Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Switch Rates and Total Cost of Care Associated with Apremilast and Biologic Therapies in Biologic-Naive Patients with Plaque Psoriasis
Authors Kaplan DL, Ung BL, Pelletier C, Udeze C, Khilfeh I , Tian M
Received 28 February 2020
Accepted for publication 11 June 2020
Published 17 July 2020 Volume 2020:12 Pages 369—377
DOI https://doi.org/10.2147/CEOR.S251775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
David L Kaplan,1 Brian L Ung,2 Corey Pelletier,2 Chuka Udeze,2 Ibrahim Khilfeh,3 Marc Tian2
1Adult & Pediatric Dermatology, Overland Park, Kansas, USA; 2US HEOR, Celgene Corporation, Summit, New Jersey, USA; 3US HEOR, Amgen Inc, Thousand Oaks, California, USA
Correspondence: Corey Pelletier Email [email protected]
Purpose: Compare treatment switching rates and costs among biologic-naive psoriasis patients initiating apremilast or biologics.
Methods: This retrospective claims analysis used IBM MarketScan Commercial and Medicare Supplemental databases to identify patients who initiated apremilast or a biologic (ie, tumor necrosis factor [TNF] or interleukin [IL] inhibitor) for psoriasis treatment between January 1, 2015, and December 31, 2016. A 1:1 propensity score matching was used to adjust for possible selection bias and maximize the number of patients available for analysis. Treatment switching, days to switch, and healthcare costs were assessed at 12 months. T-test and chi-square test were used to evaluate differences between cohorts for continuous and categorical variables as appropriate; Wilcoxon rank-sum tests were used to assess cost differences.
Results: In total, 88,025 patients newly initiated apremilast, a TNF inhibitor, or an IL inhibitor. After inclusion/exclusion criteria were applied and patients were propensity score matched, 1645 (apremilast), 1207 (TNF inhibitor), and 438 (IL inhibitor) patients were included in this analysis. Twelve-month switch rates were significantly lower for apremilast initiators compared with TNF inhibitor initiators (14% vs 25%; p< 0.01) and comparable to IL inhibitors (14% vs 11%; p> 0.05). No statistical difference was observed in days to switch at 12 months for any treatment group. Total healthcare costs were lower for apremilast initiators compared with TNF and IL inhibitor initiators ($34,028 vs $55,973 and $64,430; p< 0.0001). Per-patient per-month (PPPM) costs were significantly lower for apremilast initiators compared with TNF inhibitor and IL inhibitor initiators ($2834 vs $4662 and $5366; p< 0.0001).
Conclusion: Over a 12-month follow-up, biologic-naive psoriasis patients initiating apremilast had significantly lower switching rates compared with patients on TNF inhibitors and similar rates as patients on IL inhibitors. PPPM and total healthcare costs were significantly lower for patients initiating apremilast vs TNF or IL inhibitors, primarily due to lower pharmacy costs.
Keywords: adherence, apremilast, healthcare costs, IL inhibitors, TNF inhibitors, treatment pattern
Introduction
Psoriasis is a chronic inflammatory disease characterized by scaling erythematous plaques.1,2 In the United States, an estimated 3.2% of adults are affected by psoriasis,3 with plaque psoriasis accounting for >80% of cases.1 As a multisystem inflammatory disorder that follows a relapsing course, psoriasis is associated with clinically significant comorbidities such as psoriatic arthritis, cardiovascular disease, inflammatory bowel disease, malignancy, renal disease, sleep apnea, chronic obstructive pulmonary disease, uveitis, and hepatic disease.4 Treatment options for mild to moderate disease include topical agents or phototherapy.2
In moderate to severe disease, these treatment options are often insufficient to manage psoriasis, at which time worsening disease may be managed with a systemic or biologic therapy.2 Currently approved biologic therapies, such as tumor necrosis factor (TNF) or specific interleukin (IL) inhibitors (ie, IL-12/23, IL-23, IL-17), target specific inflammatory mediators.2 Apremilast, an oral phosphodiesterase 4 inhibitor, was approved by the US Food and Drug Administration in 2014 for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.5 Unlike biologic agents that target specific inflammatory mediators, including IL-23, TNF-α, IL-17A, or IL-22, apremilast does not target a single inflammatory mediator.6,7
Because of its chronic disease course, psoriasis is associated with high direct medical costs that increase with severity.8 Switching between treatments is common and has been seen in real-world studies.9,10 The most common reasons for treatment discontinuation are lack of effectiveness and adverse events.9 Switch rates may reflect whether patients are responding to or tolerating their prescribed therapies. Poor treatment persistence and adherence, as well as the associated costs, are of interest to US payers. The primary aim of this retrospective, real-world claims analysis of a US payer database is to compare the rates of treatment switching and the associated direct healthcare costs among biologic-naive patients with psoriasis initiating treatment with apremilast or a biologic (IL or TNF inhibitor).
