Back to Journals » Infection and Drug Resistance » Volume 17
Synergistic Activity of 3-Hydrazinoquinoxaline-2-Thiol in Combination with Penicillin Against MRSA
Authors Elfadil A , Ibrahem K , Abdullah H , Mokhtar JA , Al-Rabia MW, Mohammed HA
Received 8 November 2023
Accepted for publication 16 January 2024
Published 31 January 2024 Volume 2024:17 Pages 355—364
DOI https://doi.org/10.2147/IDR.S448843
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Abdelbagi Elfadil, Karem Ibrahem, Hani Abdullah, Jawahir A Mokhtar, Mohammed W Al-Rabia, Hafsa Alawad Mohammed
Department of Clinical Microbiology and Immunology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabic
Correspondence: Abdelbagi Elfadil, Department of Clinical Microbiology and Immunology, Faculty of Medicine, King Abdulaziz University, P.O. Box 80205, Jeddah, 21589, Saudi Arabia, Tel +96612 6952000 Ext:21062, Email [email protected]
Background: The growing resistance seen in various antibiotics, including those considered as last-resort options, underscores the pressing need for novel approaches and new substances to address MRSA infections. Combining antibiotics as a treatment approach can enhance effectiveness, expand the range of targeted bacteria, and minimize the likelihood of resistance emergence. This approach holds promise in addressing the escalating issue of antibiotic resistance.
Purpose: This study seeks to investigate the potential synergy between 3-hydrazinoquinoxaline-2-thiol and penicillin against a diverse array of MRSA isolates, thereby providing insights into their combined antimicrobial action.
Methods: Twenty-two clinical MRSA isolates subjected to broth microdilution to determine the Minimum Inhibitory Concentrations (MICs) of 3-hydrazinoquinoxaline-2-thiol and penicillin. Subsequently, a checkerboard assay was employed to evaluate the interaction between 3-hydrazinoquinoxaline-2-thiol and penicillin, focusing on the Fractional Inhibitory Concentration Index (FICI).
Results: The MICs of penicillin and 3-hydrazinoquinoxaline-2-thiol were determined for 22 clinical MRSA strains. Penicillin exhibited MICs within a range of 1024 to 128 μg/mL, while 3-hydrazinoquinoxaline-2-thiol displayed MICs varying from 64 to 8 μg/mL. Remarkably, the combination of 3-hydrazinoquinoxaline-2-thiol and penicillin yielded a synergistic effect, resulting in a significant reduction of MICs by up to 64-fold.
Conclusion: The potential of 3-hydrazinoquinoxaline-2-thiol in combination with penicillin as a viable solution against MRSA appears promising. However, to establish its practical utility, further extensive testing and experiments are essential.
Keywords: penicillin, antibiotic combination therapy, drug discovery, 3-hydrazinoquinoxaline-2-thiol
Introduction
Bacterial infections continue to pose a substantial global threat and remain a leading cause of mortality. This dire situation is exacerbated by the growing challenge of antibiotic resistance. This evolving resistance makes it increasingly difficult to effectively treat these infections, emphasizing the urgent need for novel therapeutic strategies and the development of innovative antibiotics.1 Methicillin-resistant Staphylococcus aureus (MRSA) is accountable for a wide spectrum of diseases and stands out as a primary culprit behind hospital-acquired infections. These infections frequently lead to elevated levels of illness and death, prolonged hospital stays, and escalated treatment expenses. The formidable impact of MRSA on healthcare systems underscores the urgency of addressing this growing concern to mitigate its adverse effects on both patients and healthcare resources.2
β-lactam antibiotics exert their action by interfering with penicillin-binding proteins (PBPs), key players in the process of peptidoglycan crosslinking in bacterial cell walls.3 In the case of MRSA, resistance mechanisms are attributed to the presence of the mecA gene, responsible for encoding penicillin-binding protein 2A (PBP2A). PBP2A distinguishes itself by having a diminished affinity for the majority of β-lactam antibiotics, rendering them less effective against MRSA strains. This lowered susceptibility to these antibiotics underscores the significance of alternative treatment strategies to combat MRSA infections effectively.4 Handling MRSA infections has proven to be a formidable challenge, primarily due to the limited therapeutic choices available. MRSA exhibits resistance to a range of antibiotics, including β-lactams, as well as last-resort antibiotic options. This resistance profile significantly narrows down the arsenal of effective treatments, amplifying the complexity of managing MRSA infections.5
