Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Sydenham Chorea in Sudan; Presentation Panorama
Authors A Ibrahim EA, Mohamed RH, Abbasher Hussien Mohamed Ahmed K , AbdAlla Mohamed MT , Fadelallah Eljack MM
Received 15 April 2023
Accepted for publication 25 July 2023
Published 26 July 2023 Volume 2023:19 Pages 1657—1663
DOI https://doi.org/10.2147/NDT.S417326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Etedal Ahmed A Ibrahim,1,2 Rogia Hussein Mohamed,3 Khabab Abbasher Hussien Mohamed Ahmed,4 Malaz Tarig AbdAlla Mohamed,4 Mohammed Mahmmoud Fadelallah Eljack5
1Department of Medicine, Al-Neelain University, Khartoum, Sudan; 2Department of Neurology, The National Centre for Neurological Sciences, Khartoum, Sudan; 3Department of Medicine, Latifa Hospital for Women and Children, Dubai, United Arab Emirates; 4Department of Medicine, University of Khartoum, Khartoum, Sudan; 5Department of Community Medicine, University of Bakht Alruda, Ad Duwaym, White Nile State, Sudan
Correspondence: Mohammed Mahmmoud Fadelallah Eljack, Department of Community Medicine, University of Bakht Alruda, Ad Duwaym, White Nile State, Sudan, Tel +249964656914, Email [email protected]
Introduction: Sydenham’s chorea (SC) is the most common form of acquired chorea in childhood, it is considered a neurological complication of streptococcal pharyngitis. In this study, we aimed to determine the clinical pattern, association of Sydenham’s chorea with other manifestations of acute rheumatic fever, and the laboratory findings of Sydenham’s chorea among Sudanese patients.
Methods: A prospective cross-sectional study involving fifty patients of various ages diagnosed with Sydenham’s chorea and followed up at The National Center for Neurological Sciences from January 2017 to November 2019. Data were obtained after patients’ consent through personal interviews or personal review of patients’ records via a structured questionnaire composed of demographic data, symptoms, co-morbid illness, risk factors, physical examination, and related investigations.
Results: About 50 patient was enrolled in this study with a median age of 13.7 years. Females were (n=35) (70%) and (30%) (n=15) s were males. Generalized chorea was seen in 33 (66%) and hemichorea in 17 (34%) patients. Weakness (38%) and hypotonia (46%) were common, such as behavior change (44%), dysarthria (70%), gait change (18%), and deterioration of handwriting (12%). Arthritis occurred in (36%), carditis in 30 (60%), both arthritis and carditis in 18 (36%), and pure chorea in 14 (28%). Erythema marginatum and subcutaneous nodules were not observed in our patients. Only 13 patients (26%) gave a history of pharyngitis.
Conclusion: Sydenham chorea is more common in young female Sudanese, with a familial predominance and a tendency towards mitral valve disease. All pediatric Patients with chorea should be screened for Sydenham’s chorea.
Keywords: Sydenham, chorea, acute rheumatic fever, presentations, psychiatric
Introduction
Chorea “derived from the Latin word choreus means dance” and refers to involuntary non -stereotactic, arrhythmic, and purposeless movements.1,2 It was first mentioned in the Middle Ages and was thought to be psychological, while others reported it to be post-infectious, which is now known as Sydenham’s chorea.1 Sydenham’s chorea, also known as St. Vitus dance, St. Johannis’ chorea, chorea minor, and rheumatic chorea, is one of the most common forms of acquired chorea in childhood.1
It occurs in 10% to 40% of patients with acute rheumatic fever (ARF), with females dominating seven times more than males.3 It began 1–6 months after streptococcal infections as generalized in the face and limbs, while 20% to 30% develop hemichorea.3 It is sometimes associated with dysarthria, gait disturbance, hypometric saccades, hypotonia, weakness, loss of fine motor controls, motor impersistence, and hemiballismus.3 The behavioral changes range from outbursts of abusive behavior, such as crying and restlessness, to transient psychotic episodes in severe cases; in some cases, these behavioral changes may even precede chorea by weeks or months.3
The exact pathogenesis is unknown, but an antibody-mediated immune response attacking basal ganglia tissues in response to group A beta-hemolytic streptococci antigen remains the most likely theory, as do other major features of ARF such as arthritis, carditis, subcutaneous nodules, and erythema marginatum.2
