Back to Journals » Infection and Drug Resistance » Volume 16
Synergistic Effects of Gentamicin, Cefepime, and Ciprofloxacin on Biofilm of Pseudomonas aeruginosa
Authors Usman M , Marcus A, Fatima A , Aslam B, Zaid M, Khattak M, Bashir S, Masood S , Rafaque Z , Dasti JI
Received 11 July 2023
Accepted for publication 19 August 2023
Published 6 September 2023 Volume 2023:16 Pages 5887—5898
DOI https://doi.org/10.2147/IDR.S426111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Muhammad Usman,1 Arooj Marcus,1 Aimen Fatima,1 Bushra Aslam,1 Maryam Zaid,1 Muska Khattak,1 Sidra Bashir,1 Safia Masood,1 Zara Rafaque,2 Javid Iqbal Dasti1
1Lab of Microbial Genomics and Epidemiology, Department of Microbiology, Quaid-I-Azam University, Islamabad, 45320, Pakistan; 2Department of Microbiology Hazara University Mansehra, Mansehra 21120, Pakistan
Correspondence: Javid Iqbal Dasti, Lab of Microbial Genomics and Epidemiology, Department of Microbiology, Quaid-I-Azam University, Islamabad, 45320, Pakistan, Tel +92-5190644175, Email [email protected]
Background: Pseudomonas aeruginosa is an opportunistic pathogen involved in number of hospital-acquired infections such as catheter-associated urinary tract infections, bacteremia, septicemia, skin infections, and ventilator-associated pneumoniae. Biofilm formation is an important trait implicated in chronic infections, such as cystic fibrosis and chronic pulmonary obstruction. We evaluated effects of gentamicin, cefepime, and ciprofloxacin on biofilm of P. aeruginosa.
Materials and Methods: A total of 266 isolates were collected from the Armed Forces Institute of Pathology (AFIP). Antibiotic susceptibility was assessed by double disk synergy testing. ESBL and carbapenemase detection was performed by phenotypic testing. Molecular screening of the genes was done by PCR. Micro-dilution broth method was used to determine minimum inhibitory concentrations of antibiotics. Biofilm formation was done by micro-titer plate assay.
Results: Overall, 20% of the P. aeruginosa isolates were extensively drug-resistant (XDR-PA), and 25% were multi-drug-resistant (MDR-PA). Likewise, 43% of the isolates were ESBL producers, and carbapenemase production was detected in 40% of the isolates. Molecular analysis confirmed occurrence of different resistant factors in ESBL-positive isolates; 67% carried blaTEM, 62% blaCTXM-15, 41% blaSHV, 34% blaCTXM-14, and 33% blaOXA-1. In addition, 68% of the carbapenem-resistant isolates were positive for blaNDM-1, 25% for blaOXA-48, and 22% for blaKPC-2. Biofilm formation was assessed for 234 isolates, out of which 28% were strong biofilm formers. Moderate and weak biofilm formers constituted 46% and 23%, respectively. Overall, ciprofloxacin, levofloxacin, and cefepime showed inhibitory effects on P. aeruginosa biofilms. Antibiotics in combination showed strong synergistic effects (ciprofloxacin and cefepime), while gentamicin and cefepime resulted in complete eradication of P. aeruginosa biofilm.
Conclusion: We confirm strong synergistic effects of gentamicin and cefepime that completely eradicated P. aeruginosa biofilm. We further confirm inhibitory effects of ciprofloxacin, levofloxacin, and cefepime on P. aeruginosa biofilms. Hence, combination therapy can be more effective against biofilm-associated infections.