At present, the arsenal of antibiotics suitable for MRSA treatment is quite limited, including options like vancomycin, linezolid, daptomycin and ceftaroline. These antibiotics have frequently been employed as the final resort for managing MRSA infections. However, it’s important to note that they come with certain constraints in their clinical utility, and resistance to them has been frequently observed.3,6,7
The process of developing novel antibiotics encounters a multitude of obstacles, encompassing regulatory hurdles, economic constraints, and substantial time investments. In light of these challenges, a pragmatic approach involves repurposing existing antibiotics and combining them synergistically in a well-thought-out manner. This strategy not only caters to the immediate and pressing clinical requirements but also benefits from the advantage that these agents have already gained approval for human use, streamlining the path to effective treatment solutions.8,9 Undoubtedly, the strategy of antibiotic combination therapy has proven to be efficacious in addressing the challenges posed by multidrug-resistant pathogens, exemplified by its successful application in treating organisms like Mycobacterium tuberculosis and Helicobacter pylori. This approach has enabled more comprehensive and effective treatment regimens, mitigating the impact of drug resistance and enhancing therapeutic outcomes in these cases.10,11
Taking into account the utilization of antibiotic combinations offers a plethora of benefits, including the enhancement of treatment efficacy, the expansion of the range of pathogens targeted, a reduction in the likelihood of adverse side effects and toxicity, and a decreased risk of resistance development. This approach proves advantageous by concurrently addressing multiple aspects of bacterial infections, resulting in more effective and safer therapeutic strategies.11
In a prior investigation, Elfadil et al demonstrated the notable efficacy of quinoxaline derivatives in combating various clinical strains of MRSA.12 Quinoxalines, known for intercalating into DNA, exhibit preferential action against Gram-positive bacteria. Synthetic structures with the quinoxaline moiety possess antibacterial properties against a wide range of pathogens, acting through DNA interaction and inducing reactive oxygen species (ROS).13 Expanding on this, we propose that these quinoxaline derivatives have the capacity to restore the efficacy of penicillin when used in combination therapy against various MRSA clinical strains. This approach leverages the prior findings, presenting an innovative strategy to address the challenges posed by MRSA infections and potentially pave the way for more effective treatment options.
The aim of this study is to evaluate the in vitro antimicrobial activity of 3-hydrazinoquinoxaline-2-thiol when used in conjunction with penicillin against a range of clinical MRSA strains. This research endeavours to shed light on the potential synergy between these compounds and how it impacts the efficacy of treatment, further contributing to the understanding of innovative approaches to combat MRSA infections.
Materials and Methods
Sample Collection Growth Media and Condition
This study involved the assessment of 20 MRSA isolates, which were sourced from King Abdulaziz University hospital at King Abdulaziz University in Jeddah, Saudi Arabia. These isolates had been preserved in glycerol and stored at a temperature of −80°C. Prior to testing, all isolates were carefully thawed and cultured on Mannitol salt agar (provided by HiMedia, India) and incubated overnight at a temperature of 37°C within an aerobic environment.
Identification of the colonies was carried out using standard procedures, involving tests for catalase and tube coagulase. The process of sample collection was executed in strict adherence to the ethical guidelines and research protocols established by the Faculty of Applied Medical Sciences at King Abdulaziz University, under the reference number 38–712-456. The research was conducted in accordance with the principles outlined in the Declaration of Helsinki.
It’s important to note that, as the clinical isolates utilized in this study were obtained as part of routine hospital laboratory procedures, the ethics committee granted an exemption from the requirement for informed consent.
Antibacterial Agents
The MRSA-targeting medications assessed in this study encompassed a 3-hydrazinoquinoxaline-2-thiol compound, sourced from Fluorophen Ltd in the United Kingdom and penicillin powder, acquired from Med ChemExpress.com
Susceptibility Testing
To assess antibiotic sensitivity, a broth microdilution test was conducted. The procedure involved creating a two-fold serial dilution of the antibiotics under examination in Mueller Hinton Broth obtained from Sigma-Aldrich in the United States. Subsequently, 100 µL of the prepared antibiotic solution was dispensed into each well of 96-well plates from Corning, Italy.
