Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Symptom Burden and GOLD Classification in Medicare Advantage Patients with COPD Initiating Umeclidinium/Vilanterol or Fluticasone Propionate/Salmeterol Therapy
Authors Moretz C, Hahn B , White J, Goolsby Hunter A, Essoi B, Elliott C, Ray R
Received 28 May 2020
Accepted for publication 23 September 2020
Published 29 October 2020 Volume 2020:15 Pages 2715—2725
DOI https://doi.org/10.2147/COPD.S265037
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Chad Moretz,1 Beth Hahn,1 John White,2 Alyssa Goolsby Hunter,2 Breanna Essoi,2 Caitlin Elliott,2 Riju Ray3
1US Value Evidence & Outcomes, GlaxoSmithKline, Research Triangle Park, NC, USA; 2Optum, Eden Prairie, MN, USA; 3US Medical Affairs, GlaxoSmithKline, Research Triangle Park, NC
Correspondence: Beth Hahn
GSK, 5 Moore Drive, Research Triangle Park, Durham, NC, USA
Tel +1 919 274 0660
Email [email protected]
Background: Long-acting muscarinic antagonist/long-acting β2-agonist (LAMA/LABA) provide greater improvements in lung function and symptoms than inhaled corticosteroid (ICS)/LABA in patients with chronic obstructive pulmonary disease (COPD). This study evaluated symptom burden and Global Initiative for Obstructive Lung Disease (GOLD) categorization among patients who recently initiated umeclidinium/vilanterol (UMEC/VI; LAMA/LABA) or fluticasone propionate/salmeterol (FP/SAL; ICS/LABA) single-inhaler dual therapy.
Methods: COPD-diagnosed Medicare Advantage enrollees aged ≥ 65 years were identified from the Optum Research Database (ORD). Eligible patients had ≥ 1 pharmacy claim for UMEC/VI or FP/SAL in the 6-month period before sample identification, with no evidence of triple therapy (ICS/LAMA/LABA), asthma, or lung cancer. Symptom burden was assessed via cross-sectional surveys using the COPD Assessment Test (CAT) and modified Medical Research Council (mMRC) dyspnea scale. Patients were classified into GOLD categories using patient-reported symptoms and claims-based exacerbation history. Treatment groups were balanced on potential confounders using inverse probability of treatment weighting (IPTW). CAT and mMRC scores were analyzed with generalized linear regression models using IPTW propensity scores.
Results: The final analytic sample included 789 respondents (UMEC/VI: N=392; FP/SAL: N=397). Approximately 66% patients were classified as GOLD B when assessing symptoms with CAT and mMRC together, or CAT alone; more patients were classified as GOLD A (∼ 40%) than GOLD B (∼ 36%) using mMRC alone. Proportions of patients in each GOLD group were similar between treatment cohorts. Post-IPTW multivariable modeling showed similar symptom burden between treatment groups.
Conclusion: After controlling for baseline characteristics, symptom burden was similar between patients receiving UMEC/VI or FP/SAL. GOLD classification using mMRC produced more conservative results compared with CAT, potentially underestimating patient symptoms. Many patients receiving FP/SAL were classified as GOLD A or B, despite GOLD recommending non-ICS-containing therapy in these patients. These findings support the need for routine assessment of symptoms in patients with COPD.
Keywords: COPD, fluticasone propionate/salmeterol, umeclidinium/vilanterol, CAT, mMRC, GOLD group
Plain Language Summary
Long-term treatment for patients with chronic obstructive pulmonary disease (COPD) includes steroids, to reduce inflammation in the airways, and non-steroid bronchodilators, to make breathing easier. We compared COPD symptoms between patients receiving a steroid-containing medication called fluticasone propionate/salmeterol (FP/SAL) and a non-steroid dual bronchodilator medication called umeclidinium/vilanterol (UMEC/VI).
We used a large US healthcare claims database to identify patients with COPD who started treatment with UMEC/VI or FP/SAL in the past 6 months, and asked them to complete a survey describing their treatment and symptoms. The survey included two different questionnaires; the modified Medical Research Council (mMRC) dyspnea scale that measures patients’ breathlessness, and the more comprehensive COPD Assessment Test (CAT) that assesses a range of symptoms. After controlling for other differences in health and background, patients had similar levels of self-reported symptoms regardless of which medication they were prescribed. Based on their symptoms and recent history of disease flare-ups, we separated patients into categories that physicians use to decide which treatment patients should receive. We found that mMRC may underestimate patients’ symptoms compared with CAT, and that over three-quarters of patients receiving FP/SAL could have been treated with non-steroid bronchodilator medications instead.
