Back to Journals » Veterinary Medicine: Research and Reports » Volume 8
Swine brucellosis: current perspectives
Received 28 May 2016
Accepted for publication 1 July 2016
Published 20 December 2016 Volume 2017:8 Pages 1—12
DOI https://doi.org/10.2147/VMRR.S91360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Young Lyoo
SC Olsen, FM Tatum
Infectious Bacterial Diseases of Livestock Research Unit, National Animal Disease Center, Agricultural Research Service, United States Department of Agriculture, Ames, IA, USA
Abstract: Brucella suis is a significant zoonotic species that is present in domestic livestock and wildlife in many countries worldwide. Transmission from animal reservoirs is the source of human infection as human-to-human transmission is very rare. Although swine brucellosis causes economic losses in domestic livestock, preventing human infection is the primary reason for its emphasis in disease control programs. Although disease prevalence varies worldwide, in areas outside of Europe, swine brucellosis is predominantly caused by B. suis biovars 1 and 3. In Europe, swine are predominantly infected with biovar 2 which is much less pathogenic in humans. In many areas worldwide, feral or wild populations of swine are important reservoir hosts. Like other Brucella spp. in their natural host, B. suis has developed mechanisms to survive in an intracellular environment and evade immune detection. Limitations in sensitivity and specificity of current diagnostics require use at a herd level, rather for individual animals. There is currently no commercial vaccine approved for preventing brucellosis in swine. Although not feasible in all situations, whole-herd depopulation is the most effective regulatory mechanism to control swine brucellosis.
Keywords: livestock, transmission, pathogenicity, vaccine, host, infection
Introduction
Brucellosis in swine, a disease caused by infection with intracellular bacteria from the genus Brucella, is a disease of economic importance with worldwide distribution. Although the disease is associated with reproductive losses in swine worldwide, its primary importance is related to its zoonotic capability of causing clinical symptoms in humans. For this reason, many countries have regulatory programs to address swine brucellosis. Several studies have indicated that addressing brucellosis in livestock reservoirs is the most efficient and economical approach for reducing human brucellosis.1–3
Taxonomy of the pathogen
The genus Brucella encompasses a group of Gram-negative bacteria that survive almost exclusively in infected hosts with preference for localization in intracellular compartments of phagocytic, reticuloendothelial, and specialized epithelial cells. Including Brucella suis, the genus currently is composed of ten species (Brucella abortus, Brucella canis, Brucella ceti, Brucella inopinata, Brucella melitensis, Brucella microti, Brucella neotomae, Brucella ovis, and Brucella pennipedalis) with several additional new species under consideration for inclusion.4 Although genomic techniques in the 1980s suggested that Brucella strains may comprise a single genetic “species”,5 the epidemiologic and diagnostic benefits obtained by splitting Brucella strains to separate “nomenspecies” based on their distinctive phenotypic characteristics and host preferences are more compelling for classification purposes. The current classification system is further supported by the observation that newly identified Brucella spp. demonstrate greater genetic variability when compared to the six traditional Brucellae species6 and whole-genome sequencing data demonstrate distinct species-specific lineages.7 However, it should be recognized that the most virulent Brucella spp. can naturally infect multiple hosts.4 Clinical disease caused by infection with bacteria in this genus, brucellosis, is generally associated with chronic but somewhat asymptomatic infections in native hosts with pathologic effects predominantly associated with reproductive tissues.
Brucella spp. are further divided into biovars which are determined by metabolic characteristics, growth in the presence of thionin or basic fuschin dyes, and patterns of agglutination by monoclonal antibodies against the A, M, or R forms of the lipopolysaccharide (LPS) O side-chain.8 Isolates of B. suis are invariably in the smooth form (expressing the LPS O side-chain), and colonies of B. suis cannot be visually differentiated from isolates of other smooth Brucella spp. B. suis is currently subdivided into five biovars with biovars 1, 2, and 3 being responsible for brucellosis in swine. While biovars 1 and 3 are pathogenic in humans, biovar 2 appears to be a very rare cause of human infection.9 Although isolates of biovar 4 are considered to be zoonotic, their distribution is exclusively limited to subarctic areas where they primarily infect reindeer and wild caribou (Rangifer tarandus and various species). This biovar has also been recovered from moose (Alces alces), arctic foxes (Alopex lagopus), and wolves (Canis lupus) in subarctic areas.10 Isolates in biovar 5 also have very limited geographic distribution as they have only been recovered from rodents in the USSR.8 As biovars 4 and 5 are not natural pathogens for swine, they will not be emphasized in this review of swine brucellosis.
