Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Synergistic Effects of Resistive Breathing on Endotoxin-Induced Lung Injury in Mice
Authors Toumpanakis D, Glynos C, Schoini P, Vassilakopoulou V , Chatzianastasiou A, Dettoraki M, Mizi E, Tsoukalas D, Perlikos F , Magkou C, Papapetropoulos A, Vassilakopoulos T
Received 2 July 2023
Accepted for publication 16 October 2023
Published 19 October 2023 Volume 2023:18 Pages 2321—2333
DOI https://doi.org/10.2147/COPD.S424560
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Dimitrios Toumpanakis,1 Constantinos Glynos,1 Pinelopi Schoini,2 Vyronia Vassilakopoulou,1 Athanasia Chatzianastasiou,1 Maria Dettoraki,1 Eleftheria Mizi,1 Dionysios Tsoukalas,1 Fotis Perlikos,1 Christina Magkou,3 Andreas Papapetropoulos,4,5 Theodoros Vassilakopoulos1,6
1“Marianthi Simou” Applied Biomedical Research and Training Center, Medical School, National and Kapodistrian University of Athens, Evangelismos Hospital, Athens, Greece; 2 4th Respiratory Clinic, “Sotiria” General Hospital for Thoracic Diseases of Athens, Athens, Greece; 3Department of Pathology, Evangelismos Hospital, Athens, Greece; 4Biomedical Research Foundation of the Academy of Athens, Athens, Greece; 5Division of Pharmaceutical Chemistry, Department of Pharmacy, National and Kapodistrian University of Athens, Athens, Greece; 6Department of Physiology, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Correspondence: Dimitrios Toumpanakis, “Marianthi Simou” Applied Biomedical Research and Training Center, Medical School, National and Kapodistrian University of Athens, Evangelismos Hospital, 3rd Ploutarchou Street, 2nd Floor, Athens, 10675, Greece, Tel +302107235521, Email [email protected]
Introduction: Resistive breathing (RB) is characterized by forceful contractions of the inspiratory muscles, mainly the diaphragm, resulting in large negative intrathoracic pressure and mechanical stress imposed on the lung. We have shown that RB induces lung injury in healthy animals. Whether RB exerts additional injurious effects when added to pulmonary or extrapulmonary lung injury is unknown. Our aim was to study the synergistic effect of RB on lipopolysaccharide (LPS)-induced lung injury.
Methods: C57BL/6 mice inhaled an LPS aerosol (10mg/3mL) or received an intraperitoneal injection of LPS (10 mg/kg). Mice were then anaesthetized, the trachea was surgically exposed, and a nylon band of a specified length was sutured around the trachea, to provoke a reduction of the surface area at 50%. RB through tracheal banding was applied for 24 hours. Respiratory system mechanics were measured, BAL was performed, and lung sections were evaluated for histological features of lung injury.
Results: LPS inhalation increased BAL cellularity, mainly neutrophils (p < 0.001 to ctr), total protein and IL-6 in BAL (p < 0.001 and p < 0.001, respectively) and increased the lung injury score (p = 0.001). Lung mechanics were not altered. Adding RB to inhaled LPS further increased BAL cellularity (p < 0.001 to LPS inh.), total protein (p = 0.016), lung injury score (p = 0.001) and increased TNFa levels in BAL (p = 0.011). Intraperitoneal LPS increased BAL cellularity, mainly macrophages (p < 0.001 to ctr.), total protein levels (p = 0.017), decreased static compliance (p = 0.004) and increased lung injury score (p < 0.001). Adding RB further increased histological features of lung injury (p = 0.022 to LPS ip).
Conclusion: Resistive breathing exerts synergistic injurious effects when combined with inhalational LPS-induced lung injury, while the additive effect on extrapulmonary lung injury is less prominent.