In developed countries, incidences of Sydenham chorea and ARF have decreased in recent years due to improvements in socioeconomic and sanitary conditions, as well as the availability of penicillins, whereas in developing countries remains a major health issue with a lack of epidemiological data regarding the situation.4
Even though serological tests for streptococci infection are positive in approximately 70% to 80% of cases, the diagnosis of Sydenham chorea is primarily clinical, with symptomatic treatment offered either alone or in conjunction with other forms of treatment such as high-dose corticosteroids, valproate, neuroleptics, immunotherapy, and plasmapheresis in severe cases.5
Clinical knowledge of Sydenham chorea has recently been expanded by studies describing associated motor, and ocular abnormalities, cognitive function, and neuropsychiatric symptoms; these studies also contradicted previously held beliefs that Sydenham chorea is a benign self-limiting condition.6 The application of this clinical knowledge is critical for early diagnosis to avoid the disease’s long duration, relapses, a decline in school performance, accompanying heart diseases, and the use of prophylaxis -which can last for years- as much as possible.6,7
Despite this, there is a significant lack of knowledge regarding the clinical patterns of Sydenham chorea in Sudanese patients, which may affect the long-term outcome of such a disease. Our goal is to determine the clinical pattern of Sydenham’s chorea, its association with other manifestations of Acute rheumatic fever, and the laboratory findings of Sydenham’s chorea in Sudanese patients.
Methods
Study Design
A Prospective cross-sectional study was conducted between January 2017 and November 2019 including fifty patients with different age groups, representing the whole patients who were diagnosed as having Sydenham’s chorea and followed up at the national center for neurological sciences, Khartoum, Sudan, which is a tertiary hospital for the referral of all neurological cases in Sudan. Those with an undetermined type of movement disorder and those who refused to participate were excluded.
Rheumatic fever has been Established aided by judicious application of the modified Jones criteria; including three components:
major manifestations including carditis, polyarthritis, chorea, erythema marginatum, and subcutaneous nodules minor manifestations including arthralgia, fever, Elevated acute phase reactants (erythrocyte sedimentation rate, C-reactive protein), and Prolonged PR interval (first-degree heart block).
Evidence of a preceding group A streptococcal infections including Elevated or rising streptococcal antibody titer, most often Antistreptolysin O (ASO) titer.
Data Collection
A total coverage, prospective collection of data from the patients with Sydenham’s chorea or their records through a designed questionnaire including demographic data, symptoms, risk factors, physical examination and related investigations.
Echocardiography and electrocardiography were performed in all patients, and brain image was done in 11 patients.
Analysis
All collected data were entered into the computer using the statistical package program for social science (SPSS v24) to analyze the data via simple descriptive statistics. (Analyzer is specialized personnel in SPSS).
Results
Sydenham’s chorea can present in different age groups, the age range of patients in this study was between 7 −17 years (Table 1). The patients’ median age was 13.7 years. In this study, 50 patients were diagnosed with Sydenham’s chorea, among them (70%) (n=35) were females, and (30%) (n=15) s were males (Table 1). There was a positive family history of acute rheumatic fever in 17 (34%) of the studied group.
 |
Table 1 The Age Group & Sex Distribution in Patients with Sydenham’s Chorea |
In all patients, the movements were suppressed during sleep (100%) and stress was found to be the provocation factor of chorea in 12 patients (24%) (Figure 1).
 |
Figure 1 The provocation factors for Sydenham’s chorea. |
Sydenham’s chorea had an acute onset (less than six weeks) in 34 patients (68%), while 16 patients (32%) had gradual onset (more than six weeks). Generalized chorea was seen in 33 patients (66%) and hemichorea in 17 patients (34%).