Keywords: synergistic effects, gentamicin, cefepime, biofilm, Pseudomonas aeruginosa
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen associated with different nosocomial infections.1 This bacterium is classified as a “critical pathogen” by WHO and is one of the “ESKAPE” organisms. P. aeruginosa causes different types of infections including respiratory tract infections (RTIs), urinary tract infections (UTIs), wound infections, dermatitis, skin/soft tissue infections, bone/joint infections, septicemia, and bacteremia.2 Mortality associated with P. aeruginosa infections is reported to exceed well beyond many other Gram-negative bacteria, including Staphylococcus aureus.3 In the case of hospital-acquired pneumonia, multi-drug-resistant strains of P. aeruginosa (MDR-PA) are an independent risk factor for mortality.4 Quite recently, MDR-PA, resistant to levofloxacin, ciprofloxacin, imipenem-cilastatin, meropenem, aztreonam, cefepime, ceftazidime, and piperacillin-tazobactam, are labelled as difficult-to-treat (DTR) infections.5,6 In the current scenario of antimicrobial resistance (AMR), combination therapy has been recommended to treat bloodstream infections caused by P. aeruginosa.7,8 Combining any conventional or novel β-lactam with aminoglycosides or a fluoroquinolone is prioritized.9,10 Yet, for the use of empiric combination versus monotherapy, no consensus has been reached among the scientific community.11 Although one study described synergistic effects of β-lactam in combination with an aminoglycoside and β-lactam with a fluoroquinolones on planktonic Pseudomonas aeruginosa, no study has focused on biofilm for investigating synergistic effects of these antibiotics.12 Bacterial biofilms are a formidable barrier against the efficacy of antibiotics that makes biofilm-associated infections notoriously difficult to treat. The three-dimensional structure of the biofilm results in an anaerobic environment, hence some antibiotics such as fluoroquinolones, β-lactams, and aminoglycosides alone lose efficacy in such environment.13 On the other hand, exposure to sub-lethal or sub-minimal inhibitory concentrations of certain antibiotics significantly expedites biofilm formation.14,15 In short, not much is known about the efficacy of antibiotic therapy in combination against infections involving pseudomonal biofilms. Therefore, in the current study, we evaluated effects of ciprofloxacin (CIP), gentamicin (GEN), and cefepime (CEF) on biofilm formation of Pseudomonas aeruginosa. It is one of the very few studies focusing on synergistic effects of multiple antibiotics on the biofilm of P. aeruginosa.
Materials and Methods
A total of 266 samples were collected from the Armed Forces Institute of Pathology (AFIP), Rawalpindi. The study was carried out from June 2019 to June 2022. Approval for this study was granted by the Ethical Board of National Institute of Health (NIH) Islamabad (letter no. F.1–5/RAPiD/2020-21/ERC). Verbal consent was taken from the participating patients. For each patient, data such as name, age, gender, and location were recorded. A unique identification number was assigned to each sample. Samples were exclusively collected for this study. The strains were isolated from different specimens such as pus, urine, sputum, non-directed bronchial lavage (NBL), pleural fluid, ear swab, tissue, CVP tip, tracheal tube tip, endo-bronchial secretion, blood, chest tube tip, catheter tip, and peritoneal fluid. Antibiotic testing was performed by disc diffusion methods according to the CLSI guidelines.16 Significant differences between parameters were calculated by a chi-square cut-off value of p < 0.05.
Phenotypic and Genotypic Detection of ESBLs and Carbapenemases
Phenotypic detection of ESBLs and carbapenemases was performed by double disc synergy and modified Hodge testing.17,18 Genotypic screening of the ESBLs genes, blaCTXM-15, blaCTXM-14, blaSHV, blaTEM, and blaOXA-1, was done as described elsewhere.19 The genes, blaNDM-1, blaOXA-48, and blaKPC-2, were screened by using PCR. Amplification conditions were as follows: denaturation at 95°C for 1 min, annealing at 55°C, 56°C, and 57°C (for blaNDM-1, blaOXA-48, and blaKPC-2, respectively) for 1.5 min, extension at 72°C for 1 min, and the final extension was done at 72°C for 10 min (Table 1).
 |
Table 1 Primer Sequences Used in This Study |
Minimum Inhibitory Concentrations Planktonic (MIC-p)
For ciprofloxacin, levofloxacin, and cefepime, MIC-p was determined by using broth micro-dilution method as per CLSI recommendation.16 Briefly, a 100 μL of standardized inoculum of P. aeruginosa was added into tissue culture plate (TCP) containing 100 μL of two-fold serial dilution of tested antibiotics. Each bacterial isolate was tested against a series of antibiotic dilutions: final concentration, 256 µg/mL, 128 µg/mL, 64 µg/mL, 32 µg/mL, 16 µg/mL, 8 µg/mL, 4 µg/mL, 2 µg/mL, 1 µg/mL, 0.5 µg/mL, 0.25 µg/mL, and 0.125 μg/mL. The TCP were incubated overnight at 37°C, and MICs were noted as lowest concentration that inhibited the visible growth of the bacterium. P. aeruginosa ATCC 27853 was used as a control.