Patients and Methods
Study Design and Data Source
This retrospective claims analysis used the IBM MarketScan Commercial and Medicare Supplemental databases (IBM Watson Health, Cambridge, MA) to identify patients who initiated apremilast or a biologic agent for the treatment of psoriasis between January 1, 2015, and December 31, 2016. The entire study period was from January 1, 2011, to December 31, 2017. The MarketScan Commercial Claims and Encounters Database contains the inpatient, outpatient, and outpatient prescription drug experience of several million employees and their dependents (annually), covered under a variety of fee-for-service and managed care health plans. The MarketScan Medicare Supplemental and Coordination of Benefits Database contains the healthcare experience (both medical and pharmacy) of individuals with Medicare supplemental insurance paid for by employers. The Commercial database contains 137.6 million covered lives and the Medicare Supplemental database contains 10.2 million covered lives from 1995 to 2016. Both databases are de-identified, consistent with the Health Insurance Portability and Accountability Act of 1996, and provide detailed utilization, outcomes, and cost data for healthcare services provided in the inpatient, outpatient, and outpatient pharmacy settings. Because the analysis uses only de-identified patient records and does not involve the collection, use, or transmittal of individually identifiable data, institutional review board approval to conduct this study was not required.
Study Population
Adult patients, ≥18 years of age on the index date, were eligible for inclusion if they initiated a new treatment with apremilast or a biologic agent (ie, adalimumab, certolizumab, etanercept, golimumab, infliximab, ixekizumab, secukinumab, or ustekinumab) for treatment of psoriasis between January 1, 2015, and December 31, 2016. Patients were required to have two International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM/ICD-10-CM) diagnosis codes for psoriasis during the 12 months before the initiation of new treatment. The start date of a new treatment episode was considered to be the index date. A minimum 12-month baseline period that was free of any index agent was required to identify the baseline patient demographics and clinical characteristics, as was a minimum follow-up period of 12 months continuous enrollment to assess switch rates and costs. Patients were included if they did not have a history of biologic use in the pre-index period. All available data were used to identify previous biologic- and psoriasis-related therapy exposure. Key exclusion criteria included a diagnosis of psoriatic arthritis, other biologic-indicated autoimmune conditions (eg, ulcerative colitis, Crohn’s disease, rheumatoid arthritis and other inflammatory polyarthropathies, ankylosing spondylitis, or juvenile idiopathic arthritis), or cancer at any time during the pre- or post-index period.
Switch Rates and Cost Outcomes
Switch rate was defined as the proportion of patients who switched to or added a new systemic treatment, either apremilast or biologic therapy (categorized into either TNF or IL inhibitors) after the initiation of the index treatment. Time to switch was defined as the time from the date of initiation of the index treatment to the date of initiation of a new treatment for those patients who did switch within the 12-month follow-up period. Adherence while on the index treatment was calculated utilizing the proportion of days covered and the medication possession ratio. Healthcare costs were based on the paid amounts of adjudicated claims, including insurer and health plan payments, as well as patient cost-sharing in the form of copayment, deductible, and coinsurance. Healthcare costs reflect actual paid costs based on patient adherence to treatment and treatment switching. Total healthcare costs were defined as the total sum of healthcare costs from the initiation of treatment. Per-patient per-month (PPPM) total healthcare costs were defined as the average total monthly healthcare costs while patients remained on the index treatment.