Historically, B. suis was first isolated from aborted porcine fetuses in Indiana in 1914.11 Additional isolates obtained from swine fetuses in 1916 were used to demonstrate pathogenicity of the bacterial isolates in swine.12 Limitations in microbiologic techniques resulted in initial isolates being misidentified as B. abortus, and recognition of the isolates as a separate Brucella sp. did not occur until 1929.13 In a similar manner, limitations in microbiologic tests prior to the 1960s (lack of phage testing and oxidative metabolic tests) led to the misidentification of B. suis biovar 3 isolates as B. melitensis biovar 2 for many years.8
It should also be noted that B. abortus field strains and the B. abortus strain 19 vaccine strain have also been isolated from feral swine populations.14,15 The recovery of B. abortus and strain 19 from a feral swine population that had been isolated from domestic livestock for at least 40 years suggests that feral swine may be able to naturally maintain B. abortus infections. Recently, a newly recognized Brucella sp., B. microti, was isolated from an asymptomatic female wild boar, although the significance of this finding is unknown.16 As these observations are insignificant in relationship to the high prevalence of B. suis biovars 1, 2, and 3 in swine, the remainder of this review concentrates on these three biovars.
Genetic characteristics
Representative genomes of all biovars of B. suis have been sequenced and analyzed.17,18 With the exception of B. suis biovar 3, which has a single chromosome of ~3.1 Mbp, all other biovars have two circular chromosomes of ~2.1 and 1.2 Mbp, respectively. The origin of replication of the large chromosome is typical of other bacteria, while the origin of replication of the small chromosome is plasmid like. The G+C content of the two chromosomes is nearly identical with an average GC content of ~58%–59% and encodes ~3,200–3,400 open reading frames.7 Although the genus is highly homogeneous, the B. suis clade is reported to exhibit the most intraspecific genetic diversity as compared to the six classical Brucella spp. (B. abortus, B. suis, B. neotomae, B. melitensis, B. ovis, and B. canis). Phylogenetic analysis suggests early separation of the B. suis strains from B. abortus and B. melitensis clades.7 Comparative bioinformatics suggest that the acquisition of the VirB type 4 secretion system and adaptation to a limited-metal environment were critical evolutionary steps for development of Brucella from soil bacteria ancestors and adaptation to the intracellular environment of eukaryotes.19 Although Brucella have no known natural plasmids which might allow transfer of genetic material or antibiotic resistance, a VirB type 4 secretion system similar to that in Brucella was found on a plasmid with a broad host range which was isolated from an unidentified bacterium in the rhizosphere of alfalfa.20 Evolution of the Brucellae was also associated with an ~30% genome reduction particularly in proteins involved in carbohydrate and amino acid utilization, metabolism, and biosynthesis.7,19 Brucella possess genomic islands which encode pathogenicity factors, mostly hypothetical proteins and enzymes commonly found associated with horizontally acquired DNA such as transposases and integrases. A recent analysis of 54 published B. suis genomes identified 16,756 single-nucleotide polymorphisms between strains, including biovar-specific single-nucleotide polymorphisms that may have value as diagnostic targets.21 With the exception of biovar 5, genomes of B. suis isolates cluster together.