It was seen that 41 patients (82%) had their chorea initially started with limb involvement only, while nine patients (18%) had their chorea first appear with oro-facial movement followed by limb involvement. Weakness occurred in 19 patients (38%), hypotonia in 23 patients (46%), dysarthria in 35 patients (70%), gait change in nine patients (18%), and deterioration of handwriting in six patients (12%) (Figure 2). Darting tongue and milking signs were found in 21 patients (42%), emotional liability was found in 26 patients (52%) followed by restlessness in 22 patients (44%) and depression occurred in patients two patients (4%).
 |
Figure 2 The abnormalities in neurologic examination in patients of Sydenham’s chorea. |
Regarding the association of Sydenham’s chorea and other manifestations of acute rheumatic fever, carditis occurred in 30 patients (60%), of them mitral regurgitation was found in 20 patients (83%) and a combination of mitral and aortic regurgitation occurred in ten patients (27%) (Figure 3). Prolonged PR interval (or first-degree block) was noted in nine patients (18%). About 18 patients had arthritis only (36%), arthritis and carditis in 18 patients (36%), and pure chorea was observed in 14 patients (28%). Erythema marginatum and subcutaneous nodules were not observed in our patients (Figure 3).
 |
Figure 3 The association of Sydenham’s chorea and other manifestation of acute rheumatic fever. |
Regarding investigations, Elevated ASO levels were detected in 44 patients (88%). Only 13 patients (26%) gave a history of pharyngitis. A highly elevated Erythrocyte sedimentation rate (ESR) was detected (>100 mm/h) in 18 patients (36%) and moderately elevated ESR (30–70 mm/h) in 30 patients (60%) patients. Brain imaging, which was performed in only 11 patients, was evaluated as normal in all.
As for the duration of illness, the chorea lasted from two to four weeks in (52%) (n=26) patients, five to ten weeks in 19 patients (38%), and 11 to 15 weeks in five patients (10%) (Table 2). Ten of the 50 patients (20%) had a recurrent attack, in all cases, it was a second time attack. The interval between attacks varied from two to three years. Carditis was detected in all patients with recurrences.
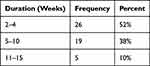 |
Table 2 The Duration of Symptoms in Patients with Sydenham’s Chorea |
Discussion
This study is a prospective cross-sectional study that aimed to determine the clinical pattern of Sydenham’s chorea among Sudanese patients, the association of Sydenham’s chorea with other manifestations of acute rheumatic fever, and the laboratory findings of Sydenham’s chorea among Sudanese patients.
The age range between 7 and 17 years, the mean age was 13.7 years while the age of diagnoses in literature ranged from 62 to 17 years7 with a general female preponderance in most series.1,6,8,9
There was a positive family history of acute rheumatic fever in 17 (34%) of the studied group in this study. Jordan et al found that it is common for (5%) to (18%) of patients with rheumatic fever to have a positive family history of rheumatic fever.10,11 This higher percentage of positive family history might be explained by the higher prevalence of rheumatic fever in Sudan than in other countries.
As is expected with chorea movement,5,12 all patients’ movements resolved during sleep (100%). Stress was a provoking factor of chorea in (24%) (n=12) of patients. Chorea’s random and continuous contraction flow usually rapidly spreads to become generalized (61% to 80%), but in 20% and up to 26.3% in some literature, it remains as hemichorea.6,8,10 In this case of patients, generalized chorea was seen in 33 patients (66%) and hemichorea in 17 patients (34%).
While Sydenham’s chorea almost solely presents as an acute onset disease, a sub-acute onset of presentation is also possible.1 Of the 50 patients included in this study, (68%) (n=34) cases presented with acute onset. While (32%) (n=16) of patients presented with a gradual and progressive presentation of the disease.
Regarding the onset and progression of symptoms of chorea, it was observed that 41 patients (82%) had their chorea initially started with limb involvement, (18%) (n=9) of which started with the oro-facial movement and then were followed by limb involvement.