This study shows that patients with COPD who are prescribed UMEC/VI and FP/SAL appear to have similar levels of symptoms; many patients on either medication had symptoms or flare-ups that were not controlled by their current treatment. Physicians should regularly assess patients’ symptoms, preferably using a comprehensive questionnaire like CAT, to ensure that they are treated appropriately.
Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report includes a simple assessment tool for health-care providers (HCP) to determine the appropriate therapy for patients with chronic obstructive pulmonary disease (COPD). The assessment tool categorizes patients into four mutually exclusive groups (A, B, C, or D) based on their symptom burden and risk of future exacerbations.1 A comprehensive measure such as the COPD Assessment Test (CAT) is recommended for the assessment of symptoms, rather than solely assessing breathlessness using the modified Medical Research Council (mMRC) dyspnea scale. However, the mMRC dyspnea scale is still included in the GOLD assessment tool, as it is recognized that this measure is widely used to assess symptom burden.1 Patients with a CAT score ≥10 and/or mMRC grade ≥2 are considered to have a considerable symptom burden (GOLD B and D), while patients with CAT <10 and mMRC ≤1 are considered to have less severe symptoms (GOLD A and C).1
Long-acting muscarinic antagonist/long-acting β2-agonist (LAMA/LABA) combination therapy is recommended as initial maintenance therapy in symptomatic patients at low risk for future exacerbations (GOLD B) who have severe breathlessness, or in symptomatic patients with high exacerbation risk (GOLD D) who have particularly severe symptoms (CAT score ≥20).1 Inhaled corticosteroid (ICS)/LABA combinations are recommended as initial maintenance therapy to reduce exacerbations in patients at high risk of exacerbation who have elevated blood eosinophil counts and/or a history of asthma.1 In support of these recommendations, head-to-head clinical trials have demonstrated that LAMA/LABA combination therapy is associated with significantly greater improvements in lung function and symptoms than ICS/LABA therapy,2–7 without the elevated risk of pneumonia associated with ICS-containing maintenance treatments.2–5,8–11 However, ICS/LABA treatment is frequently prescribed as initial maintenance therapy in US patients across all GOLD categories.12 This suggests that the GOLD recommendations are not being consistently implemented in clinical practice, which may impact treatment outcomes and medication adherence.13,14
Better odds of adherence, as measured by proportion of days covered, and a lower risk of escalation to multiple-inhaler triple therapy have been demonstrated among patients initiating treatment with the once-daily LAMA/LABA umeclidinium/vilanterol (UMEC/VI) compared with the twice-daily ICS/LABA fluticasone propionate/salmeterol (FP/SAL).15 Further studies are required to determine whether these differences are associated with a disparity in patient-reported burden of illness between patients initiating therapy with FP/SAL and those initiating UMEC/VI. The present study evaluated symptom severity and GOLD classification among patients who recently initiated UMEC/VI or FP/SAL to better understand their symptom burden.
Methods
Study Design
This was a claims-linked cross-sectional survey of patients diagnosed with and treated for COPD. Patients were identified using retrospective claims data from the Optum Research Database (ORD) for the 12-month baseline period prior to the completion of the study survey instruments. Sample identification used enrollment records and medical and pharmacy claims between June 1, 2017 and September 30, 2018 (Figure 1); four waves of sample identification and data collection were conducted to obtain the target sample size.
Survey data collection was conducted using the Dillman Tailored Design Method.16 Eligible patients were contacted by mail and invited to participate in the survey, with a total data collection period of 8 weeks per wave. Respondents were mailed a $25 post-paid incentive for their study participation. Survey data were merged with administrative claims data for the 12-month baseline period following the data collection lag period (approximately 6 months for medical claims data and 6 weeks for pharmacy claims data).