Several molecular procedures have been implemented to understand epidemiologic and genetic relationships between B. suis and other Brucella spp. Polymerase chain reaction (PCR) assays have been developed that discriminate B. suis from other Brucella spp. and allow differentiation of different biovar types.22 Identification of multiple-locus variable-number tandem-repeat sequences has also allowed differentiation between strains and genetic comparisons of strain lineages.23,24 More recently, advances in whole-genome sequencing have allowed this approach to be utilized for more detailed comparisons across Brucella strains.25 Although publications using whole-genome sequencing to compare B. suis isolates are limited, this approach was recently used to determine that a man with clinical brucellosis was actually infected with B. suis in Tongo prior to emigration to the US.26
Distribution
Data from numerous countries indicate widespread distribution of B. suis in both domestic livestock and wildlife populations. Although regulatory efforts have reduced disease prevalence in domestic swine, the high prevalence of brucellosis in the estimated six million feral swine population in the US is of concern for reemergence. Seroprevalence of brucellosis in feral swine in the US differs by study and location with estimates ranging from 18% to 53%.27–29 The high prevalence of biovars 1 and 3 in feral swine in the US has led to frequent transmission of disease to not only domestic swine raised in outside pens but also domestic cattle where it induces serologic titers that cannot be differentiated from titers caused by infection with B. abortus. Similar issues are reported in Australia where B. suis infection in feral swine has been frequently reported and multiple transmission events from feral swine to cattle have been documented.30
The epidemiology of swine brucellosis in Europe differs from the US. On the European continent, England and Scandinavian countries appear to be free of porcine brucellosis, and biovar 3 has only been reported in Croatia.31 There are no recent reports documenting the isolation of B. suis biovar 1 from swine in Europe. In the majority of Europe, porcine brucellosis almost exclusively results from transmission of B. suis biovar 2 from Eurasian wild boar (Sus scrofa scrofa) and European brown hare (Lepus europaeus) reservoirs.32 Disease prevalence in Eurasian wild boar is high with estimates ranging from 8% to 32% throughout continental Europe.10 In some regions, such as the north of Spain,33 molecular patterns suggest that the B. suis biovar 2 strains infecting wild boar and European brown hares may be different, whereas other studies have suggested that biovar 2 genotypes cluster based on country and year.24 Venereal transmission is proposed as the main route of transmission of B. suis biovar 2 from wild boars to domestic swine, whereas transmission from brown hares is probably through oral consumption. Reemergence of swine brucellosis in continental Europe is predominantly related to production systems in which swine are raised outdoors under conditions where contact with wildlife reservoirs may occur.24,34
In some parts of the world, prevalence of swine brucellosis appears to be influenced by religious or cultural preferences that influence consumption of pork and impact populations of the preferred host species. Current data suggest a wide distribution of B. suis in domestic swine in Central and South America (isolations from swine in Argentina, Brazil, Columbia, Cuba, Chile, Honduras, Paraguay, and Peru) with infections predominantly caused by biovar 1.31,35–39 Feral swine are present in some South American countries, but the prevalence of swine brucellosis in these populations and their role in transmission of B. suis remain uncharacterized.
Swine brucellosis also appears to be endemic in parts of Central and Southeast Asia with greatest economic impact in the People’s Republic of China due to high levels of swine production, sporadic epidemics of B. suis (biovars 1 and 3) in swine, and recent reports of B. suis biovar 3 infections in humans.40,41 In India, reports of B. suis isolation from swine (biovar 1) and humans (biovar not reported) are available.42,43 A recent report of the isolation of B. suis biovar 1 from a man in Turkey suggested a possible link to increasing populations of wild boars in that country.44 Epidemiologic data from other parts of Southeast Asia are limited but suggest occurrence of swine brucellosis in many areas including Indonesia, Philippines, Taiwan, French Polynesia, Malaysia, Tonga, and other islands in the Pacific.8,23,26,45,46
Populations of swine in Africa are relatively small and epidemiologic data are limited,47 but B. suis biovar 1 has been isolated from cattle in Egypt and Zimbabwe.48,49 Porcine brucellosis is believed to be widespread across sub-Saharan Africa, but epidemiologic data are limited.