Weakness occurred in 19 patients (38%), hypotonia in 23 patients (46%), dysarthria in 35 patients (70%), gait change in nine patients (18%), and deterioration of handwriting in six patients (12%). Darting tongue and milking signs were found in 21 patients (42%), emotional liability was found in 26 patients (52%) followed by restlessness in 22 patients (44%) and depression occurred in patients two patients (4%). In a paper by Tumas et al, behavioral abnormalities were reported in (40%) of patients, dysarthria in (38%), and gait abnormalities in (34%). With the most prevalent functional abnormalities being, deteriorated handwriting (17%) and feeding (16%). No tics or obsessive-compulsive symptoms.6
The association of Sydenham’s chorea and other manifestations of acute rheumatic fever:
The association of Sydenham’s chorea with other manifestations of acute rheumatic fever such as cardiac lesions is the only cause of concerning morbidity in Sydenham’s chorea.1 Cardoso1 mentions that 80% of cases of Sydenham’s chorea are associated with carditis. In our study, carditis was found in (60%) of patients (n=30), of the 30 patients, mitral regurgitation was found in 20 patients (83%) and a combination of mitral and aortic regurgitation occurred in ten patients (27%). Prolonged PR (or first-degree block) was noted in nine patients (18%). In Demiroren et al7 –a similar study- (70.5%) of patients had carditis. Mitral involvement was found in (92.3%) of patients, aortic in (23.3%), and (7%) and (2.3%) for tricuspid and pulmonary.7
As for other patients who did not have carditis only, (36%) (n=18) of patients had arthritis only, (36%) (n=18) had arthritis and carditis, and (28%) (n=14) had pure chorea. None of the patients had erythema marginatum or subcutaneous nodules.
To diagnose a patient with Sydenham’s chorea, history and family history and drug use should be taken from the patients and a physical exam should be performed. Patients with an atypical history of hemichorea undergo brain imaging to exclude other causes first. Patients with typical history who meet the Jones criteria are diagnosed directly, while other patients undergo additional investigations such as Anti-streptolysin “O” (ASO) titer or Anti-DNase B, total blood cell count, and other investigations.4,6 Regarding investigations, a highly elevated Erythrocyte sedimentation rate (ESR) was detected (>100 mm/h) in 18 patients (36%) and moderately elevated ESR (30–70 mm/h) in 30 patients (60%). Elevated ASO levels were detected in 44 patients (88%). Only 13 patients (26%) gave a history of pharyngitis. These results come not far from a study by Nair et al13 conducted in the tropics found that high Erythrocyte sedimentation rate (ESR) had a good correlation with the clinical profile of children diagnosed with Sydenham’s chorea. It also stated that Anti-streptolysin “O” (ASO) titer was significant in (68%) of cases. However, much higher rates of associated sore throats were reported in the latter (67%).
Brain imaging, which was performed in only 11 patients, was normal in all. All brain MRI studies of patients in Demiroren et al’s study7 were normal, while Tumas et al’s study6 described brain abnormalities in four out of 11 patients. It is fair to say that patients in this study had a lower rate of chorea-associated brain abnormalities.
Typically, Treatment of chorea shows response within four to 12 weeks with the use of carbamazepine, with 77% of patients showing response within 4 weeks. Recurrence is possible as well.14,15 In our study, the chorea lasted from two to four weeks in 26 patients (52%), five to ten weeks in 19 patients (38%), and 11 to 15 weeks in five patients (10%).
Ten of 50 patients (20%) had a recurrent attack; to all of them, it was the second attack. The interval between attacks varied from two to three years. Carditis was detected in all patients with recurrences. Recurrence however is not a feature of chorea itself but is rather either associated with a second streptococcal infection or irregular use of prophylactic antibiotics.6 The high recurrence rate found in this study is worthy of more attention and further study and investigation.
Conclusion
Sydenham chorea is more common in young female patients with or without a positive family history. It manifests acutely in the majority of patients and is suppressed during sleep, with stress being the most precipitating factor.
Chorea was predominantly generalized, typically beginning with oro-facial movement and progressing to limb involvement. Other presentations such as weakness, hypotonia, darting tongue, and milking sign were common. Psychiatric symptoms included emotional vulnerability, restlessness, and depression. Our patients did not have erythema marginatum or subcutaneous nodules. Most patients had elevated ASO and ESR levels, and a small percentage had a history of pharyngitis. Carditis was very common among patients with predominantly mitral involvement. Cases of prolonged PR or first-degree heart block were found.
Recommendations
As Sydenham’s chorea is a common disease in Sudan, further studies are needed to be conducted in this direction. All Patients with chorea should be screened for Sydenham’s chorea.
The prevalence of recurrence of chorea among patients and its association with long-term adherence to secondary prophylaxis is an area of major importance in a country like Sudan and requires further attention and investigation.