The study was designed and conducted in accordance with the ethical standards of the institutional and/or national research committee and the principles of the 1964 Declaration of Helsinki and its later amendments. The study was approved by the New England Institutional Review Board (NEIRB; IRB# 120180129) on May 30, 2018. All patients who were contacted by mail were provided with a statement of informed consent with the survey packet, which included consent for the patients’ claims data to be accessed.
Patients
The identified sample included Medicare Advantage enrollees ≥65 years of age with continuous enrollment throughout the 12-month baseline period. Eligible patients had recently initiated UMEC/VI or FP/SAL, with ≥1 pharmacy claim for fixed-dose combination therapy with either UMEC/VI or FP/SAL in the 6 months prior to sample identification (and no claims for these treatments in the first 6 months of the baseline period). Patients were also required to have a diagnosis of COPD, as indicated by ≥2 International Classification of Diseases 10th Edition Clinical Modification (ICD-10-CM) COPD diagnosis codes ≥30 days apart, within the 12-month baseline period. Patients with evidence of COPD triple therapy (ICS/LAMA/LABA, including combined monotherapy formulations and fixed-dose combinations), ICD-10-CM codes for asthma during the baseline period, or missing demographic data (age, sex, or geographic region) were excluded.
After the survey was completed and the claims lag had elapsed, claims data for the baseline period were re-extracted and patient eligibility for inclusion in the final analytic sample was assessed. Exclusion criteria based on claims data were medical claims for asthma in the baseline period, claims for diagnosis or treatment of lung cancer in the baseline period, or pharmacy claims for triple therapy. From the survey, patients were also required to have a self-reported HCP diagnosis of COPD, self-reported treatment with UMEC/VI or FP/SAL that corresponded to claims-identified treatment at the time of sample identification, and evaluable responses to the CAT and mMRC.
Outcomes
For the primary objective, patient-reported symptom burden was assessed using CAT and mMRC scores (collected via the cross-sectional survey). CAT is an 8-item validated questionnaire that assesses the impact of COPD symptoms on patients’ well-being and daily life.17 Individual CAT items measure salient symptoms of COPD, including cough, chest tightness, breathlessness, and activity limitation. Each item is measured on a scale of 0–5, with higher scores indicating worse symptom burden, and these scores are summed to produce a total score ranging from 0 (least symptom burden) to 40 (greatest symptom burden). The mMRC, a single-item measure assessing patients’ current level of dyspnea, is comprised of five statements of respiratory disability, graded from none (grade 0) to almost complete incapacity (grade 4).18 Both CAT and mMRC have been shown to correspond well with the St. George’s Respiratory Questionnaire, a comprehensive tool for assessing COPD-related health status impairments.18,19
For the secondary objective, patient-reported symptoms and claims-based evidence of exacerbations during the 12-month baseline period were used to categorize patients according to the GOLD assessment tool.1 Patients were classified into four mutually exclusive categories (GOLD A, B, C, or D) based on the assessment of symptoms using either CAT score or mMRC score, or both measures in combination, to determine the effect of each measure.
The following clinical and treatment characteristics were extracted from claims data for the baseline period: baseline Quan-Charlson comorbidity score, baseline Agency for Healthcare Research and Quality (AHRQ) comorbidities, baseline COPD-related comorbidities, baseline medications, and baseline medication dispensings. The following clinical and treatment characteristics were collected via the cross-sectional survey: diagnosis, general health, COPD duration, COPD treatment, height, weight, body mass index, and smoking behavior (smoking status, cigarettes smoked per day, pack-years, age started and stopped smoking, total years smoked). Age, sex, and geographic region were extracted from claims data, while other demographic and sociodemographic characteristics were collected via the survey.
Statistical Analysis
The final analysis population included all respondents with complete, evaluable survey data who met all study inclusion and exclusion criteria. Statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA; version 9.4) on a Unix platform.
Inverse probability of treatment weighting (IPTW) was applied to reduce confounding of observed clinical, demographic, and sociodemographic characteristics. Weights were based on propensity scores, defined as each individual’s probability of receiving a specific treatment for a given pattern of confounders. A logistic regression model was implemented, with the treatment cohort as the dependent or response variable conditioned on a set of predetermined covariates. A combination of patient-reported and claims-based variables were included in the IPTW model. Each patient was assigned a propensity score weight, and the expected value of an outcome of interest in the corresponding multivariable model was weighted by a function of the inverse propensity score and the sample size of the cohort to adjust for potential treatment selection bias. The estimated probability from this model was used to weight the data from the FP/SAL cohort such that it better resembled the UMEC/VI cohort. The quality of the propensity score determination was descriptively assessed. Where outliers were identified, the study team determined whether these patients should be retained in the model based on whether their clinical and analytic characteristics would unduly impact the subsequent multivariable analysis. Standardized differences determined from the weighted and unweighted descriptive analysis of the covariates included in the IPTW analysis were compared.