The inoculum suspension density was meticulously adjusted to 0.5 McFarland using a suspension turbidity detector, specifically the Biosan Densitometers DEN-1B. Following this, 5 µL of the prepared inoculum was introduced into each well containing varying concentrations of antibiotics. The plates were then incubated overnight at a temperature of 37°C. The antibiotic susceptibility testing was conducted in triplicate, and the resulting mean values were recorded for analysis.
The Minimum Inhibitory Concentration (MIC) refers to the lowest drug concentration capable of restraining the observable growth of a microorganism. The MIC results for the two antibacterial agents were assessed using the broth microdilution technique and were interpreted in accordance with the guidelines outlined by the Clinical and Laboratory Standards Institute (CLSI).14,15
Checkerboard Assay
We employed the checkerboard broth assay to assess the interactions between antibiotics. To perform this, a two-fold serial dilution was prepared for each antibiotic using Muller-Hinton broth (MHB) Sigma, and 50 μL of each dilution was dispensed into 96-well plates from Italy Inc.
The density of the inoculum suspension was carefully adjusted to 0.5 McFarland using a suspension turbidity detector, specifically the Biosan Densitometers DEN-1B. Subsequently, 5 μL of the diluted bacteria were introduced into each well of the 96-well plates.
To evaluate the interactions, we calculated the fractional inhibitory concentration index (FICI) using the following equations: (MIC of drug A when used in combination) divided by (MIC of drug A when used alone) plus (MIC of drug B when used in combination) divided by (MIC of drug B when used alone). A FIC index of ≤0.5 was considered indicative of a synergistic effect.1 The checkerboard assay was meticulously performed in triplicate to ensure robustness and reliability. The ensuing mean values obtained from these replicates were meticulously recorded, forming the basis for subsequent detailed analysis. This stringent approach to triplicate testing and subsequent mean calculation adds a layer of precision to the experimental process, ensuring a comprehensive and accurate assessment of the data.
Results
Mics of Penicillin and 3-Hydrazinoquinoxaline-2-Thiol Were Evaluated
It is essential to have determined the MICs of penicillin and 3-hydrazinoquinoxaline-2-thiol before proceeding with the checkerboard method. The MICs for penicillin range from 1024 to 128 µg/mL (Table 1), high MIC values for penicillin are expected when dealing with MRSA strains. On the other hand, the MICs for 3-hydrazinoquinoxaline-2-thiol range from 64 to 8 µg/mL (Table 2). With this information, we were able to design the checkerboard assay by combining various concentrations of penicillin and 3-hydrazinoquinoxaline-2-thiol to assess their potential synergistic or additive effects against the resistant strains. This assay will help you determine the interaction of these agents against different MRSA clinical strains.
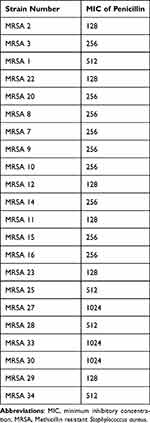 |
Table 1 MICs of Penicillin in μg/mL Against Different MRSA Clinical Strains |
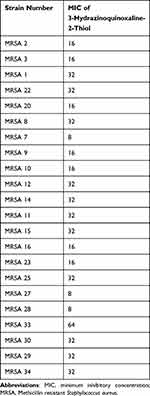 |
Table 2 MICs of 3-Hydrazinoquinoxaline-2-Thiol in μg/mL Against Different MRSA Clinical Strains |
3-Hydrazinoquinoxaline-2-Thiol Synergises Penicillin Against Different MRSA Clinical Strains
A checkerboard assay was employed to evaluate the combined impact of 3-hydrazinoquinoxaline-2-thiol and penicillin on various clinical MRSA strains. When penicillin was administered in isolation, it failed to restrain the growth of MRSA. However, the intriguing observation was that when penicillin was used in conjunction with 3-hydrazinoquinoxaline-2-thiol, the MICs of penicillin decreased significantly, in some cases by up to 64-fold. Similarly, the MICs of 3-hydrazinoquinoxaline-2-thiol exhibited a noteworthy reduction when used in combination with penicillin, up to 8-fold lower (Figure 1).