Keywords: endotoxin, resistive breathing, lung, inflammation, injury
Introduction
COPD exacerbations are devastating episodes in the natural course of the disease, leading acutely to increased morbidity and mortality.1 Moreover, in COPD, the rate of exacerbations has been correlated with an accelerated decline in lung function.2 Although the mechanism for this association is not determined, increased lung and systemic inflammation during an exacerbation could promote long term lung functional decline in COPD patients, especially in frequent exacerbators.3 A frequent cause of COPD exacerbation is pulmonary infection with Pseudomonas aeruginosa, especially in patients with severely impaired lung function, bronchiectasis and in COPD exacerbations leading to mechanical ventilation.4 In general, COPD exacerbations are characterized by increased airway inflammation and the bacterial load during COPD exacerbations has been also associated with augmented pulmonary inflammation.5
Resistive breathing, ie breathing through increased airway resistance, leading to severe airflow limitation and hyperinflation, is the main characteristic of the pathophysiology of COPD exacerbations. Forceful contractions of the diaphragm during resistive breathing are associated with large negative intrathoracic pressures that together with hyperinflation lead to increased mechanical stress, imposed on the lung.6,7 We have shown that resistive breathing through tracheal banding (as a model of severe airway obstruction) induces acute lung injury and inflammation, possible due to exposure of the lung to increased mechanical stress.8 The aforementioned study was performed in previously healthy animals and whether resistive breathing could augment pulmonary inflammation in mice suffering an underlying inflammation due to prior pulmonary lipopolysaccharide (LPS) exposure, as would be more clinically relevant, is unknown.
COPD exacerbations are a frequent cause of hospitalizations in intensive care units, where the incidence of nosocomial infections with gram-negative bacteria, such as Pseudomonas aeruginosa, is high, leading to sepsis and indirect lung injury.9 Again, whether the mechanical consequences of a COPD exacerbation, could have an additive effect on pulmonary inflammation, following a systemic exposure to LPS has not been tested before.
Therefore, we hypothesized that resistive breathing could have a synergistic effect on pulmonary inflammation and injury, when combined to either inhale of systemic exposure to endotoxin (LPS) from Pseudomonas aeruginosa bacteria. To achieve our aim, we have combined our model of severe airway obstruction (via tracheal banding) with the model of endotoxin exposure, administered either as an aerosol (direct pulmonary insult) or intraperitoneally (systemic – secondary pulmonary insult).
Methods
Animals
Male C57BL/6 mice [8–12 weeks old (Biomedical Sciences Research Center “A. Fleming”)] were housed in a 12-hour day/night cycle and had an ad libitum access to water and food. The number of animals per group is stated in figure legends.
Endotoxin Exposure
Two independent sets of experiments were performed with lipopolysaccharide (LPS) from Pseudomonas aeruginosa serotype 10 (Sigma-Aldrich) being administered either through inhalation (aerosol challenge) or intraperitoneally.
Aerosol Administration (Inhalational)
An LPS solution was prepared (10 mg/3 mL in sterile normal saline) and was administered over a 20-min period in a nebulization chamber under continuous oxygen flow, as previously described.10 Animals exposed to nebulized sterile normal saline alone were used as control.
Intraperitoneal Administration
LPS was administered via an intraperitoneal injection (10 mg/kg) in sterile normal saline. This dose was based on a pilot study (5, 10 and 20 mg/kg ip doses) showing that animals develop significant pulmonary injury with minimal mortality (data not shown). Animals receiving an equal volume of sterile normal saline alone served as controls.
Animal Model of Resistive Breathing Through Tracheal Banding
Animals were anaesthetized [ketamine (90 mg/kg) and xylazine (5mg/kg) ip], an incision was performed in the anterior cervix and the trachea was exposed. Then, the diameter of the trachea was measured using a caliber gauge through a surgical microscope (Zeiss Inc.). Following, a new perimeter of the trachea was calculated that corresponds to a total surface area, reduced to half of the initial, and a nylon band of the pre-specified length was sutured around the trachea, to provoke resistive breathing.8 The animals recovered from the anesthesia and returned to their cage.
The animals underwent tracheal banding 30 minutes following inhalation or intraperitoneal LPS (or sterile normal saline-control) administration and resistive breathing lasted for 24h in total. Sham operation included anesthesia and skin incision, but no band placement.
Respiratory System Mechanics
A small animal ventilator (FlexiVent, Scireq) was employed to estimate the mechanics of the respiratory system at the end of the resistive breathing session. The animals received a dose of anaesthesia [ketamine (90 mg/kg) and xylazine (10 mg/kg)] and a tracheal tube was inserted to the trachea, after removal of the nylon band. The dynamic compliance and the total respiratory system resistance were measured (single compartment linear model,11 values reported are the average of 3 snapshot perturbations). Prior to measurements, a deep inspiration in the ventilator, pressure-limited to 30 cmH20, was used to establish the lung volume history and succinylcholine (8 mg.kg−1) was administered to inhibit spontaneous breathing.