Abbreviations
SC, Sydenham chorea; ARF, Acute Rheumatic Fever; ASO, Antistreptolysin O; ESR, Erythrocyte Sedimentation Rate; SPSS, Statistical Package for Social Sciences.
Data Sharing Statement
The materials datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
After they informed about the purpose of the study, Informed consent was obtained from all the participants and also from parents/legal guardians of minors and illiterates.
The protocol of this study was approved by the research and ethics committee, Sudan Medical Specialization Board (SMSB), Khartoum, Sudan. We confirm that all methods were carried out in accordance with the Declaration of Helsinki. Participants were assured about the confidentiality of any obtained information. The responses were kept confidentially and data from this research was managed only researchers in this study, Results will be used only for research and data cannot be traced back to their original sources.
Acknowledgments
This paper published as preprint in research square on July 2021 with the following link: DOI:10.21203/rs.3.rs-715605/v1.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be
accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no competing interests.
References
1. Cardoso F. Sydenham’s chorea. In: Handbook of Clinical Neurology.
2. De Queiroz Campos Araújo AP, Pádua PAB, Maia Filho HS. Management of rheumatic chorea: an observational study. Arq Neuropsiquiatr. 2002;60(2 A):231–233. doi:10.1590/S0004-282X2002000200008
3. Jordan LC, Singer HS. Chorea in children. Treat Pediatr Neurol Disord. 2005;2005:133–138.
4. Oosterveer DM, Overweg-Plandsoen WCT, Roos RAC. Sydenham’s chorea: a practical overview of the current literature. Pediatr Neurol. 2010;43(1):1–6. doi:10.1016/j.pediatrneurol.2009.11.015
5. Risavi BL, Iszkula E, Yost B. Sydenham’s Chorea. J Emerg Med. 2019;56(6):e119–21. doi:10.1016/j.jemermed.2019.02.012
6. Tumas V, Caldas CT, Santos AC, Nobre A, Fernandes RMF. Sydenham’s chorea: clinical observations from a Brazilian movement disorder clinic. Park Relat Disord. 2007;13(5):276–283. doi:10.1016/j.parkreldis.2006.11.010
7. Demiroren K, Yavuz H, Cam L, Oran B, Karaaslan S, Demiroren S. Sydenham’s Chorea: a clinical follow-up. Joural Child Neurol. 2007;22(5):550–554. doi:10.1177/0883073807302614
8. Cardoso F, Eduardo C, Silva AP, Mota CC. Chorea in fifty consecutive patients with rheumatic fever. Mov Disord. 1997;12(5):701–703. doi:10.1002/mds.870120512
9. Cardoso F, Seppi K, Mair KJ, Wenning GK, Poewe W. Seminar on choreas. Lancet Neurol. 2006;5(7):589–602. doi:10.1016/S1474-4422(06)70494-X
10. Nausieda PA, Grossman BJ, Koller WC, Weiner WJ, Klawans HL. Sydenham chorea: an update. Neurology. 1980;30(3):331–334. doi:10.1212/WNL.30.3.331
11. Chorea SS. Original articles. J Am Med Assoc. 1889;XII(21):730–737.
12. Rodopman-Arman A, Yazgan Y, Berkem M, Eraksoy M. Are sensory phenomena present in Sydenham’s Chorea? Evaluation of 13 cases. Neuropediatrics. 2004;35(4):242–245. doi:10.1055/s-2004-820917
13. Nair PM, Philip E, Bahuleyan CG, Thomas M, Shanmugham JS, Suguna Bai NS. The first attack of acute rheumatic fever in childhood--clinical and laboratory profile. Indian Pediatr. 1990;27(3):241–246.
14. Harel L, Zecharia A, Straussberg R, Volovitz B, Amir J. Successful treatment of rheumatic chorea with carbamazepine. Pediatr Neurol. 2000;23(2):147–151. doi:10.1016/S0887-8994(00)00177-6
15. Genel F, Arslanoglu S, Uran N, Saylan B. Sydenham’s chorea: clinical findings and comparison of the efficacies of sodium valproate and carbamazepine regimens. Brain Dev. 2002;24(2):73–76. doi:10.1016/S0387-7604(01)00404-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