Biofilm Formation Assay
Biofilm formation assays were done by using the micro-titer plate method as described elsewhere.20 For the biofilm formation assays, P. aeruginosa strains were cultured on fresh media plates and incubated overnight. The incubated cultures were then diluted 1:100 into fresh Mueller Hinton broth (MHB). A 200 µL of the culture dilution was added per well in a sterile micro-titer plate and incubated overnight at 37°C. Every isolate was tested in triplicate. After incubation, media were discarded and plates were washed thrice with phosphate-buffered saline (PBS). After initial washing, 200 µL methanol was added in each well for fixation of biofilm. Subsequently, biofilms were stained using 1% crystal violet (CV) stain. The wells were again rinsed with PBS three times and then allowed to air dry. Afterwards, acetic acid (33%) was added to each well for solubilization of crystal violet dye. The plate was incubated at room temperature for 20–30 min, and absorbance was checked in plate reader (RT-6000) at 540 nm. The cut off OD was 0.055 for the crystal violet.
Effect of Sub-MICs of CIP, LEV, and CEF on Biofilm Formation
The effect of sub-MICs of different antibiotics on biofilm was evaluated by micro-titer plate assay. Six CIP, LEV, and CEF-sensitive P. aeruginosa clinical strains were selected for this assay. Biofilm formation was analyzed in the presence and absence of ciprofloxacin, levofloxacin, and cefepime. All three antibiotics were tested at the following sub-minimal concentrations: final concentration, 0.5 µg/mL, 0.25 µg/mL, 0.125 µg/mL, 0.0625 µg/mL for different time intervals, i.e 4 hours, 8 hours, 12 hours, and 24 hours.
Evaluation of Synergistic Effects of Antibiotics on Biofilm by Checkerboard Method
Two different combinations of antibiotics, namely (i) ciprofloxacin with cefepime, and (ii) gentamicin with cefepime, were selected to determine minimum biofilm inhibitory concentration (MBIC) and minimum biofilm eradication concentration (MBEC) by checkerboard assay as described elsewhere.21–23 Briefly, three strong biofilm-producing isolates of P. aeruginosa simultaneously sensitive to selected antibiotics were processed for the checkerboard assay. Serial two-fold dilutions of the selected antibiotics were prepared in MHB ranging from final concentration 8 µg/mL to 2048 µg/mL in sterile test tubes, and 100 µL of each serially diluted antibiotic was added into the wells of a micro-titer plate containing preformed biofilm of the selected isolates. After incubation at 37°C for 24 hours MBIC was visualized, antibiotic dilutions were then discarded, followed by washing of wells with PBS (phosphate saline buffer). After washing, 100 μL recovery media (MHB) was added into each well, and the micro-titer plate was again incubated for 24 hours. Following the overnight incubation, the lowest antibiotic concentration in a combination that inhibited the re-growth of the biofilm was considered as MBIC.24,25 Experiments were performed in triplicate. Wells containing only bacterial suspension and MHB with antibiotics were considered as positive and negative controls, respectively. MBEC was determined the same way as MBIC, except that after the incubation step (of recovery media), wells with no visible growth were thoroughly scrapped and suspended in 1 mL PBS. The suspensions were then vortexed to disrupt the biofilm, followed by plating on fresh tryptic soy agar (TSA). Plates were incubated overnight. MBEC was recorded as the minimum concentration showing no growth on TSA plates. In order to confirm the synergistic effects at which eradication occurred, fractional inhibitory concentrations were determined using the formula:
FIC value of ≤0.5 indicated synergy, FIC between 0.5–4 indicated indifference, and FIC ˃4 indicated antagonism.26
Evaluation of Synergistic Effects of Antibiotics on Biofilms at Sub-MIC Level by Checkerboard Method
Two-fold serial dilutions of ciprofloxacin, cefepime, and gentamicin were prepared at final concentrations; 0.5 µg/mL, 0.25 µg/mL, 0.125 µg/mL, and 0.0625 µg/mL in MHB. A 50 µL of antibiotic solution was added to treat wells containing 100 µL of standardized bacterial suspension, making the final volume in the well 200 µL. The TCP was then incubated for 2 hours, 4 hours, 6 hours, and 24 hours at 37°C.21,27 Biofilm quantification was done at 540 nm following the crystal violet staining. All experiments were performed in triplicate. Wells containing only bacterial suspension were considered positive control, and MHB with antibiotics was taken as negative control.