Bronchoalveolar Lavage (BAL)
Following the measurement of the respiratory system mechanics, the animals received a further dose of anesthesia and were sacrificed by vena cava dissection (exsanguination). Then, the thoracic cavity was opened, the left main bronchus was temporally ligated, and BAL was performed at the right lung with 3 aliquots of 0.5 mL normal saline. BAL fluid was centrifuged (300xg, 5 min, 4°C), the cell pellet was resuspended, and the BAL fluid supernatant was stored at −80°C for further analysis.
Total Protein in BAL Fluid
The total protein levels in the BAL fluid supernatant were estimated with a colorimetric protein assay (BioRad). Standard curves were created using bovine serum albumin.
Cell Count (Total – Differential)
After BAL fluid centrifugation, the cell pellet was diluted in 1 mL normal saline and the total cells were counted. Following, May-Grűnwald-stained cytospins were prepared to measure the percentages of macrophages, neutrophils, lymphocytes and eosinophils/basophils. Eosinophils/basophils were omitted from analysis, due to minimal counts.
Cytokine Levels in BAL Fluid
IL-6 and TNF-α protein levels, central cytokines in the pathogenesis of acute lung injury, were estimated in samples of BAL fluid supernatant by ELISA (DuoSet, R&D Systems).
Lung Histology
Following BAL, the right main bronchus was ligated, and the right lung was removed, and the left lung was fixed with 4% formaldehyde under 20 cm H2O pressure. Haematoxylin and eosin-stained lung tissue sections were prepared, and a lung injury score was determined, as previously described.12 Briefly, (i) focal alveolar membrane thickening, (ii) capillary congestion, (iii) intra-alveolar haemorrhage, (iv) interstitial and (v) intra-alveolar leukocyte infiltration were evaluated and scored from 0 to 3 based on their absence (0) or presence to a mild (1), moderate (2), or severe (3) degree. Moreover, the mean linear intercept (Lm) was estimated, as previously described by our group.13 Briefly, 4 random optical fields were selected per lung tissue section, avoiding large vessels or airways. Then, seven equally distributed horizontal lines were superimposed on each field, using the ImageJ software. The total length of each line of the grid was divided by the number of alveolar intercepts, to provide the mean linear intercept (Lm).
cGMP Measurement
cGMP levels were measured in the BAL fluid, as previously described by our group.10 Briefly, following BAL collection, cells were pelleted and incubated in Hanks’ balanced salt solution for 15 min and then lysed with 0.1 N HCl. cGMP levels were analyzed in the extracts using a commercially available enzyme immunoassay kit (Direct cGMP Elisa Kit; Enzo Life Sciences), according to the manufacturer’s instructions. cGMP values were normalized per milligram of total protein.
Statistical Analysis
Data are presented as mean ± standard error of the mean. The one-way analysis of variance (ANOVA) was used for statistical analysis and when significant, with Fisher’s least significant difference (LSD) test for post hoc comparisons (Statistica Software, StatSoft). Analysis of the histological data was performed with the non-parametric Kruskal–Wallis ANOVA. When significant, between two group comparisons were performed with the Mann–Whitney U-test and a Holm–Bonferroni method was followed to correct for multiple comparisons. A p <0.05 is considered as statistically significant. For all measurements (except BAL fluid cytokine levels), sham operated or tracheal banding groups that received normal saline, either inhaled or intraperitoneally, were pooled into one group.
Results
The Effect of Resistive Breathing on BAL Cellularity Following Endotoxin Exposure
LPS aerosol exposure resulted in a significant increase of the total cell count in BAL fluid (p < 0.001 to ctr, Figure 1), due to raised neutrophil numbers (p < 0.001 to ctr) (differential cell percentages are presented in Table 1). Superimposed RB for 24h on LPS aerosol exposure significantly augmented both macrophage (p < 0.001 to LPS inh) and neutrophil count (p = 0.004 to LPS inh). Total cell count in BAL fluid was increased following intraperitoneal LPS exposure (p < 0.001 to ctr), caused by increased macrophage numbers (p < 0.001 to ctr). RB for 24h did not affect BAL cellularity following LPS ip exposure. Lymphocyte count was not affected by either LPS aerosol exposure (ANOVA, p = 0.185), or LPS ip administration (ANOVA, p = 0.354).