Statistical Analysis
Baseline patient demographics and clinical characteristics between the apremilast and biologics cohorts were compared using a t-test for continuous variables and a chi-square test for categorical variables. A 1:1 propensity score matching was used to adjust for possible selection bias and to maximize the number of patients available for analysis. Logistic regression was used to estimate the propensity score for individual patients with the following variables: age, gender, region, payer, plan type, index year, pre-index days, Charlson Comorbidity Index score, pre-index cost, and a limited number of clinical characteristics, including number of prior biologics, number of prior systemic agents, previous usage of non-steroidal anti-inflammatory drugs or cyclooxygenase-2 inhibitors, previous use of corticosteroids or phototherapy, and total healthcare cost in 12 months prior to the index period. A p value of ≤0.05 was considered statistically significant. Generalized linear models were used to assess the relationship between the treatment groups and total healthcare costs, controlling for baseline patient demographics and clinical characteristics. The Wilcoxon rank-sum test was used to assess the cost differences between patients initiating apremilast and a biologic.
Results
In total, 88,025 patients in the IBM database had initiated treatment with apremilast or a biologic agent between January 1, 2015, and December 31, 2016. After inclusion and exclusion criteria were applied and patients were propensity score matched, 1645 biologic-naive patients were included in the apremilast group and 1645 biologic-naive patients were included in the biologic group (Figure 1). The biologic group comprised 1207 patients in the TNF inhibitor group and 438 in the IL inhibitor group. After propensity score matching, patients treated with apremilast had a mean age of 47.5 years, 51.7% were female, and had a mean CCI score of 0.45 compared with patients treated with TNF inhibitors, who had a mean age of 48.1 years, 51.7% were female, and had a mean CCI score of 0.47, or IL inhibitors, who had a mean age of 46.4 years, 47.7% were female, and had a mean CCI score of 0.39 (Table 1). The majority of patients treated with a TNF inhibitor had an index treatment with adalimumab (79.9%) or etanercept (20.0%), and the majority of patients treated with an IL inhibitor had an index treatment of ustekinumab (80.8%) or secukinumab (15.8%).
 |
Table 1 Patient Demographics Post-Matcha |
Switch Rates
At 12 months, switch rates were significantly lower in biologic-naive patients receiving apremilast compared with biologic-naive patients receiving TNF inhibitors (14% vs 25%; p<0.0001), and comparable to patients receiving IL inhibitors (14% vs 11%; p=0.09). In the patients with data up to 24 months post-index, switch rates increased in all treatment groups, but the overall trend remained the same (Figure 2). The majority of patients treated with apremilast switched to adalimumab (51.5%) or ustekinumab (24.7%). Patients treated with an IL inhibitor most often switched to adalimumab (27.3%) or apremilast (26.0%), while the patients treated with a TNF inhibitor switched to ustekinumab (37.1%) or apremilast (22.0%; Table 2). No significant difference was observed in days to switch at 12 months between treatment groups (Table 2). The mean adherence rate while on the index treatment, measured by proportion of days covered, was 0.81 for apremilast initiators, 0.84 for TNF inhibitor initiators (p=0.0022 vs apremilast), and 0.73 for IL inhibitor initiators (p<0.0001 vs apremilast) over the 12-month follow-up period (Table 3).
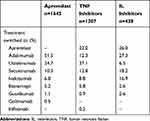 |
Table 2 Treatment Switch Details |
 |
Table 3 12-Month Mean Adherence Rates in Biologic-Naive Patients Treated with Apremilast or a Biologic |
 |
Figure 2 Switch rates of biologic-naive patients initiating apremilast or biologics. Notes: *p<0.01. †p=0.02. |
Healthcare Costs
Total healthcare costs at 12 months were significantly lower for biologic-naive patients treated with apremilast compared with biologic-naive patients treated with a TNF inhibitor or an IL inhibitor ($34,028 vs $55,973 and $64,430, respectively; p<0.05; Table 4). Most total healthcare costs were pharmacy related in all treatment groups, although pharmacy costs were significantly lower for patients treated with apremilast compared with TNF and IL inhibitors ($27,818 vs $50,070 and $58,871, respectively; both p<0.0001). Outpatient costs for all treatment groups were similar. Among the patients who switched treatment at 12 months, total healthcare costs increased regardless of the index treatment (Figure 3). Total healthcare costs for patients who did not switch at 12 months were lower with apremilast compared with a TNF inhibitor and an IL inhibitor ($30,566 vs $52,156 and $63,667, respectively; both p<0.0001). In patients who switched from their index treatment, total healthcare costs at 12 months were lowest for patients treated with apremilast compared with a TNF inhibitor and an IL inhibitor ($55,222 vs $67,412 and $70,623, respectively; both p<0.0001).