Results
Susceptibility Profile
Samples were collected from the Armed Forces Institute of Pathology (AFIP), Islamabad, Pakistan. The most frequent sources of P. aeruginosa were pus samples, n=102 (38%); followed by urine samples, n=49 (18%); ear/throat/wound swabs, n=27 (10%); tissue cultures, n=18 (7%); sputum n=14 (5%); endobronchial secretion, n=10 (4%); and NBL, n=9 (3%). P. aeruginosa isolates were resistant to ciprofloxacin, n=114 (43%); levofloxacin, n=116 (44%); meropenem, n=99 (37%); ceftazidime, n=97 (36%); imipenem, n=94 (35%); aztreonam, n=94 (35%); gentamicin, n=87 (32%); amikacin, n=72 (27%); cefepime, n=69 (26%); and piperacillin/tazobactam, n=45 (17%). Least resistance was observed against polymyxin B and colistin, since 98% of the isolates were sensitive to both antibiotics. Moreover, 25% of the isolates were MDR and 20% XDR. The distribution of MDR, XDR, and non-MDR strains of P. aeruginosa is shown in (Table S1).
Molecular Identification of ESBL and Carbapenemase Genes
Out of 266 isolates, 43% of the isolates were confirmed as ESBL producers by double disc synergy testing. Molecular analysis revealed that 62% of the isolates carried blaCTXM-15, and 34% were positive for blaCTXM-14. Other molecular factors including blaSHV, blaTEM, and blaOXA-1 were detected in 41%, 67%, and 33% of the isolates, respectively. In addition, 40% of the isolates were carbapenemase producers. Overall, 68% of the isolates carried the blaNDM-1 gene, and 25% were positive for blaOXA-48; blaKPC-2 was detected in 22% of the P. aeruginosa isolates.
Biofilm Formation Assay
P. aeruginosa isolates were assessed for biofilm formation using the micro-titer plate method. Out of 234 tested isolates, 227 (97%) were biofilm producers. Out of these, 65 (28%) were strong biofilm formers, 108 (46%) produced biofilms at moderate levels, and 54 (23%) showed weak biofilm formation. Each isolate was categorized as strong, moderate, and weak biofilm former according to the following criteria:
Weak biofilm producer: OD=2×ODc
Moderate biofilm producer: 2×ODc≤4×ODc
Strong biofilm producer: OD≥4×ODc
Minimum Inhibitory Concentration
Minimum inhibitory concentrations were measured against ciprofloxacin, levofloxacin, and cefepime using broth micro-dilution method. Six isolates, simultaneously strong biofilm formers and sensitive to ciprofloxacin, levofloxacin, and cefepime, were selected for measuring the minimum inhibitory concentration of biofilm (MIC-b). It was observed that MIC-b values for all six tested isolates were 128–1000 times higher, revealing that the isolates became tolerant to antibiotics in their biofilm forms. Furthermore, MBEC values indicated a ~2000-fold increase when compared with the respective MIC-p, which clearly showed that complete eradication of biofilms of P. aeruginosa was difficult to achieve even at much higher concentration of antibiotics (Table 2).
 |
Table 2 MIC-p, MIC-B, and MBEC Analysis on Selected Pseudomonas aeruginosa Isolates |
Effects of Sub-MICs of CIP, LEV, and CEF on Biofilm Formation
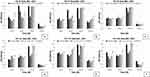
All six P. aeruginosa isolates, simultaneously strong biofilm formers and sensitive to ciprofloxacin, levofloxacin, and cefepime, were used to determine sub-minimal inhibitory concentrations of ciprofloxacin, levofloxacin, and cefepime at different time intervals, i.e 4 hours, 8 hours, 12 hours, and 24 hours. It was observed that biofilm formation was significantly (p<0.05) and consistently reduced upon treatment with antibiotic at sub-minimal level. Reduction was observed in a dose-dependent manner. In the case of ciprofloxacin, maximum reduction of biofilm was shown at 0.5 µg/mL (Figure 1). In the case of levofloxacin, only two isolates showed significant reduction (p<0.05) in biofilm formation at two different concentrations (0.125 µg/mL and 0.5 µg/mL) after 24 hours of incubation. Overall, however, the effect of levofloxacin on the biofilm-forming ability of tested isolates was inhibitory (Figure 2). In the case of cefepime, inhibitory effects were not observed except one isolate that showed significant reduction at 0.125 µg/mL and 0.5 µg/mL as shown in Figure 3.