For the primary objective, CAT total score and mMRC score were analyzed as continuous variables using generalized linear regression models. If CAT scores for 1–2 items were missing, the total score was imputed as the within-subject average of the non-missing item scores; if >2 items were missing, the patient was excluded from the final analytic sample. Surveys with missing mMRC scores were considered incomplete and not included in the final analytic sample. For each model, regression diagnostics were performed to assess goodness of fit and violations of the model assumptions such as multicollinearity or heteroskedasticity. The fitted and observed data were also examined to uncover outliers, their effect on the analysis, and possible misspecification of the initial equation. Predictors included in the multivariable models were treatment cohort, sex, age category, race, ethnicity, urban/suburban/rural residence, marital status, education level, body mass index, smoking history in pack-years, COPD duration, baseline Quan–Charlson comorbidity score, count of all-cause ambulatory visits, count of all-cause emergency room visits, count of COPD-related fixed-dose combination therapy fills, and count of COPD exacerbations. Post-IPTW matched covariates were used.
For the secondary objective, the proportion of patients meeting the criteria for each GOLD category based on pre-IPTW symptom burden (CAT, mMRC, or both) and exacerbation history is presented for the complete population and by treatment cohort.
Results
Study Population
Of 3335 patients who met claims-based sample identification criteria and were invited to participate in the study, 891 respondents met all eligibility criteria and were included in the survey sample (UMEC/VI: N=441; FP/SAL: N=450; Figure 2). The overall response rate based on the American Association for Public Opinion Research (AAPOR) Response Rate 4 formula was 39.2%, calculated as the number of returned surveys divided by the total number eligible (comprising returned surveys, eligible non-surveys, and estimated eligible).20
The final analytic sample included 789 respondents (UMEC/VI: N=392; FP/SAL: N=397). Patient demographics and clinical characteristics are described in Table 1. Before IPTW, certain variables included in the model were not balanced between treatment groups (indicated by absolute standardized differences >10%), such as: race, smoking pack-years, time since COPD diagnosis, COPD-related emergency room visits, and COPD-related ambulatory visits (Table 1). Following IPTW, all covariates were balanced between treatment cohorts (absolute standardized differences ≤10%) and there were no significant between-treatment differences in post-weighting covariates.
 |
Table 1 Patient Demographics by Treatment Cohort |
All results extracted from claims data, including all healthcare utilization variables, represent the baseline period. During this period, 44.1% of patients had ≥1 moderate/severe exacerbation (UMEC/VI: 41.8%; FP/SAL: 46.4%). All patients had ≥1 all-cause ambulatory visit, almost all had ≥1 office visit (98.4%), and nearly half had an emergency room visit (47.5%). Approximately one-third of patients had ≥1 all-cause hospitalization (overall: 30.2%; UMEC/VI: 28.8%; FP/SAL: 31.5%), with a mean (standard deviation [SD]) stay of 12 (15) days and a median stay of 6 days among those with at least one admission. More patients receiving FP/SAL had a COPD-related emergency room visit than those receiving UMEC/VI (16.4% vs 10.7%; P=0.022), while more patients in the UMEC/VI cohort had an outpatient visit (39.0% vs 29.0%; P=0.003). On average, patients receiving FP/SAL had claims for 14.5 unique medications, which was significantly higher than the corresponding average of 13.4 unique medications for patients receiving UMEC/VI (P=0.013).
Patient-Reported Symptom Burden
Symptom Burden
Overall, mean (SD) CAT score was 18.9 (7.8); 692/789 (87.7%) patients had a CAT score ≥10, and 365/789 (46.3%) had a score ≥20. Before IPTW, mean (SD) patient-reported CAT score was significantly higher in the FP/SAL cohort compared with the UMEC/VI cohort (19.7 [8.3] vs 18.1 [7.2]; P=0.006; Figure 3). Significantly more patients receiving FP/SAL had CAT scores ≥20 than those receiving UMEC/VI (50.6% vs 41.8%; P=0.015). Multivariable modeling using the propensity scores from the IPTW analysis showed comparable CAT scores in the UMEC/VI and FP/SAL cohorts (mean difference [95% CI]: −0.3 [−1.4, 0.9]; P=0.662).
