi R, Wang Y, et al. Isoforskolin and forskolin attenuate lipopolysaccharide-induced inflammation through TLR4/MyD88/NF-κB cascades in human mononuclear leukocytes. Phytother Res. 2019;33(3):602–609. doi:10.1002/ptr.6248
19. Holden NS, Bell MJ, Rider CF, et al. β 2 -Adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proc Natl Acad Sci U S A. 2011;108(49):19713–19718. doi:10.1073/pnas.1110226108
20. Xie Y, Jiang H, Nguyen H, et al. Regulator of G protein signaling 2 is a key modulator of airway hyperresponsiveness. J Allergy Clin Immunol. 2012;130(4):968–976. doi:10.1016/j.jaci.2012.05.004
21. Greer S, Page CW, Joshi T, Yan D, Newton R, Giembycz MA. Concurrent agonism of adenosine A 2B and glucocorticoid receptors in human airway epithelial cells cooperatively induces genes with anti-inflammatory potential: a novel approach to treat chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2013;346(3):473–485. doi:10.1124/jpet.113.206284
22. BinMahfouz H, Borthakur B, Yan D, George T, Giembycz MA, Newton R. Superiority of combined phosphodiesterase PDE3/PDE4 inhibition over PDE4 inhibition alone on glucocorticoid- and long-acting β 2 -adrenoceptor agonist–induced gene expression in human airway epithelial cells. Mol Pharmacol. 2015;87(1):64–76. doi:10.1124/mol.114.093393
23. Jiang H, Xie Y, Abel PW, et al. Regulator of G-protein signaling 2 repression exacerbates airway hyper-responsiveness and remodeling in asthma. Am J Respir Cell Mol Biol. 2015;53(1):42–49. doi:10.1165/rcmb.2014-0319OC
24. Holden NS, George T, Rider CF, et al. Induction of regulator of G-protein signaling 2 expression by long-acting β 2 -adrenoceptor agonists and glucocorticoids in human airway epithelial cells. J Pharmacol Exp Ther. 2014;348(1):12–24. doi:10.1124/jpet.113.204586
25. George T, Chakraborty M, Giembycz MA, Newton R. A bronchoprotective role for Rgs2 in a murine model of lipopolysaccharide-induced airways inflammation. Allergy Asthma Clin Immunol. 2018;14(1):40. doi:10.1186/s13223-018-0266-5
26. Liu S, Feng G, Wang G-L, Liu G-J. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-κB pathway. Eur J Pharmacol. 2008;584(1):159–165. doi:10.1016/j.ejphar.2008.02.009
27. Shen W, Gan J, Xu S, Jiang G, Wu H. Penehyclidine hydrochloride attenuates LPS-induced acute lung injury involvement of NF-κB pathway. Pharmacol Res. 2009;60(4):296–302. doi:10.1016/j.phrs.2009.04.007
28. Tu G-W, Shi Y, Zheng Y-J, et al. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J Transl Med. 2017;15(1):181. doi:10.1186/s12967-017-1284-7
29. Smith KM, Mrozek JD, Simonton SC, et al. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit Care Med. 1997;25(11):1888–1897. doi:10.1097/00003246-199711000-00030
30. Li J, Wang W, Tong P, et al. Autophagy induction by HIV-tat and methamphetamine in primary midbrain neuronal cells of tree shrews via the mTOR signaling and ATG5/ATG7 pathway. Front Neurosci. 2018;12:921. doi:10.3389/fnins.2018.00921
31. Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3(3):e149. doi:10.1002/prp2.149
32. Holtzman MJ, Byers DE, Alexander-Brett J, Wang X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol. 2014;14(10):686–698. doi:10.1038/nri3739
33. Sciacca FL, Rizzo A, Bedini G, Capone F. Microduplication of 15q13.3 and microdeletion of 18q21.32 in a patient with moyamoya syndrome. Int J Mol Sci. 2019;21(1):20. doi:10.3390/ijms21010020
34. Ferraro M, Di Vincenzo S, Dino P, et al. Budesonide, aclidinium and formoterol in combination limit inflammaging processes in bronchial epithelial cells exposed to cigarette smoke. Exp Gerontol. 2019;118:78–87. doi:10.1016/j.exger.2019.01.016
35. Mostafa MM, Rider CF, Shah S, et al. Glucocorticoid-driven transcriptomes in human airway epithelial cells: commonalities, differences and functional insight from cell lines and primary cells. BMC Medical Genomics. 2019;12(1):29. doi:10.1186/s12920-018-0467-2
36. Newton R, Giembycz MA. Understanding how long-acting β 2 -adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma – an update. Br J Pharmacol. 2016;173(24):3405–3430. doi:10.1111/bph.13628
37. Ward PA. Oxidative stress: acute and progressive lung injury. Ann N Y Acad Sci. 2010;1203(1):53–59. doi:10.1111/j.1749-6632.2010.05552.x
38. Guo R-F, Ward PA. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal. 2007;9(11):1991–2002. doi:10.1089/ars.2007.1785
39. Mokra D, Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol. 2015;209:52–58. doi:10.1016/j.resp.2014.10.006
40. Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108(5):1303–1314. doi:10.1378/chest.108.5.1303
41. Mowery NT, Terzian W, Nelson AC. Acute lung injury. Curr Probl Surg. 2020;57(5):100777. doi:10.1016/j.cpsurg.2020.100777
42. McNabb HJ, Zhang Q, Sjogren B. Emerging roles for regulator of G protein signaling 2 in (Patho)physiology. Mol Pharmacol. 2020;98(6):751–760. doi:10.1124/molpharm.120.000111
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.