 |
Table 4 Healthcare Costs in Biologic-Naive Patients Treated with Apremilast or Biologics Over 12 Months |
 |
Figure 3 Mean total healthcare costs over 12-month follow-up in biologic-naive patients treated with apremilast and biologics. Notes: *p<0.01 vs apremilast. †p=0.09 vs apremilast. |
Over the 12-month follow-up period, PPPM healthcare costs were significantly lower for patients treated with apremilast compared with a TNF inhibitor and an IL inhibitor ($2834 vs $4662 and $5366, respectively; both p<0.05), largely due to pharmacy costs. PPPM healthcare costs at 12 months for patients who did not switch index treatments were significantly lower for apremilast compared with a TNF inhibitor and an IL inhibitor ($2546 vs $4344 and $5303, respectively; both p<0.0001). This trend was consistent with PPPM healthcare costs in patients who did switch treatment at 12 months ($4599 [apremilast] vs $5615 [TNF inhibitor] and $5882 [IL inhibitor]; both p<0.0001). Although patients treated with apremilast had significantly lower PPPM healthcare costs before switching compared with patients treated with a TNF inhibitor and an IL inhibitor ($2910 vs $5488 and $6028, respectively; both p<0.0001), no significant differences were observed between treatment groups in total PPPM healthcare costs after switching for patients treated with apremilast compared with a TNF inhibitor ($8724 vs $9300; p=0.1127) and an IL inhibitor ($8724 vs $7708; p=0.3057). Regardless of the index treatment, in patients who did switch, PPPM costs increased after treatment switch (Figure 4).
Discussion
Our study investigated apremilast and biologic agents in a real-world setting, differentiating between biologic mechanisms of action to demonstrate the switch rate and cost differences associated with initiating apremilast, a TNF inhibitor, or an IL inhibitor in biologic-naive plaque psoriasis. Total healthcare and PPPM costs at 12 months were significantly lower for patients treated with apremilast compared with biologic-naive patients treated with a TNF inhibitor or an IL inhibitor. These trends were due to differences in pharmacy costs between apremilast, a TNF inhibitor, or an IL inhibitor. In this analysis of claims data, biologic-naive patients with psoriasis initiating apremilast had lower rates of treatment switching at 12 months compared with biologic-naive patients initiating a TNF inhibitor and comparable rates to biologic-naive patients initiating an IL inhibitor.
Switch rates may reflect whether patients were responding to or tolerating prescribed psoriasis treatment. Our results show significant differences between patients initiating apremilast and TNF inhibitors. A previous analysis using a different database found that the proportion of patients who switched treatment at 12 months was similar for biologic-naive patients initiating apremilast compared with those initiating a biologic (24.6% vs 27.0%; p=0.30) (Wu JJ, et al, personal communication). This study expands on previous analyses of treatment patterns by differentiating between treatment with TNF and IL inhibitors. The previous lack of differences seen between the apremilast and biologic treatment patterns may be due to combining TNF and IL inhibitor targeted treatments and reduced sample size of other studies.