What’s even more remarkable is that this combination displayed a synergistic interaction against 22 distinct clinical MRSA strains, as evidenced by the FICI values consistently falling below 0.5 (Table 3). This strongly suggests that 3-hydrazinoquinoxaline-2-thiol complements penicillin effectively in combating MRSA strains, offering a potential avenue for improved treatment strategies (Figure 2).
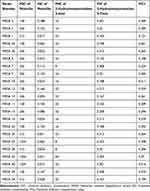 |
Table 3 The Interaction of Penicillin Against 3-Hydrazinoquinoxaline-2-Thiol 22 Clinical Different MRSA Strains |
Discussion
This study marks the pioneering demonstration of the synergistic potential between penicillin and 3-hydrazinoquinoxaline-2-thiol derivatives when confronting diverse MRSA strains. It’s noteworthy that these MRSA strains exhibited varying Minimum Inhibitory Concentration (MIC) values in response to penicillin, highlighting the diverse resistance profiles within the MRSA population. The revelation of this synergistic interaction not only expands our understanding of MRSA treatment but also offers a promising avenue for addressing antibiotic resistance in this context.
Our research has unveiled a striking outcome: when used in combination with 3-hydrazinoquinoxaline-2-thiol, penicillin’s Minimum Inhibitory Concentrations (MICs) saw a significant reduction, up to 64-fold. Likewise, a similar pronounced effect was observed when penicillin was paired with 3-hydrazinoquinoxaline-2-thiol, leading to a considerable decrease in the MICs of the 3-hydrazinoquinoxaline-2-thiol derivatives themselves. This potent cooperative action of 3-hydrazinoquinoxaline-2-thiol and penicillin was further substantiated in our experiments against a variety of clinical MRSA strains. These findings strongly indicate that the combined therapy of penicillin and 3-hydrazinoquinoxaline-2-thiol elicits a more robust response against MRSA strains compared to the efficacy of each individual drug.
Introducing a second antibiotic into the treatment regimen can potentially compensate for the limitations of the first antibiotic.16 Our results support the proposed concept: Penicillin alone could not halt MRSA growth, but in combination with 3-hydrazinoquinoxaline-2-thiol, it demonstrated inhibitory efficacy. This synergy highlights the therapeutic potential of combined treatments against MRSA challenges.
Given the potential for toxic effects associated with high concentrations of 3-hydrazinoquinoxaline-2-thiol, penicillin, and other antibiotics, the utilization of reduced doses of each drug in a synergistic manner offers a promising strategy to mitigate potential toxicity.9 Our study supports the concept that penicillin, when combined with 3-hydrazinoquinoxaline-2-thiol, achieved MRSA growth inhibition at a significantly lower dose (32 μg/mL) compared to penicillin alone (512 μg/mL). This implies a potential for lower doses in combined therapy, potentially reducing adverse effects. Further research is needed to validate this promising result.
In our study, we made a noteworthy discovery regarding the efficacy of 3-hydrazinoquinoxaline-2-thiol in restoring the activity of penicillin against various clinical strains of MRSA. The synergistic effect observed between 3-hydrazinoquinoxaline-2-thiol and penicillin appears to be underpinned by a dual mechanism involving the inhibition of two distinct pathways. Firstly, penicillin hinders the action of penicillin-binding proteins (PBPs),17 and secondly, 3-hydrazinoquinoxaline-2-thiol impede DNA synthesis.18
The significance of this synergy lies in the fact that β-lactam antibiotics, like penicillin, primarily function by inhibiting PBPs. Importantly, each β-lactam antibiotic displays varying affinities for different bacterial PBPs. Consequently, the inhibition of PBPs disrupts the cross-linking of peptidoglycan in the bacterial cell wall.19 This disruption could potentially enhance the uptake and effectiveness of 3-hydrazinoquinoxaline-2-thiol.
It is worth noting that earlier research by Moellering and Weinberg indicated a substantial increase in the uptake of aminoglycoside antibiotics in the presence of penicillin or other antibiotics that interfere with the synthesis of bacterial cell walls.20 Based on our findings, we hypothesize that the observed synergy between penicillin and 3-hydrazinoquinoxaline-2-thiol is driven by the activity of penicillin, which results in the impairment of the cross-linked cell wall. This impairment, in turn, facilitates the penetration and uptake of 3-hydrazinoquinoxaline-2-thiol, ultimately enhancing their bactericidal effects against MRSA.