 |
Table 1 Differential Cell Percentage in BAL Fluid Following Either Aerosol (Inhalation) or Intraperitoneal Endotoxin (LPS) Administration and the Synergistic Effect of Resistive Breathing (RB) |
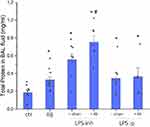
The Effect of Resistive Breathing on Total Protein Levels in BAL Fluid Following Endotoxin Exposure
Total protein levels in BAL fluid, an indirect marker of lung permeability,14 were increased by LPS aerosol inhalation (p < 0.001 to ctr) (see Figure 2). Combining 24h of RB plus LPS aerosol resulted in an additive effect on total protein levels in BAL fluid (~35% increase, p = 0.016 to LPS inh). Combining RB and LPS ip exposure did not result in a significant change in total protein levels in BAL fluid, in comparison to each treatment alone.
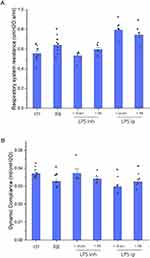
The Effect of Resistive Breathing on Respiratory System Mechanics Following Endotoxin Exposure
As we have previously reported, resistive breathing alone resulted in decreased respiratory system compliance and increased total resistance, a finding supporting the presence of acute lung injury (p = 0.009 and p = 0.01 to ctr, respectively). LPS inhalation did not affect respiratory system mechanics, neither dynamic compliance nor total resistance, either alone or after combining with resistive breathing (Figure 3). In contrast, intraperitoneal LPS resulted in decreased compliance and increased resistance of the respiratory system (p = 0.004 and p = 0.01 to ctr, respectively), although no synergy was observed when combined with resistive breathing.
The Effect of Resistive Breathing on BAL Fluid Cytokine Levels Following Endotoxin Exposure
As expected, inhaled LPS resulted in a significantly raised IL-6 protein level in BAL fluid compared to control (p < 0.001), however combining RB and LPS did not result in augmented IL-6 levels (p = 0.14 to LPS inhalation). In contrast, the addition of resistive breathing to LPS inhalation increased TNFa levels in BAL fluid, compared to endotoxin alone (~3-fold, p = 0.011) (see Figure 4). Intraperitoneal administration of LPS did not affect TNF or IL-6 in BAL fluid, under our experimental settings [TNFa (pg/mL) control 2.74 ± 1.34, LPS ip 1.84 ± 0.45, p = 0.55, IL-6 (pg/mL) control 4.89 ± 1.12, LPS ip 9.68 ± 2.10, p = 0.15].
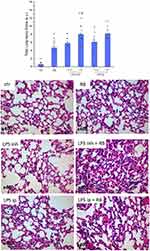
The Effect of Resistive Breathing on Histological Features of Lung Injury Following Endotoxin Exposure
Adding resistive breathing to LPS administration significantly increased the total lung injury for both inhalation (p = 0.001) and intraperitoneal (p = 0.022) LPS administration (see Figure 5). Regarding the individual histological features, resistive breathing added to inhalation LPS administration mainly increased interstitial and intra-alveolar leukocyte infiltration, while when added to intraperitoneal LPS administration, RB augmented capillary congestion and interstitial leukocyte infiltration (for a complete description of histological indices of lung injury please see Table 2). No statistically significant difference was detected for the mean linear intercept (Lm), neither after inhaled nor after intraperitoneal LPS exposure (ANOVA, p = 0.74 and p = 0.30, respectively, Table 3).
 |
Table 2 Histological Features of Lung Injury Following Either Aerosol (Inhalation) or Intraperitoneal Endotoxin (LPS) Administration and the Synergistic Effect of Resistive Breathing (RB) |
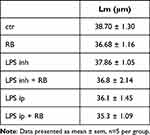 |
Table 3 Mean Linear Intercept (Lm) of Lung Tissue Sections Following Either Aerosol (Inhalation) or Intraperitoneal Endotoxin (LPS) Administration and the Effect of Resistive Breathing (RB) |
The Effect of Resistive Breathing on cGMP Levels Following Endotoxin Exposure
Combining resistive breathing with inhalational LPS exposure caused a synergistic reduction in cGMP levels, compared to LPS alone (p = 0.04) (Figure 6). On the contrary, the reduction in cGMP levels following addition of RB to intraperitoneal LPS was not statistically significant, compared to LPS alone (p = 0.11).