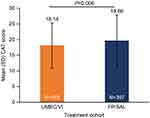 |
Figure 3 Mean CAT scores among patients receiving UMEC/VI or FP/SAL before IPTW. |
Dyspnea
Overall, mean (SD) mMRC grade was 1.7 (1.0). High levels of dyspnea, indicated by mMRC grades of 2–4, were reported by 400/789 (50.7%) patients. Mean (SD) mMRC grades before IPTW were not significantly different between the UMEC/VI and FP/SAL cohorts (1.6 [1.0] vs 1.7 [1.0]; P=0.602). Multivariable modeling based on the IPTW propensity scores revealed comparable mMRC grades in the UMEC/VI and FP/SAL cohorts (mean difference [95% CI]: 0.1 [−0.1, 0.2]; P=0.385).
GOLD Categorization
The mMRC score, CAT score, and exacerbation history were used to categorize patients as described in the GOLD strategy report.1 The majority of patients (66–67%) in both treatment groups were classified as GOLD B (high symptom burden and low exacerbation risk), and approximately 10% were classified as GOLD A (low symptom burden and low exacerbation risk) (Figure 4). Approximately one-fifth of patients (22–23%) had severe symptoms and a high risk of exacerbations, and were classified as GOLD D. A similar pattern was observed when only CAT score and exacerbation history were used, with approximately two-thirds of patients classified as GOLD B, approximately 10% categorized as GOLD A, and 22% classified as GOLD D (Figure 5). In contrast, when only mMRC score and exacerbation history were used, approximately 40% of patients were categorized as GOLD A, with fewer patients meeting the criteria for GOLD B (36%) or GOLD D (~15%) (Figure 5). Whether symptoms were assessed using mMRC, CAT, or both measures, the proportions of patients meeting the criteria for each GOLD category were similar between the UMEC/VI and FP/SAL cohorts (Figure 4; Supplementary Table 1).
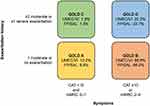 |
Figure 4 GOLD classification according to symptom burden (assessed by CAT and mMRC) and exacerbation history. |
 |
Figure 5 Comparison of GOLD classification according to different patient-reported measures of symptom burden (CAT or mMRC). |
Discussion
In this study, patients receiving single-inhaler combination therapy with either UMEC/VI or FP/SAL reported a substantial burden of illness. Before IPTW, symptom burden as determined by CAT score (but not mMRC score) was significantly less severe in patients receiving UMEC/VI than in patients receiving FP/SAL. However, after controlling for potential confounders using IPTW, symptom burden as determined by both CAT and mMRC scores was similar between patients receiving UMEC/VI and those receiving FP/SAL.