Another plausible explanation for the enhanced effectiveness of the combination of penicillin and 3-hydrazinoquinoxaline-2-thiol against various MRSA strains could involve the increased production of reactive oxygen species (ROS). These ROS interfere with target-specific cellular processes and eventually lead to cell death. It is well-documented that bactericidal antibiotics, particularly β-lactams, can trigger the generation of ROS, which plays a significant role in bacterial cell killing.21 Interestingly, 3-hydrazinoquinoxaline-2-thiol are also known to have the capacity to generate reactive oxygen species.22
Recent research has highlighted the phenomenon of β-lactam antibiotics inducing ROS production in Enterococcus faecalis.23 Based on these findings, we speculate that the observed synergy between penicillin and 3-hydrazinoquinoxaline-2-thiol may be linked to the excessive production of ROS. However, further experiments are necessary to validate this hypothesis.
Additional investigations are imperative to gain a more comprehensive understanding of the synergistic interaction between penicillin and 3-hydrazinoquinoxaline-2-thiol. This entails conducting a series of crucial experiments.
Firstly, a Time Kill assay is warranted to meticulously evaluate the bactericidal effects of this combination. This assay will help us understand how effectively the combination eradicates bacteria over time, providing insights into its potential as a treatment strategy.
Furthermore, a resistance assay is essential to assess the likelihood of bacteria developing resistance to the penicillin and 3-hydrazinoquinoxaline-2-thiol combination. Understanding the resistance potential is crucial for the long-term effectiveness of this treatment approach.1,24 Incorporating an in vivo model is of paramount importance to assess the efficacy of the penicillin and 3-hydrazinoquinoxaline-2-thiol combination in a living organism. This model will not only allow us to gauge how well the treatment works in a complex biological environment but also delve into the pharmacokinetic and pharmacodynamic aspects of the combination, shedding light on its behaviour within the body and its interaction with bacteria at a molecular level.25,26
Conclusion
In summary, for the first time we have demonstrated that the synergistic potential of 3-hydrazinoquinoxaline-2-thiol in combination with penicillin against a diverse spectrum of clinical MRSA strains. The findings from our study strongly indicate that this synergy could be advanced into clinical application. Nonetheless, there are several crucial steps that lie ahead to transition these combined antibiotics into clinical practice. Further research and clinical trials are imperative to fully explore the therapeutic potential, optimize dosing regimens, and ensure the safety and efficacy of this promising combination in real-world medical settings.
Abbreviations
MHB, Muller-Hinton broth; MRSA, Methicillin resistant Staphylococcus aureus; MIC, minimum inhibitory concentration; FIC, Fractional inhibitory concentration; FICI, Fractional inhibitory concentration index; ROS, Reactive oxygen species; PBP2A, penicillin-binding protein 2A.
Funding
Grant number IFPIP:1448-140-1443- 260671 from the Instructional Improvement Fund supported this study. The authors would like to express their appreciation to the Saudi Arabian Moe and King Abdulaziz University, DSR in Jeddah for their technical and financial aid.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Hu Y, Liu Y, Coates A. Azidothymidine produces synergistic activity in combination with colistin against antibiotic-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2019;63(1):1–11. doi:10.1128/AAC.01630-18
2. Butterly A, Schmidt U, Ph D, Wiener-kronish J. Methicillin-resistant Staphylococcus aureus colonization, its relationship to nosocomial infection, and efficacy of control methods. Anesthesiol J Am Soc Anesthesiol. 2010;113:1453–1459.
3. Bush K, Bradford PA. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med. 2016;6(8):a025247. doi:10.1101/cshperspect.a025247
4. Stapleton PD, Taylor PW. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci Prog. 2007;85:1–14.
5. Meyer E, Schwab F, Gastmeier P. Nosocomial methicillin resistant Staphylococcus aureus pneumonia-epidemiology and trends based on data of a network of 586 German ICUs (2005–2009). Eur J Med Res. 2010;15(12):514–524. doi:10.1186/2047-783X-15-12-514
6. Nguyen HM, Graber CJ. Limitations of antibiotic options for invasive infections caused by methicillin-resistant Staphylococcus aureus: is combination therapy the answer? J Antimicrob Chemother. 2009;65(1):24–36. doi:10.1093/jac/dkp377
7. Pantosti A, Sanchini A, Monaco M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007;2(3):323–334. doi:10.2217/17460913.2.3.323