Discussion
The key outcome of our study is that resistive breathing exaggerates pulmonary inflammation and injury caused by either inhaled or intraperitoneally administered LPS, with the more significant synergistic effect being noticed in inhalational LPS injury.
Resistive breathing is amongst the main pathophysiological features of obstructive airway diseases, such as COPD, especially in severe stable state and while on exacerbation. In stable COPD, mainly due to cigarette smoke exposure, small airway inflammation and remodelling and loss of alveolar attachments results in airway obstruction and airflow limitation15,16 During exacerbations, excessive inflammation triggered by various stimuli, including bacterial infections, exaggerates airflow limitation leading to resistive breathing.6 RB results in severe mechanical stress on the lung, due to forceful contractions of the diaphragm to overcome increased resistance, that causes more negative intrathoracic pressures and/or the presence of hyperinflation, due to raised expiratory resistance.7 Although increased mechanical stress has been clearly shown to promote lung injury, especially during the application of mechanical ventilation,17 the significance of mechanical forces in the natural course of airway diseases is largely unknown. Indeed, recent data have suggested that mechanical stress may promote the progress of COPD.18
We have shown in a series of studies that the induction of resistive breathing (to mimic severe airway obstruction) provokes pulmonary inflammation and injury.8,19 However, since these studies were performed in previously healthy animals, the effect of resistive breathing in the presence of acute inflammation, such as during an infectious exacerbation, is largely unknown. Pseudomonas aeruginosa infection is a common cause of a COPD exacerbation,20 especially amongst severe patients21 and bacterial colonization is associated with increased frequency of COPD exacerbation.22 The net outcome is an augmentation of systemic and pulmonary inflammation during a COPD exacerbation, compared to baseline.23
Our results showed that combining resistive breathing with inhalational LPS exposure resulted in a significant increase in pulmonary inflammation. As expected, LPS exposure induced neutrophilic inflammation that was further augmented when combined with resistive breathing. Interestingly, when resistive breathing was added to LPS exposure, the macrophage number also increased, compared to inhaled LPS alone. In accordance, Rizzo et al reported increased BAL neutrophilia, when intratracheal LPS was combined with raised mechanical stress applied to the lung, through high tidal volume mechanical ventilation.24 As previously reported, inhaled LPS induced the expression of proinflammatory cytokines in BAL fluid, including IL-6 and TNF-a in vivo,25,26 and in vitro, treatment with LPS resulted in increased expression of IL-6 and TNF-a in both macrophages and neutrophils.27 Our data suggest that resistive breathing exerts a synergistic effect by augmenting pro-inflammatory cytokine expression in the lung in combination with LPS exposure.
The increased levels of total protein in BAL fluid suggest that combining resistive breathing with inhaled LPS also aggravated the derangement of lung permeability, although it must be acknowledged that the total protein level in BAL fluid is an indirect and not specific marker of lung epithelial permeability.28 This is in accordance with a previously presented study in mice, where a “two-hit” model of lung injury was established by combining LPS or acid (HCL) intratracheal exposure and increased mechanical stress applied to the lung, through high tidal volume ventilation.29 Exposure to LPS aerosol alone was not associated with a derangement in the mechanical properties of the respiratory system in our experimental settings. In contrast, resistive breathing, as has been previously shown by our group,8 resulted in decreased compliance. When combined with RB, inhaled LPS numerically decreased the dynamic compliance of the respiratory system, probable to the greater increase in alveolar-capillary permeability, compared to LPS alone, although this finding did not reach statistical significance (p = 0.09).