Recovery of B. suis from non-porcine hosts has been reported in a number of countries. In addition to the frequent isolations of B. suis biovar 1 from naturally infected cattle in several countries,30,50 B. suis biovar 1 has also been recovered from European hares (L. europaeus), possums (Dideiphis marsupialis), armadillos (Chaetophractus villosus), and sheep in Argentina,51,52 and from dogs in the US and Australia.53 B. suis biovar 2 has been recovered from roe deer in Germany54 and biovar 3 from horses in Croatia.55 Although the biovar was not determined, B. suis was also recovered from blue sheep near the Qinghai–Tibet Plateau of the People’s Republic of China.56
Intracellular infection/trafficking
Brucella generally enter the host through penetration of mucous membranes, transported either free or in phagocytic cells to regional draining lymph nodes where initial replication occurs, followed by dissemination throughout the body. Smooth strains of Brucella, including B. suis, are internalized through interactions with lipid rafts which are present on the surface of phagocytic cells and contain glycosphingolipids, cholesterol, and glycosyl-phosphatidylinositol-anchored proteins.57 The internalized bacteria initially localize within phagocytes in a membrane-bound compartment (phagosome) where the acidified environment induces a type IV secretion system of Brucella which interferes with phagosomal maturation. This process also neutralizes the pH of the compartment resulting in a modified, nonmaturing phagosome that does not fuse with lysosomes. By bypassing the endocytic pathway, Brucella reach the endoplasmic reticulum (ER) and create a replicating niche (Brucella-containing vacuole [BOV]) where the bacteria evade host defenses.58 By attaching ER proteins/chaperons and other proteins from secretory vesicles that traffic between the ER and Golgi apparatus (Rab GTPase and glyceraldehyde-3-phosphate dehydrogenase) onto the BOV, interaction with the ER is maintained that allows establishment of a replicative niche.58 Although ~70%–85% of the internalized bacteria may be eliminated by phagolysosome fusion, the creation of the BOV allows intracellular survival of some bacteria. Intracellular Brucella bacteria then use stationary-phase physiology and other nutrient-scavenging mechanisms (ie, siderophores to scavenge iron) as a strategy for long-term survival within the nutrient-poor environment.59,60 Brucella spp. have multiple molecular mechanisms to detoxify free radicals since oxidative killing is the primary mechanism employed by host phagocytes to control replication of intracellular pathogens. The O side-chain on the LPS appears to be a key molecule for invasion, and protection from oxidative killing, cationic peptides, and complement-mediated lysis.61 In addition, B. suis can also inhibit programmed cell death in phagocytic cells, allowing persistence of their preferred intracellular niche and preventing exposure to host immune defenses.62 The ability of Brucella to adapt to long-term intracellular survival in a nutrient-starved environment, while also evading immune recognition by the host, is the basis for Brucella spp. establishing and maintaining chronic infections.59
Epidemiology/pathophysiology of B. suis
It is important to recognize that the pathogenesis of disease caused by B. suis in swine significantly differs from characteristics of brucellosis in large or small ruminants (B. abortus or B. melitensis).10,63 Such differences include a prolonged bacteremia, capability of venereal transmission, and prolonged shedding from mucosal surfaces or in urine. Unlike ruminant brucellosis, males and nonpregnant swine appear capable of contributing to disease transmission. Environmental persistence is generally accepted to be of low epidemiological importance as direct or close contact is required for transmission.4 Maintenance of B. suis in a population is considered to require continued infection of susceptible hosts.
Contrary to perceptions that abortion is a key clinical symptom associated with B. suis infection in swine, abortion is generally a minor component of disease presentation under field conditions. After studying the natural course of disease in large groups of swine, some authors have blamed this misconception of the fact that B. suis was first isolated from an aborted fetus.64 Reproductive losses are associated with B. suis in swine, but clinical signs are not pathognomonic for brucellosis as fetal and placental lesions are difficult to differentiate from other infectious agents.65 When females become infected through natural breeding, lesions of placentitis of variable severity develop which can cause embryonic death between days 21 and 27, resulting in small fetuses that are expelled in placental tissues and rarely detected by farmers. The first evidence of early abortions may be a return to estrus at 40–45 days after natural breeding. This is also supported by experimental infection data in which sows inoculated with infected semen in early gestation demonstrated reproductive losses as early as 22 days after infection, and irregular return to estrus at 30–45 days after infection.66 Experimental data also suggest that infection after day 40 of pregnancy results in abortions in mid-to-late gestation. Under field conditions, abortions are generally associated with oral exposure between 50 and 100 days of gestation. Occasionally, sows expel stillborn or weak fetuses from 100 to 110 days of gestation.65 Although uterine infection usually does not persist for >30–40 days after abortion,65 variable uterine or vaginal shedding can occur for up to 36 months.66 In the small percentage of females that have persistent uterine infections, shedding may occur for up to 36 months and be associated with temporary or permanent infertility.65 Metritis and placental retention may occur in infected sows. Infertility is directly related to the duration of infection and the severity of uterine lesions.66,67
Increased neonatal mortality can be observed in pigs born to B. suis-infected sows.68,69 The majority of pigs infected in utero cleared B. suis infection by 6 months of age, but a small percentage (8% of 230 pigs) were blood culture positive beyond 3 months of age and 2.5% were culture positive at slaughter at 2 years of age.70 As documented with B. abortus in its native hosts, the onset of puberty appears to influence disease pathogenesis. Prior to the onset of sexual maturity, clinical signs are rare and generally limited to swollen joints and lameness. Less common signs include posterior paralysis, spondylitis, and abscess formation in various organs. After sexual maturity, the course of infection appears to be longer, with a greater impact on chronicity of infection in males as compared to females. In experimentally infected mature boars, B. suis was recovered from 66.7% of tissue samples after 6 months and from 50% at 42 months after infection.71 In comparison, ~25% of experimentally infected females were culture positive between 6 and 42 months after experimental infection.71 Data from one study of feral swine suggest a similar sex difference with Brucella recovered from 93% of males as compared to 61% of females.14 Some infected animals remain asymptomatic.