8. Ventola CL. The antibiotic resistance crisis part 1: causes and threats. Pharm Ther. 2015;40:277–283.
9. Gonzales PR. Synergistic, collaterally sensitive β-lactam combinations suppress resistance in MRSA. Nat Chem Biol. 2015;11(11):855–861. doi:10.1038/nchembio.1911
10. Kerantzas CA, Jacobs WR, Rubin EJ, Collier RJ. Origins of combination therapy for tuberculosis: lessons for future antimicrobial development and application. MBio. 2017;8(2):1–10. doi:10.1128/mBio.01586-16
11. Ramo S, Ng C, Anderson H, et al. Synergistic drug combinations for tuberculosis therapy identified by a novel high-throughput screen. Antimicrob Agents Chemother. 2011;55(8):3861–3869. doi:10.1128/AAC.00474-11
12. Elfadil A, Alzahrani AM, Abdullah H, et al. Evaluation of the antibacterial activity of quinoxaline derivative compound against methicillin-resistant Staphylococcus aureus. Infect Drug Resist. 2023;16:2291–2296. doi:10.2147/IDR.S401371
13. Ahammed KS, Pal R, Chakraborty J, et al. DNA structural alteration leading to antibacterial properties of 6-nitroquinoxaline derivatives. J Med Chem. 2019;62(17):7840–7856. doi:10.1021/acs.jmedchem.9b00599
14. Tan CM, Therien AG, Lu J, et al. Restoring Methicillin-Resistant Staphylococcus aureus Susceptibility to β-Lactam Antibiotics. Sci Transl Med. 2012;4(126):126ra35. doi:10.1126/scitranslmed.3003592
15. Mikkelsen K, Sirisarn W, Alharbi O, et al. The novel membrane-associated auxiliary factors AuxA and AuxB modulate β -lactam resistance in MRSA by stabilizing lipoteichoic acids. Int J Antimicrob Agents. 2021;57(3):106283. doi:10.1016/j.ijantimicag.2021.106283
16. Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol. 2019;10. doi:10.3389/fmicb.2019.00539
17. Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482–501. doi:10.3934/microbiol.2018.3.482
18. Cheng G, Sa W, Cao C, et al. Quinoxaline 1,4-di-N-oxides: biological activities and mechanisms of actions. Front Pharmacol. 2016;7:1–21. doi:10.3389/fphar.2016.00064
19. Uddin TM, Chakraborty AJ, Khusro A, et al. Antibiotic resistance in microbes: history, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021;14(12):1750–1766. doi:10.1016/j.jiph.2021.10.020
20. Moellering RC, Weinberg AN, Weinberg AN. Studies on antibiotic synergism against enterococci: II. Effect of various antibiotics on the uptake of 14 C-labeled streptomycin by enterococci. J Clin Invest. 1971;50(12):2580–2584. doi:10.1172/JCI106758
21. Dwyer DJ, Belenky PA, Yang JH, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci. 2014;111(20):E2100–E2109. doi:10.1073/pnas.1401876111
22. Chacón-Vargas KF, Andrade-Ochoa S, Nogueda-Torres B, et al. Isopropyl quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives induce regulated necrosis-like cell death on Leishmania (Leishmania) mexicana. Parasitol Res. 2018;117(1):45–58. doi:10.1007/s00436-017-5635-3
23. Léger L, Budin-Verneuil A, Cacaci M, Benachour A, Hartke A, Verneuil N. β-lactam exposure triggers reactive oxygen species formation in enterococcus faecalis via the respiratory chain component DMK. Cell Rep. 2019;29(8):2184–2191. doi:10.1016/j.celrep.2019.10.080
24. Rani R, Sharma D, Chaturvedi M, Parkash Yadav J. Antibacterial activity of twenty different endophytic fungi isolated from calotropis procera and time kill assay. Clin Microbiol Open Access. 2017;06(03). doi:10.4172/2327-5073.1000280
25. Duan L, Zhang J, Chen Z, et al. Antibiotic combined with epitope-specific monoclonal antibody cocktail protects mice against bacteremia and acute pneumonia from methicillin-resistant staphylococcus aureus infection. J Inflamm Res. 2021;14:4267–4282. doi:10.2147/JIR.S325286
26. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–12. doi:10.1086/516284
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