Evaluation of Synergistic Effects of Antibiotics by Checkerboard Method
Strong biofilm former P. aeruginosa isolates, simultaneously sensitive to ciprofloxacin, cefepime, and gentamicin, were used to evaluate effects of antibiotics on biofilm formation. Antibiotics were tested individually and in combination. When testing gentamicin alone, MBIC values were 512 µg/mL and MBEC values were 1024 µg/mL. However, when testing gentamicin in combination with cefepime, MBIC for both antibiotics was substantially reduced (16 µg/mL). Similarly, MBEC of the antibiotics gentamicin and cefepime in combination was reduced (32 µg/mL). Likewise, MBIC and MBEC were significantly reduced for another tested combination, namely ciprofloxacin and cefepime (Table 3). FIC values clearly indicate a significant synergistic potential of the combinations tested in this study (Figures 4 and 5).
 |
Table 3 Analysis of MBIC and MBEC of Selected Antibiotics (Individual Vs Combination) Against Bacterial Biofilm |
Synergistic Effect of Antibiotics at Sub-MIC Level
To assess the effect of sub-MIC on synergistic potential, the antibiotics were used in combination at sub-MIC. The sub-MIC of each antibiotic was used in the following concentrations: 0.5 µg/mL, 0.25 µg/mL, 0.125 µg/mL, and 0.0625 µg/mL. An effective inhibition in biofilm formation was observed after 4–6 hours of incubation with CEF+CN and CEF+CIP, particularly at 0.5 µg/mL. Meanwhile, growth controls (in the absence of antibiotics) showed strong biofilm production (Figure 6).
Discussion
A critical ESKAPE pathogen, P. aeruginosa, is associated with a variety of infections, including skin infections, ventilator-associated pneumoniae, bacteremia, and septicemia. Severe infections such as bloodstream infections (BSI) caused by this bacterium are linked to higher mortality rates up to 30%. In the current study, 266 clinical isolates of P. aeruginosa were collected from the Armed Forces Institute of Pathology (AFIP) located in Rawalpindi. We scrutinized the antibiotic resistance profile and evaluated synergistic effects of different antibiotics on biofilms of P. aeruginosa. Out of a total of 266 isolates, the majority (38%) were recovered from pus and another 18% from urine samples. Frequent isolation of P. aeruginosa from pus and urine was reported earlier from Pakistan.28,29 In the case of severe P. aeruginosa infections such as BSI, several antibiotics are listed as the first-line therapeutic options, including piperacillin/tazobactam. In addition, aminoglycoside in combination with piperacillin/tazobactam is also recommended to treat nosocomial pneumonia. Overall, 17% of P. aeruginosa isolates were resistant to piperacillin/tazobactam. Likewise, resistance to aminoglycosides, gentamicin and amikacin, was 32% and 27%, respectively. Among cephalosporins, cefepime is the most frequently used β-lactam class of antibiotic for P. aeruginosa infections, others being ceftazidime and cefoperazone. In this study 36% of the isolates were resistant to ceftazidime and 26% to cefepime. Likewise, fluoroquinolones are considered first-line therapeutic options to treat BSI caused by P. aeruginosa. Ciprofloxacin and levofloxacin are the only options which can be administered orally during bloodstream infections. Upon testing these two antibiotics, 43% of the isolates showed resistance to ciprofloxacin and 44% to levofloxacin. Taken together, resistance to the above-mentioned frontline antibiotics indicates significant constraints on available therapeutic options to treat P. aeruginosa infections in Pakistan. Meropenem is considered as a single-agent therapy for complicated skin infections caused by P. aeruginosa. For the treatment of P. aeruginosa sepsis, carbapenems are considered second-line therapy; particularly use of meropenem is favored over imipenem because the former is linked to the induction of resistance during the continual course of treatment.30 Overall, however, cephalosporins are preferred over carbapenems because of their better potency and narrower spectrum of activity to treat sepsis. In this study, 37% of the isolates were resistant to meropenem and 35% to imipenem. P. aeruginosa is notorious for using a variety of mechanisms of antibiotic resistance including production of β-lactamase enzymes, aminoglycoside-modifying enzymes, modification of drug target sites, modification of outer membrane protein (OprD), production of a variety of carbapenemases, and regulation of efflux pump gene (MexAB, MexXY).11 In this study, 43% of the isolates were ESBL producers and 40% showed carbapenemase production. In three or more classes of antibiotics, resistance to at least one agent suffices for MDR status, while resistance to at least one agent in all antibiotic classes, except two or fewer than two classes, confers XDR status. In the current study, 25% of the isolates were MDR while 20% were XDR.