In intact boars, B. suis often localizes in accessory sexual organs or testicles with subsequent shedding in semen. Clinical evidence of orchitis and epididymitis may be infrequent, but lesions of testicular hypertrophy or testicular abscesses, sometimes severe, may be observed. Dependent upon unilateral or bilateral involvement of reproductive tissues, infected males may or may not demonstrate reduced fertility or libido even if high numbers of B. suis are present in the semen.66,72 Infection in male reproductive tissues may persist for 3–4 years,73 and lowered conception rates and fewer live pigs per litter may be observed in sows bred with infected boars.
In general, most infected swine do not demonstrate clinical illness on visual examination. Pyrexia or anorexia is usually not observed clinically. Detectable changes in leukograms are also not a common manifestation of acute or chronic B. suis infections in swine.
Immunity
Clearance and protective immunity against B. suis are associated with adaptive immune responses, particularly cellular immunity. However, Brucella spp., including B. suis, have adopted a number of mechanisms to evade immune detection in vivo.74 In addition to the establishment of its replicative niche which minimizes host recognition, Brucella spp. are noted for their ability to minimize stimulation of pathogen recognition receptors (PRRs), such as Toll-like receptors (TLRs). Not only are PRRs on the cell membrane involved in microbial detection leading to phagocytosis and the recruitment of antimicrobial activities to phagosomes, they are also present in the phagosome and can coordinate innate and adaptive immune processes.75 PRRs are also present in the cytoplasm where they sense DNA or RNA of pathogens and induce production of type I interferons and other inflammatory cytokines.75 Brucella bacteria are devoid of many classical structures involved in virulence such as pilli, fimbria, capsules, and plasmids that are known to stimulate PRRs. The Brucella cell envelope has high hydrophobicity of the cell envelope, a noncanonical structure of its LPS, and a lower stimulatory activity on Toll-like 4 receptors. The O side-chain on the LPS can form complexes with the major histocompatibility complex class II molecules that interfere with the ability of macrophages to present exogenous proteins. Proteins have been identified in Brucella with homology to TLR adaptor molecules that may interfere with, or subvert, TLR signaling. The lipid A of the LPS of Brucella strains stimulates a greatly reduced inflammatory response in mammalian hosts than does the endotoxin of other Gram-negative bacteria.76 Compared to other Gram-negative bacteria, Brucella induces a reduced innate immune response, and a lower rate of maturation and activation of dendritic cells.
The LPS of B. suis is a highly immunogenic, T-cell-independent antigen that can directly activate B-cells and elicit antibody responses in infected swine. Antibodies provide beneficial effects by opsonizing bacteria for phagocyte uptake, inducing complement-mediated killing, preventing adherence by binding of bacterial receptors, and mediating antibody-dependent cellular toxicity. However, as with Brucella spp. infection in other hosts, it is assumed that antibodies are not sufficient to provide long-term protection against B. suis in swine. Cellular immunity associated with cytotoxic T-cells that induce TH1-type patterns of cytokines (interferon, interleukin-2, TNF-α, interleukin-12) is believed to be critical for immune protection. The cross-presentation pathway in which internalized antigen gains access to the ER by fusion of phagosomes with ER-derived vesicles and subsequent loading of antigen onto major histocompatibility complex class I molecular and cell surface presentation appear to be critical for stimulating cellular immune responses.74 The innate immune system may also play a role not only as effector cells but also by producing appropriate cytokines upon encountering the pathogen that direct adaptive or acquired immune responses to a TH-1 pathway.