Total healthcare, pharmacy, and PPPM costs were lower for biologic-naive patients treated with apremilast over the 12-month follow-up in this real-world analysis. As expected, the greatest contributor to total healthcare costs, regardless of index treatment, was pharmacy. This is consistent with results seen in Feldman et al, which found healthcare costs associated with initiating treatment in biologic-naive psoriasis patients were lower with apremilast compared with biologics.11 In this analysis, an approximate 15% increase in total healthcare, pharmacy, PPPM, and pharmacy PPPM costs was observed in patients initiating IL inhibitors compared with TNF inhibitors. This finding may be due to higher drug costs associated with initiating these treatments in the first year. US payers must consider the efficacy, safety, and treatment costs when reviewing their formularies.12 Reviewing real-world treatment utilization, patterns, and associated healthcare costs may help inform formulary decision making.12
Our study had a number of limitations. The results of this analysis are only generalizable to individuals with commercial or private Medicare supplemental health coverage in the United States. We are uncertain whether similar results would be observed in patient groups with different socioeconomic status and insurance coverage and, therefore, future studies should be conducted in other populations. By utilizing claims, these analyses are subject to selection bias and miscoding. Additionally, the possibility of under-diagnosis or misdiagnosis of psoriasis among patients potentially introduces selection bias. Data on disease severity, location of psoriatic disease, or specific subtype were not available for analysis; thus, there may be unknown differences in severity between the treatment groups. Treatment switch and adherence measures were based on filled prescriptions; we were unable to confirm whether patients actually took the medication. Furthermore, differences in biologic and apremilast dosing schedules may impact adherence claims-based calculations. Patients included in this analysis were required to have continuous healthcare coverage, which may contribute to selection bias. We could not assess the reasons for treatment switching in this analysis. At the time of this analysis, not all currently available biologics were approved during the index period, and our results are only applicable to biologics approved during the index period. Our use of propensity score matching balanced the two groups in terms of measured factors, but there may be differences in unmeasured factors (eg, the intrinsic adherence behaviors of individual patients) that could have impacted switching rates and costs independent of the drugs studied. Finally, the representativeness of the study population of 3290 patients (after all inclusion and exclusion criteria were applied) is uncertain; future studies applying different patient selection criteria should be conducted.
Conclusion
Over a 12-month follow-up period, biologic-naive patients with psoriasis initiating treatment with apremilast had significantly lower rates of treatment switching than patients initiating treatment with a TNF inhibitor and similar rates to patients initiating an IL inhibitor. Total healthcare and PPPM costs in the first year were significantly lower for patients initiating apremilast compared with TNF or IL inhibitors, primarily due to lower pharmacy costs. While this analysis confirms trends seen in other real-world studies, future analyses should continue to investigate reasons for and predictors of treatment switching.
Ethics Approval and Informed Consent
Because the analysis uses only de-identified patient records and does not involve the collection, use, or transmittal of individually identifiable data, institutional review board approval to conduct this study was not required.
Disclosure
All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and declare that: DLK has served as a consultant to Celgene Corporation, and has served as a speaker for AbbVie, Celgene Corporation, Dermira, and Pfizer. BLU is an employee of Bristol-Myers Squibb and was an employee of Celgene Corporation at the time of study conduct. CP is an employee of Bristol-Myers Squibb and was an employee of Celgene Corporation at the time of study conduct. CU is an employee of Vertex and was an employee of Celgene Corporation at the time of study conduct. IK is an employee of Amgen Inc. and a former employee of Celgene Corporation. MT is an employee of Bristol-Myers Squibb and was an employee of Celgene Corporation at the time of study conduct. The authors report no other conflicts of interest in this work.
References
1. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. doi:10.1016/j.jaad.2008.02.039
2. Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi:10.1016/j.jaad.2018.11.057
3. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. doi:10.1016/j.jaad.2013.11.013
4. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113. doi:10.1016/j.jaad.2018.11.058
5. Otezla (Apremilast) [Package Insert]. Thousand Oaks, CA: Amgen Inc.; June 2020.
6. Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159(4):842–855. doi:10.1111/j.1476-5381.2009.00559.x
7. Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a Phase II open-label study. J Drugs Dermatol. 2013;12(8):888–897.
8. Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72(6):961–967.e965. doi:10.1016/j.jaad.2015.02.1099
9. Armstrong AW, Foster SA, Comer BS, et al. Real-world health outcomes in adults with moderate-to-severe psoriasis in the United States: a population study using electronic health records to examine patient-perceived treatment effectiveness, medication use, and healthcare resource utilization. BMC Dermatol. 2018;18(1):4. doi:10.1186/s12895-018-0072-2
10. Feldman SR, Tian H, Wang X, Germino R. Health care utilization and cost associated with biologic treatment patterns among patients with moderate to severe psoriasis: analyses from a large U.S. claims database. J Manag Care Spec Pharm. 2019;25:479–488.
11. Feldman SR, Pelletier CL, Wilson KL, et al. Real-world US healthcare costs of psoriasis for biologic-naive patients initiating apremilast or biologics. J Comp Eff Res. 2019;8(1):45–54. doi:10.2217/cer-2018-0097
12. Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9(9):504–513.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