Regarding the histological analysis, adding resistive breathing to LPS-induced lung injury significantly increased neutrophil infiltration in the lung. Although our study has the limitation that it did not investigate the underlying molecular mechanism for the increase in pulmonary inflammation, some assumptions can be made. Interestingly, both inhaled LPS and resistive breathing have been shown to reduce soluble guanylyl cyclase (sGC) levels in the lung and in both models further pharmaceutical inhibition of sGC, led to augmentation of lung injury.8,10 Thus, it is plausible that combining resistive breathing with LPS exposure could lead to further inhibition of the sGC pathway and worse outcomes. Indeed, combining RB with LPS resulted in a further reduction in cGMP levels in the BAL, compared to LPS alone, although this effect was statistically significant only for inhalational LPS exposure. Moreover, in vivo, expression microarray analysis revealed that when intratracheal LPS exposure was combined with mechanical stress, the great percentage of highly expressed genes belonged to the inflammatory/immunity cascade.30
The combination of intraperitoneal endotoxin with resistive breathing resulted in a modest synergy, as shown by the histological analysis ie, increased capillary congestion and interstitial leukocyte infiltration in the combined group. In contrast, no synergy was detected in lung permeability, BAL cellularity and derangement of mechanics. Although a direct comparison between inhaled and systemic (intraperitoneal) administration of LPS is out of scope of our study, differences in the features of acute lung injury between the two models (eg, the lack of intra-alveolar neutrophils in systemic LPS exposure) may account for the differential additive effect of resistive breathing. Previously, in contrast to our results, Altemeier et al reported that combining systemic endotoxin administration (intravenous LPS) with mechanical ventilation results in augmented cytokine production in the lung and altered lung permeability, compared to LPS i.v. alone.31
The development of a “two-hit” animal model allows a better simulation of complex clinical states, such as the COPD exacerbation. Hitherto, LPS exposure has been combined with models of stable COPD, such as cigarette smoke exposure, to investigate the effects of acute LPS exposure on lung inflammation and emphysema, given the central role of smoking in lung functional decline in COPD.32 In mice, the combination of sub-acute exposure to cigarette smoke and intratracheal instillation of LPS resulted in augmented pulmonary inflammation.33 Moreover, combination of a single dose of intratracheal LPS with elastase exposure resulted in worsening of pulmonary inflammation in mice and subsequently augmented emphysema progression.34 Interestingly, da Fonseca et al found a synergy between elastase and LPS in pulmonary inflammation, even when LPS was administered intraperitoneally in the same dose, as in our model.35 To our knowledge, our study is the first to combine LPS exposure with resistive breathing. Although, tracheal banding is clinically more relevant to upper airway obstruction, the mechanical consequences of resistive breathing ie, increased airway resistance and forceful contractions of the respiratory muscles, leading to large negative intrathoracic pressures,13 may provide useful insights for the pathophysiology of COPD exacerbations.
In conclusion, our findings suggest that combining LPS with a model of severe airway obstruction aggravates pulmonary inflammation and injury, mainly in intrapulmonary LPS administration, while in systemic LPS exposure, the effect is modest.
Data Sharing Statement
The data of the study are available from the corresponding author on reasonable request.
Ethics Approval
Experimental procedures and protocols were approved by the ethics committee of the Experimental Surgery Department of “Evangelismos” hospital and follow the European Union Directive (2010/63/EU) on the protection of laboratory animals used for scientific causes.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding for this study was provided by the Thorax Foundation (Athens, Greece).
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
1. Mathioudakis AG, Abroug F, Agusti A, et al. ERS statement: a core outcome set for clinical trials evaluating the management of COPD exacerbations. Eur Respir J. 2022;59(5):2102006. doi:10.1183/13993003.02006-2021
2. Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332–338. doi:10.1164/rccm.200712-1869OC
3. Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128(4):1995–2004. doi:10.1378/chest.128.4.1995
4. Miravitlles M. Exacerbations of chronic obstructive pulmonary disease: when are bacteria important? Eur Respir J Suppl. 2002;36(Supplement 36):9s–19s. doi:10.1183/09031936.02.00400302
5. Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(1):71–78. doi:10.1164/rccm.200505-704OC