Diagnosis of swine brucellosis
Bacterial isolation remains the gold standard for diagnosis of swine brucellosis.10 However, the slow growth of Brucella in vitro, the tendency for reduced recovery of isolates from chronically infected swine, high costs associated with microbiologic testing, and biosafety concerns regarding working with B. suis isolates make diagnosis by bacterial isolation unfeasible in many situations. For these reasons, serologic testing has become the standard for diagnosis of swine brucellosis. Although there is some variation between strains, the smooth LPS of B. suis strains is recognized by monoclonal antibodies against both the A and M antigens, and also against common (C) LPS epitopes shared with cross-reacting strains.77 In comparison, monoclonal antibodies against the M antigen do not bind to B. abortus strains (A dominant), and monoclonal antibodies against the A antigen do not recognize B. melitensis strains (M dominant). As serologic tests used to diagnose brucellosis were mostly developed for detection of the A dominant, B. abortus O side-chain in infected cattle, this may be one reason why sensitivity and specificity of these diagnostic tests in swine are lower when compared to detection of bovine brucellosis. Although numerous studies are described in this review in which serologic tests had moderate levels of sensitivity and specificity, limitations in performance of the tests under field conditions generally mean that test interpretation is conducted at a group or herd level, rather than on individual swine. For example, recent publications reported that a panel of serologic tests were only able to identify 52% of naturally infected feral swine as seropositive,78 and in another study, 17% of culture-positive swine were negative on all serologic tests.79 In herds with positive serology, bacterial isolation or molecular assays should be used to confirm serologic data.
Brucellosis can also be diagnosed using molecular techniques such as the PCR. However, due to the high sensitivity of the technique and the fact that DNA rather than live organisms is being identified, proper controls should be included in each assay to ensure that positive responses are not due to laboratory contamination. A number of PCR assays have been described in the literature including early assays which differentiated between Brucella spp.80,81 and subsequently refined assays that allowed biovar typing within Brucella spp. including B. suis.22,82–84 These assays were further refined using data from available B. suis genomes for the development of real-time assays that allow rapid and inexpensive identification of Brucella with high sensitivity.22,85–88 These assays have been complemented with multiple-locus variable-number tandem-repeat PCR-based methods that allow strain comparisons in addition to species/biovar identification.22,89–91 In addition, a semiautomated metabolic system has also been proposed as having high specificity in differentiating Brucella spp. and biovars by metabolic profiles.92
With regard to serologic tests, ranges for estimates of sensitivity of standard tests for detecting brucellosis in swine are as follows: standard tube, 51.1%–100%; mercaptoethanol, 38.5%–100%; rivanol, 23.1%–100%; complement fixation test (CFT), 49.1%–100%; card test (Rose Bengal test [RBT]), 20%–100%; buffered plate antigen, 61%–77.1%; competitive enzyme-linked immunosorbent assay (c-ELISA), 89%–99%; and fluorescent polarization assay (FPA), 63%–98.9%.10,71,79,93–97 Ranges for estimates of specificity (SPs) are as follows: standard tube, 62%–100%; mercaptoethanol, 81.1%–100%; rivanol, 74%–100%; CFT, 86%–100%; RBT, 76%–92%; buffered plate agglutination, 90%–95.9%; and FPA, 55%–99.9%.10,79,94–96 When comparing c-ELISAs to an indirect enzyme-linked immunosorbent assay (ELISA), FPA, and RBT for diagnosis of swine brucellosis, data from two studies indicated that the c-ELISA had the highest sensitivity (mean estimates of sensitivity 95% and 96%) and the highest specificity (mean SPs 99% and 86% but equivalent SP with RBT in one study).43,98 However, a separate study of pigs naturally infected with B. suis biovar 2 found that the c-ELISA had lower sensitivity (68 to 93% dependent upon cut-off for ELISA) and was comparable in performance to the RBT, indirect ELISA, and a blocking-ELISA.99 One study proposed combining the c-ELISA, indirect ELISA, and FPA to increase specificity and sensitivity for the serologic diagnosis of swine brucellosis.100 Another study bioengineered a recombinant glycoprotein polysaccharide antigens of B. suis and demonstrated high sensitivity (98%) and specificity (100%) in biovar 1- and biovar 2-infected swine when used as the target antigen in an ELISA.101