The biofilm-forming capacity of P. aeruginosa facilitates chronic colonization of host tissues such as in the case of cystic fibrosis and bacterial persistence in implanted medical devices. These micro-communities enhance resistance potential and protect biofilm from the host defenses. In the current study, 28% of the isolates were strong, 46% moderate, 23% weak, and 3% were non-biofilm formers. Among strong biofilm formers, 25% and 20% of the isolates were MDR and XDR, respectively. In terms of tolerance towards antibiotics, biofilms are crucial and may lead to persistent cell formation, replacement of sensitive cells with resistant phenotypes, and impairment of antibiotic diffusion due to extensive exopolysaccharide presence in matrix. In the present study, efficacy of the tested ciprofloxacin, cefepime, and levofloxacin was much higher in planktonic state when compared with the biofilm mode. For example, overall, in biofilm mode, MIC values were 128–1000-fold higher when compared with MIC values in planktonic state. We observed that at sub-MIC level fluoroquinolones inhibited biofilm at different time intervals. For A. baumannii, it was shown recently that sub-minimal concentration of antibiotics can significantly alter expression of genes involved in biofilm formation and antibiotic resistance.31 It is fascinating that in biofilm matrix bacteria can sense minute concentrations of antibiotics and alter their behavior remarkably; however, the underlying molecular mechanisms for alteration in gene expression still remain obscure. For the treatment of severe P. aeruginosa infections, empirical combination therapy as an option remains inconclusive, mainly due to lack of robust prospective studies.11 Yet, due to the AMR scenario and robust intrinsic resistance of P. aeruginosa to several different antibiotics, combination therapy is encouraged, particularly a β-lactam backbone combined with aminoglycoside or fluoroquinolones is prioritized. It is pertinent to mention that, according to the current guidelines of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), treatment with ceftolozane-tazobactam is recommended for severe P. aeruginosa infections.32 Though combination antibiotic therapy might be more advantageous in case of biofilm-mediated infections, not much is known about the synergistic effects of antibiotics on bacterial biofilms. In this study we tested strong biofilm-forming clinical P. aeruginosa strains which are simultaneously sensitive to all three antibiotics (ciprofloxacin, cefepime, and gentamicin). In this study, when testing single antibiotics, MIC-b values for all three tested antibiotics were 128–1000-fold higher and MBEC were even higher (~2000-fold). Ciprofloxacin was shown to diffuse well in K. pneumoniae biofilms, while ampicillin was neutralized by β-lactamase enzymes.33 Fourier transform infrared spectroscopy showed diffusion of fluoroquinolones in P. aeruginosa biofilms.34 In this study, all the tested isolates were sensitive to all three tested antibiotics (ciprofloxacin, cefepime, and gentamicin), and we confirm that the observed increases in MIC-b (128–1000-fold) and MBEC (~2000-fold) are independent of any intrinsic or acquired mechanisms of antibiotic resistance; hence tolerance towards antibiotics is solely dependent on sessile growth of P. aeruginosa.
In order to avoid catheter-associated infections, antibiotic lock therapy (ALT) is a highly recommended therapeutic intervention. The catheter is treated with a higher concentration of antibiotic, with the expectation of limiting a biofilm-associated infection. For a successful eradication, ALT is managed by parallel administration of systemic antibiotics to the patients as well. Given the increase in MIC-b and MBEC levels, 128–1000-fold and ~2000-fold, respectively, shown in this study indicate limited success can be expected for eradication to avoid catheter-associated biofilm infections with antibiotic lock therapy using a single antibiotic. Here we evaluated efficacy of different antibiotics in inhibiting and eradicating biofilms of P. aeruginosa in unique combinations. A substantial reduction in MBIC of gentamicin when combined with cefepime (16 µg/mL) was witnessed. Likewise, MBEC of the two antibiotics in combination (gentamicin and cefepime) was reduced substantially (32 µg/mL). We also confirm significant reduction in MBIC and MBEC of combination of ciprofloxacin and cefepime. Further, in this study we clearly observed strong synergistic effects of CEF+CN and CEF+CIP, at sub-MIC concentration, 0.5 µg/mL, for inhibition of P. aeruginosa biofilm. This is one of the few studies in which synergistic effects of CEF+CIP and CEF+CN were evaluated on P. aeruginosa biofilm for inhibition and eradication.
Conclusion
This study highlights prevalence of MDR and XDR in P. aeruginosa isolates, which have notable resistance against frontline antibiotics used to treat severe P. aeruginosa infections. In addition, we confirm strong synergistic effects of CEF+CN and CEF+CIP, at sub-MIC concentration of antibiotics.