6. O’Donnell DE, Parker CM. COPD exacerbations. 3: pathophysiology. Thorax. 2006;61(4):354–361. doi:10.1136/thx.2005.041830
7. Vassilakopoulos T, Toumpanakis D. Can resistive breathing injure the lung? Implications for COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2016;11:2377–2384. doi:10.2147/COPD.S113877
8. Glynos C, Toumpanakis D, Loverdos K, et al. Guanylyl cyclase activation reverses resistive breathing-induced lung injury and inflammation. Am J Respir Cell Mol Biol. 2015;52(6):762–771. doi:10.1165/rcmb.2014-0092OC
9. Luo L, Shaver CM, Zhao Z, et al. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151(4):755–763. doi:10.1016/j.chest.2016.09.004
10. Glynos C, Kotanidou A, Orfanos SE, et al. Soluble guanylyl cyclase expression is reduced in LPS-induced lung injury. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1448–R1455. doi:10.1152/ajpregu.00341.2006
11. Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol. 2003;94(4):1297–1306. doi:10.1152/japplphysiol.00706.2002
12. Toumpanakis D, Vassilakopoulou V, Mizi E, et al. p38 inhibition ameliorates inspiratory resistive breathing-induced pulmonary inflammation. Inflammation. 2018;41(5):1873–1887. doi:10.1007/s10753-018-0831-6
13. Toumpanakis D, Mizi E, Vassilakopoulou V, et al. Spontaneous breathing through increased airway resistance augments elastase-induced pulmonary emphysema. Int J Chron Obstruct Pulmon Dis. 2020;15:1679–1688. doi:10.2147/COPD.S256750
14. Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L231–L246. doi:10.1152/ajplung.00049.2003
15. Booth S, Hsieh A, Mostaco-Guidolin L, et al. A single-cell atlas of small airway disease in chronic obstructive pulmonary disease: a cross-sectional study. Am J Respir Crit Care Med. 2023;208(4):472–486. doi:10.1164/rccm.202303-0534OC
16. Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi:10.1056/NEJMoa032158
17. Curley GF, Laffey JG, Zhang H, Slutsky AS. Biotrauma and Ventilator-Induced Lung Injury: clinical Implications. Chest. 2016;150(5):1109–1117. doi:10.1016/j.chest.2016.07.019
18. Suki B, Sato S, Parameswaran H, Szabari MV, Takahashi A, Bartolak-Suki E. Emphysema and mechanical stress-induced lung remodeling. Physiolog. 2013;28(6):404–413. doi:10.1152/physiol.00041.2013
19. Toumpanakis D, Kastis GA, Zacharatos P, et al. Inspiratory resistive breathing induces acute lung injury. Am J Respir Crit Care Med. 2010;182(9):1129–1136. doi:10.1164/rccm.201001-0116OC
20. Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi:10.1056/NEJMoa012561
21. Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest. 1999;116(1):40–46. doi:10.1378/chest.116.1.40
22. Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57(9):759–764. doi:10.1136/thorax.57.9.759
23. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
24. Rizzo AN, Sammani S, Esquinca AE, et al. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309(11):L1294–L1304. doi:10.1152/ajplung.00031.2015
25. Blackwell TS, Lancaster LH, Blackwell TR, Venkatakrishnan A, Christman JW. Differential NF-kappaB activation after intratracheal endotoxin. Am J Physiol. 1999;277(4):L823–L830. doi:10.1152/ajplung.1999.277.4.L823
26. Ulich TR, Fann MJ, Patterson PH, et al. Intratracheal injection of LPS and cytokines. V. LPS induces expression of LIF and LIF inhibits acute inflammation. Am J Physiol. 1994;267(4 Pt 1):L442–L446. doi:10.1152/ajplung.1994.267.4.L442
27. Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol. 1994;10(2):148–153. doi:10.1165/ajrcmb.10.2.8110470
28. Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–738. doi:10.1165/rcmb.2009-0210ST
29. Hoegl S, Burns N, Angulo M, et al. Capturing the multifactorial nature of ARDS – “Two-hit” approach to model murine acute lung injury. Physiol Rep. 2018;6(6):e13648. doi:10.14814/phy2.13648
30. Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol. 2005;175(5):3369–3376. doi:10.4049/jimmunol.175.5.3369
31. Altemeier WA, Matute-Bello G, Frevert CW, et al. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287(3):L533–L542. doi:10.1152/ajplung.00004.2004
32. Pezzuto A, Tonini G, Ciccozzi M, et al. Functional benefit of smoking cessation and triple inhaler in combustible cigarette smokers with severe COPD: a retrospective study. J Clin Med. 2022;12(1):234. doi:10.3390/jcm12010234
33. Sakhatskyy P, Wang Z, Borgas D, et al. Double-hit mouse model of cigarette smoke priming for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L56–L67. doi:10.1152/ajplung.00436.2016
34. Kobayashi S, Fujinawa R, Ota F, et al. A single dose of lipopolysaccharide into mice with emphysema mimics human chronic obstructive pulmonary disease exacerbation as assessed by micro-computed tomography. Am J Respir Cell Mol Biol. 2013;49(6):971–977. doi:10.1165/rcmb.2013-0074OC
35. da Fonseca LM, Reboredo MM, Lucinda LM, et al. Emphysema induced by elastase enhances acute inflammatory pulmonary response to intraperitoneal LPS in rats. Int J Exp Pathol. 2016;97(6):430–437. doi:10.1111/iep.12214
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.