More recently, studies have been conducted to develop supplemental serologic tests for swine brucellosis, which reduce false-positive reactions, particularly related to testing for biovar 2 infections in Europe. Tests that depend on the LPS O side-chain as antigen (eg, RBT, ELISAs, and FPA) had reduced diagnostic specificity in addressing false-positive reactions.102 However, gel immunodiffusion, counterimmunoelectrophoresis, latex agglutination, and indirect ELISAs using Brucella protein extracts free of the O-polysaccharide were reported to have high specificity and moderate sensitivity (45%–63%) for detecting B. suis and differentiating false-positive reactions on serologic tests.103 The delayed-type hypersensitivity reaction (widely known as Brucellin skin test) based on the use of LPS-free cytoplasmic proteins extracted from rough B. melitensis strain B115104 is also of value as a diagnostic to discriminate between B. suis infections and infections caused by Yersinia enterocolitica O:9 or other cross-reacting bacteria. The skin test does not cause cross-reacting antibodies that are reactive in RBT, CFT, or ELISA tests and has been proven effective at the herd level in pigs.105
Control of B. suis in domestic livestock or feral swine
There are currently no commercially available vaccines for protecting domestic or feral swine against B. suis infection. This may partly be due to a decreased emphasis on B. suis as compared to B. abortus and B. melitensis. This may be due to an assumption of reduced risk of human infection of B. suis as compared to the other two Brucella spp., a perception developed 30–40 years ago in developed countries that swine brucellosis was essentially controlled in domestic swine populations, the much higher prevalence in a wildlife reservoir as compared to low prevalence in domestic livestock in many countries, and changes in production systems for raising swine that reduce risk of exposure by increased emphasis of housing in confinement. As compared to vaccine development for B. abortus and B. melitensis, development of vaccines and intervention strategies is also hindered by a lack of a standardized repeatable challenge model that replicates infection and clinical disease in swine under field conditions. Although abortion storms have been reported with B. suis, our data from experimental challenge of pregnant swine suggest that it is more common for pregnancy to be maintained for a normal gestation period as long as several fetuses remain alive. Therefore, additional work on standardizing the experimental challenge model will be useful for the development of effective vaccines to prevent swine brucellosis. Although an efficacious vaccine would be a valuable tool, it must be assumed that as with brucellosis vaccines for large and small ungulates, a vaccine alone will not be sufficient to eradicate B. suis from a swine population. Rather, vaccination will most likely require combination with some other procedure (ie, test and slaughter, selective depopulation, etc) if disease eradication is the goal. Currently, total herd depopulation is a preferred regulatory method to control swine brucellosis, but this option is expensive and not feasible in many situations.
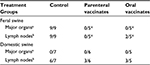
Although initial reports were promising, currently available data suggest that the oral B. suis strain 2 vaccine developed in the People’s Republic of China40 and the B. abortus strain RB51106 vaccine developed in the US do not adequately protect swine against B. suis infection. More recently, we have demonstrated the safety, immunogenicity, and efficacy of a natural rough strain of B. suis (strain 353-1) as a brucellosis vaccine for swine.107 When delivered subcutaneously (1010 colony-forming units) to swine, the strain is nonpathogenic, does not localize in undesirable tissue locations, and is cleared in ~10–12 weeks. Because it lacks expression of the O side-chain, vaccinated animals remain seronegative on brucellosis surveillance tests. We have demonstrated significantly enhanced cellular immune responses in vaccinates as compared to nonvaccinates after parenteral or oral delivery. As compared to nonvaccinated swine, parenteral-vaccinated animals demonstrated significant protection against infection following conjunctival challenge with a virulent B. suis strain.107 Similar efficacy results were noted after oral delivery of 353-1, although protection in oral vaccinates against experimental challenge (tissues infected and colonization [colony-forming units/g]) was slightly less than in parenteral vaccinates (Table 1). Infection and colonization were greater in nonvaccinated swine as compared to recovery of B. suis from from swine vaccinated with 353-1. As the studies included evaluation of the vaccine in feral swine, the 353-1 vaccine strain appears to be a promising intervention strategy for reducing the prevalence of brucellosis in wild swine populations.