Author Contributions
UM, MA, FA, AB, ZM, KM, BS executed the experimental work. MS, RZ did statistical analysis and optimization of PCR. JID conceived the idea, arranged funds, supervised experimental work, wrote and reviewed the draft. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research was funded by University Annual Research Fund (URF, 2019-20 & 2021-22), Quaid-i-Azam University, Islamabad.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barbier M, Owings JP, Martínez-Ramos I, et al. Lysine trimethylation of EF-Tu mimics platelet-activating factor to initiate Pseudomonas aeruginosa pneumonia. MBio. 2013;4(3):e00207–13. doi:10.1128/mBio.00207-13
2. Fazzeli H, Akbari R, Moghim S, Narimani T. Pseudomonas aeruginosa infections in patients, hospital means, and personnel’s specimens. Univ Med Sci. 2012;17(7):671–675.
3. Thaden JT, Park LP, Maskarinec SA, Ruffin F, Fowler VG, van Duin D. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob Agents Chemother. 2017;61(6):e02671–16. doi:10.1128/AAC.02671-16
4. Micek ST, Wunderink RG, Kollef MH, et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Critical Care. 2015;19:1–8. doi:10.1186/s13054-015-0926-5
5. Tamma P, Aitken S. Bonomo R IDSA guidance on the treatment of antimicrobial-resistant gram-negative infections: version 1.0. IDSA. 2022.
6. Kadri SS, Adjemian J, Lai YL, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67(12):1803. doi:10.1093/cid/ciy378
7. Al Salman J, Al Dabal L, Bassetti M, et al. Management of infections caused by WHO critical priority Gram-negative pathogens in Arab countries of the Middle East: a consensus paper. Int J Antimicrob Agents. 2020;56(4):106104. doi:10.1016/j.ijantimicag.2020.106104
8. Rhodes A, Evans LE, Alhazzani W, et al. Sobrevivir Sepsis Campaign: directrices internacionales para el manejo de la sepsis y el shock séptico: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
9. Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs in Context. 2018;7:1–18. doi:10.7573/dic.212527
10. Bassetti M, Vena A, Russo A, Croxatto A, Calandra T, Guery B. Rational approach in the management of Pseudomonas aeruginosa infections. Curr Opin Infect Dis. 2018;31(6):578–586. doi:10.1097/QCO.0000000000000505
11. Zakhour J, Sharara SL, Hindy J-R, Haddad SF, Kanj SS. Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis. Antibiotics. 2022;11(10):1432. doi:10.3390/antibiotics11101432
12. Burgess DS, Nathisuwan S. Cefepime, piperacillin/tazobactam, gentamicin, ciprofloxacin, and levofloxacin alone and in combination against Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 2002;44(1):35–41. doi:10.1016/S0732-8893(02)00420-0
13. Macia M, Rojo-Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infection. 2014;20(10):981–990. doi:10.1111/1469-0691.12651
14. Noreen A, Masood H, Zaib J, et al. Investigating the Role of Antibiotics on Induction, Inhibition and Eradication of Biofilms of Poultry Associated Escherichia coli Isolated from Retail Chicken Meat. Antibiotics. 2022;11(11):1663. doi:10.3390/antibiotics11111663
15. Nucleo E, Steffanoni L, Fugazza G, et al. Growth in glucose-based medium and exposure to subinhibitory concentrations of imipenem induce biofilm formation in a multidrug-resistant clinical isolate of Acinetobacter baumannii. BMC Microbiol. 2009;9(1):1–14. doi:10.1186/1471-2180-9-270
16. Clinical and Laboratory Standard Institute (CLSI). M100 Performance Standards for Antimicrobial Susceptibility Testing 30th ed.; An Informational Supplement; CLSI: Wayne, PA, USA; 2020. Available from: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf.