When categorizing patients into GOLD groups, assessment of symptoms using mMRC resulted in more patients being classified as GOLD A (40.3%) than assessment of symptoms using CAT (10.8%). In addition, fewer were classified as GOLD B or D (high symptom burden) with mMRC than with CAT. This suggests that symptom assessment using mMRC produces more conservative results than CAT, particularly with regard to classifying patients as GOLD A (low symptom burden and low exacerbation risk). This finding is consistent with a previous claims-linked survey study, in which 54.5% versus 14.5% of patients with COPD were classified as having low symptom severity (mMRC 0–1 vs CAT <10) whereas 45.5% versus 85.5% were classified as having high symptom severity (mMRC 2–4 vs CAT ≥10).21 In the same study, 40.9% of patients reported both a CAT score ≥10, indicating high symptom severity, and an mMRC grade of 0–1, indicating low symptom severity, further demonstrating the misalignment between the two measures. Another cross-sectional observational study also found that mMRC classified more patients as having low symptom severity (GOLD A and C; 57.2%) than CAT (17.2%). These findings reflect the more comprehensive assessment provided by measures such as CAT, which characterizes the impact of a range of patients’ symptoms beyond breathlessness. CAT may identify patients who have less severe dyspnea (mMRC grade of 0–1) but nevertheless have other COPD symptoms that impact their overall health.18 Our findings, therefore, strengthen the available evidence and support the GOLD recommendation that a comprehensive assessment of symptoms (such as CAT) should be used when identifying initial treatment options, rather than measuring dyspnea alone.1 This comprehensive approach is vital to accurately assess patients’ symptoms and prevent undertreatment,21,22 which can lead to poorer outcomes for patients.14
Notably, three-quarters of patients receiving FP/SAL in this study did not have evidence of one or more recent exacerbations, and consequently were categorized as GOLD A or B. Under the current GOLD strategy, ICS/LABA should only be considered as initial therapy in highly symptomatic patients at high risk of future exacerbations (GOLD D) or as follow-up treatment for patients with persistent exacerbations on bronchodilator monotherapy.1 Furthermore, ICS/LABA is generally only preferred over LAMA/LABA in patients who have elevated blood eosinophil counts and/or a history of asthma,1 due to the increased risk of pneumonia associated with ICS treatment.2–5,8–11 Our finding supports previous evidence that ICS-containing medications are frequently prescribed for patients across all GOLD groups,12,23 despite the recommendations described above. In addition, a considerable proportion of patients included in the present study were classified as GOLD B–D, suggesting that their COPD may not be well controlled with their current treatment. In both treatment groups, approximately 20% of patients met the criteria for GOLD D, the most severe group in the GOLD classification covering a considerable symptom burden and high risk of future exacerbations.1 Patients in this group may therefore be experiencing persistent symptoms and/or exacerbations while on ICS/LABA or LAMA/LABA therapy, and could benefit from escalation to triple therapy (ICS/LAMA/LABA).1 These findings suggest that current prescribing patterns are not adherent to the recommendations outlined in the GOLD report, and a considerable proportion of patients may have poorly-controlled disease with their current therapy. Inappropriate prescribing may lead to inadequate control of symptoms and an increased need for treatments such as oral corticosteroids and antibiotics,14 highlighting the importance of adherence to treatment recommendations and intensifying treatment where necessary to improve outcomes for patients.
There are general limitations associated with the use of claims data, which are collected for the purposes of payment rather than research. A claim for a filled prescription does not necessarily show that the medication was taken as prescribed, and diagnosis codes on medical claims do not conclusively demonstrate the presence of the disease. Additionally, over-the-counter medications and those provided as samples by a physician are not captured in this data source. To help mitigate these limitations, we included patients with multiple COPD diagnosis codes, who self-reported a COPD diagnosis by a HCP, and had corresponding claims-based and patient-reported current COPD treatment. Furthermore, we excluded patients who reported current prescriptions for both UMEC/VI and FP/SAL. A limitation of this study is that it was not possible to identify patients for whom ICS/LABA treatment was indicated due to blood eosinophil counts ≥300 cells/μL,1 since clinical measures such as blood eosinophils are not included in claims data. Limitations of the survey may include sampling error, coverage error, and measurement error. The study population comprised Medicare Advantage enrollees aged ≥65 years, and so the findings may not be generalizable to uninsured or younger patient populations.
Conclusions
After controlling for confounding baseline characteristics including exacerbations, symptom burden in patients with COPD receiving UMEC/VI or FP/SAL was similar. GOLD classification varied depending on the measure used to assess symptoms, with the mMRC appearing to produce more conservative results and potentially underestimating symptom burden compared with CAT. When both measures were used, a considerable proportion of patients receiving either treatment were classified as GOLD B, despite GOLD recommendations that patients in these categories should be initiated on a non-ICS-containing therapy. These findings support the need for routine assessment of symptoms in patients with COPD.
Abbreviations
AAPOR, American Association for Public Opinion Research; AHRQ, Agency for Healthcare Research and Quality; ASD, absolute standardized difference; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FP/SAL, fluticasone propionate/salmeterol; GOLD, Global Initiative for Obstructive Lung Disease; HCP, health-care provider; ICD-10, International Classification of Diseases, 10th Edition; ICS, inhaled corticosteroids; IPTW, inverse probability of treatment weighting; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MA, Medicare Advantage; mMRC, modified Medical Research Council; SD, standard deviation; UMEC/VI, umeclidinium/vilanterol.