17. Jarlier V, Nicolas M-H, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Clin Infect Dis. 1988;10(4):867–878. doi:10.1093/clinids/10.4.867
18. Anderson K, Lonsway D, Rasheed J, et al. Evaluation of Methods to Identify the Klebsiella pneumoniae in. J Clin Microbiol. 2007;45(8):2723–2725. doi:10.1128/JCM.00015-07
19. Ali I, Rafaque Z, Ahmed I, et al. Phylogeny, sequence-typing and virulence profile of uropathogenic Escherichia coli (UPEC) strains from Pakistan. BMC Infect Dis. 2019;19(1):1–9. doi:10.1186/s12879-019-4258-y
20. Rafaque Z, Abid N, Liaqat N, et al. In-vitro investigation of antibiotics efficacy against uropathogenic Escherichia coli biofilms and antibiotic induced biofilm formation at sub-minimum inhibitory concentration of ciprofloxacin. Infect Drug Resist;2020. 2801–2810. doi:10.2147/IDR.S258355
21. Drago L, De Vecchi E, Nicola L, Colombo A, Guerra A, Gismondo MR. Activity of levofloxacin and ciprofloxacin in combination with cefepime, ceftazidime, imipenem, piperacillin-tazobactam and amikacin against different Pseudomonas aeruginosa phenotypes and Acinetobacter spp. Chemotherapy. 2004;50(4):202–210. doi:10.1159/000081033
22. Thiyagarajan D, Das G, Ramesh A. Amphiphilic Cargo‐Loaded Nanocarrier Enhances Antibiotic Uptake and Perturbs Efflux: effective Synergy for Mitigation of Methicillin‐Resistant Staphylococcus aureus. ChemMedChem. 2017;12(14):1125–1132. doi:10.1002/cmdc.201700260
23. Wang L, Di Luca M, Tkhilaishvili T, Trampuz A, Gonzalez Moreno M. Synergistic activity of fosfomycin, ciprofloxacin, and gentamicin against Escherichia coli and Pseudomonas aeruginosa biofilms. Front Microbiol. 2019;10:2522. doi:10.3389/fmicb.2019.02522
24. Dosler S, Karaaslan E, Alev Gerceker A. Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against Gram-negative bacteria. J Chemother. 2016;28(2):95–103. doi:10.1179/1973947815Y.0000000004
25. Almaaytah A, Abualhaijaa A, Alqudah O. The evaluation of the synergistic antimicrobial and antibiofilm activity of AamAP1-Lysine with conventional antibiotics against representative resistant strains of both Gram-positive and Gram-negative bacteria. Infect Drug Resist. 2019;1371–1380. doi:10.2147/IDR.S204626
26. Ghorbani H, Memar MY, Sefidan FY, Yekani M, Ghotaslou R. In vitro synergy of antibiotic combinations against planktonic and biofilm Pseudomonas aeruginosa. GMS Hygiene Infection Control. 2017;12. doi:10.3205/dgkh000302
27. Gupta P, Chhibber S, Harjai K. Subinhibitory concentration of ciprofloxacin targets quorum sensing system of Pseudomonas aeruginosa causing inhibition of biofilm formation & reduction of virulence. Indian J Med Res. 2016;143(5):643. doi:10.4103/0971-5916.187114
28. Ellappan K, Narasimha HB, Kumar S. Coexistence of multidrug resistance mechanisms and virulence genes in carbapenem-resistant Pseudomonas aeruginosa strains from a tertiary care hospital in South India. J Global Antimicrobial Resistance. 2018;12:37–43. doi:10.1016/j.jgar.2017.08.018
29. Ullah W, Qasim M, Rahman H, et al. Multi drug resistant Pseudomonas aeruginosa: pathogen burden and associated antibiogram in a tertiary care hospital of Pakistan. Microb Pathog. 2016;97:209–212. doi:10.1016/j.micpath.2016.06.017
30. Gimeno C, Cantón R, García A, Gobernado M. Comparative activity of doripenem, meropenem, and imipenem in recent clinical isolates obtained during the COMPACT-Spain epidemiological surveillance study. Revista Espanola de Quimioterapia. 2010;23(3):144–152.
31. Shenkutie AM, Zhang J, Yao M, Asrat D, Chow FW, Leung PH. Effects of sub-minimum inhibitory concentrations of imipenem and colistin on expression of biofilm-specific antibiotic resistance and virulence genes in Acinetobacter baumannii sequence type 1894. Int J Mol Sci. 2022;23(20):12705. doi:10.3390/ijms232012705
32. Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infection. 2022;28(4):521–547. doi:10.1016/j.cmi.2021.11.025
33. Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44(7):1818–1824. doi:10.1128/AAC.44.7.1818-1824.2000
34. Vrany JD, Stewart PS, Suci PA. Comparison of recalcitrance to ciprofloxacin and levofloxacin exhibited by Pseudomonas aeruginosa biofilms displaying rapid-transport characteristics. Antimicrob Agents Chemother. 1997;41(6):1352–1358. doi:10.1128/AAC.41.6.1352
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.