Data Sharing Statement
Information on GlaxoSmithKline’s (GSK) data sharing commitments and requesting access to anonymized individual participant data and associated documents from GSK-sponsored studies can be found at www.clinicalstudydatarequest.com. The data reported in this publication are contained in a database owned by Optum and contains proprietary elements. Therefore, it cannot be broadly disclosed or made publicly available at this time. The disclosure of this data to third-party clients assumes certain data security and privacy protocols are in place and that the third-party client has executed Optum’s standard license agreement which includes restrictive covenants governing the use of the data.
Ethics Approval and Informed Consent
The study was designed and conducted in accordance with the ethical standards of the institutional and/or national research committee and principles of the 1964 Declaration of Helsinki and its later amendments. Prior to data collection, the study (including access to the Optum Research Database) was approved by the New England Institutional Review Board (NEIRB, #120180129) on May 30, 2018. Patients were provided with a statement of informed consent (which included consent for the patients’ claims data to be accessed) in the study packet, and consented to participation in the study by returning the completed survey.
Consent for Publication
Not applicable.
Acknowledgments
Editorial support (in the form of writing assistance during development of the initial draft, assembling tables and figures, collating authors comments, grammatical editing, and referencing) was provided by Mark Condon, DPhil, of Fishawack Indicia Ltd, UK, and funded by GSK.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GSK (study number 208782). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report.
Disclosure
CM was an employee of GSK at the time of the study. BH and RR are employees of GSK and hold stocks/shares in GSK. JW and BE are employees of Optum, which was contracted by GSK to conduct the study. AGH was an employee of Optum at the time of the study and holds stocks/shares in Optum’s parent company, UnitedHealth Group (UNH). CE was an employee of Optum at the time of the study. The authors report no other potential conflicts of interest for this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
2. Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi:10.2147/COPD.S130482
3. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066.
4. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
5. Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a Phase 3 COPD study. Eur Respir J. 2016;48(4):1030–1039. doi:10.1183/13993003.00216-2016
6. Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109(7):870–881. doi:10.1016/j.rmed.2015.04.018
7. Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol–fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2016;48(4):51–60. doi:10.1016/S2213-2600(12)70052-8
8. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD010115.
9. Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029–1036. doi:10.1136/thoraxjnl-2012-202872
10. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
11. Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
12. Wallace AE, Kaila S, Bayer V, et al. Health care resource utilization and exacerbation rates in patients with COPD stratified by disease severity in a commercially insured population. J Manag Care Spec Pharm. 2019;25(2):205–217.
13. Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J COPD. 2008;3(3):371–384. doi:10.2147/COPD.S3036
14. Mannino DM, Yu T-C, Zhou H, Higuchi K. Effects of GOLD-adherent prescribing on COPD symptom burden, exacerbations, and health care utilization in a real-world setting. COPD. 2015;2(3):223–235. doi:10.15326/jcopdf.2.3.2014.0151
15. Moretz C, Sharpsten L, Bengtson LG, et al. Real-world effectiveness of umeclidinium/vilanterol versus fluticasone propionate/salmeterol as initial maintenance therapy for chronic obstructive pulmonary disease (COPD): a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:1721–1737. doi:10.2147/COPD.S204649
16. Dillman DA, Smyth JD, Christian LM. Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method.
17. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
18. Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J. 2013;42(3):647–654. doi:10.1183/09031936.00125612
19. Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. doi:10.1183/09031936.00177210
20. The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. Deerfield, IL: AAPOR; 2016. Available from: http://www.aapor.org/AAPOR_Main/media/publications/Standard-Definitions20169theditionfinal.pdf.
21. Ray R, Hahn B, Stanford RH, White J, Essoi B, Hunter AG. Classification of patients with COPD on LAMA monotherapy using the GOLD criteria: analysis of a claims-linked patient survey study. Pulmonary Ther. 2019;5(2):191–200. doi:10.1007/s41030-019-00099-0
22. Price DB, Baker CL, Zou KH, Higgins VS, Bailey JT, Pike JS. Real-world characterization and differentiation of the Global Initiative for Chronic Obstructive Lung Disease strategy classification. Int J Chron Obstruct Pulmon Dis. 2014;9:551–561.
23. Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi:10.2147/COPD.S62750
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