Although other vaccine strains have been proposed as efficacious against B. suis, most have only been evaluated in laboratory animal models, and data on their immunogenicity and/or efficacy in swine are not currently available in the literature. Although inbred mice have some value as laboratory animal models of brucellosis, they are not natural hosts of B. suis, and immune mechanisms, disease pathogenesis, and vaccine efficacy significantly differ when comparing heterozygous swine populations to inbred mouse lines. Although mechanisms of protection against Brucella may have similarities across natural hosts, studies have demonstrated significant differences between species in immunologic responses and/or immune regulation after vaccination, which indicates that vaccine development must be conducted in the targeted host species.
With some diseases, there is interest in whether natural (genetic) resistance to disease occurs within natural hosts. Of interest was the observation that this hypothesis was explored decades ago in a study which identified swine that were resistant to B. suis infection.108 Although we question whether this approach would be an effective approach to control brucellosis in the swine industry, we found the publication to be of historical interest and relevant to this review.
As test and removal strategies are not economically feasible under all circumstances, a number of studies have evaluated the ability of antibiotic treatment to control brucellosis in host species, including swine.109–112 In general, these studies reported that although disease prevalence was reduced, cost of treatment and persistence of infection in some treated animals made long-term antibiotic therapy nonviable as a regulatory strategy. More recently, a small study of naturally infected swine (n=8/treatment) found that oral treatment with oxytetracycline (20 mg/kg/daily for 21 days) eliminated B. suis biovar 2 infection from only 50% of infected swine.113 When oral oxytetracycline therapy was combined with the macrolide antibiotic tilidipirosin (4 mg/kg administered intramuscularly on days 1 and 10), the authors were unable to recover B. suis from treated swine at 21 days. In a larger field study, oral treatment of naturally infected swine (B. suis biovar 2) with oxytetracycline (20 mg/kg/daily) was not sufficient to eradicate brucellosis in infected herds.114 However, when combined with removal of infected animals, as identified by one of three diagnostic tests (Brucellin skin test or RBT and indirect ELISA serologic tests), brucellosis was eradicated from infected herds in ~16 months.114
B. suis as a human pathogen
Swine brucellosis in humans is most frequently a disease of farm workers, veterinarians, and abattoir workers, but it can also be contracted through other activities such as hunting or other associations with feral swine.115–116 Direct contact with infected animals, or materials associated with abortion, can lead to human infection through aerosolization into respiratory tissues, oral consumption, or opportunistic penetration through breaks in the epidermis. Processing of infected swine through an abattoir setting has been known for decades to be associated with a high risk of infection of human workers and differs from the low risks associated with handling of seropositive natural hosts of other Brucella spp. (ie, cattle, sheep, and goats).117–120 Some data suggest that a risk of zoonotic infection with B. suis is associated with handling meat from infected swine.40 Zoonotic infection with B. suis through raw milk remains a public health concern as infected cattle are generally asymptomatic but can shed high levels of bacteria in milk.50,121–123 Historically, consumption of unpasteurized milk from B. suis-infected cattle resulted in numerous human infections as compared to sporadic occurrence of infection associated with consumption of unpasteurized milk from cattle infected with B. abortus.123 As mentioned previously, addressing the disease in the animal host has been demonstrated to be the most economical approach for prevention of human brucellosis, and countries in which brucellosis is controlled in animals are associated with a low incidence of human brucellosis.
Human brucellosis is generally a chronic disease with insidious onset and clinical symptoms developing over a period of weeks to months after exposure. The pathophysiology of brucellosis in humans generally differs from the characteristics of brucellosis in reservoir hosts. Clinical symptoms in humans are not pathognomonic and can include recurrent pyrexia (undulant fever), cephalagia, malaise, joint and muscle pain, night sweats, and even neurologic manifestations. Brucella can distribute to almost any tissue or in vivo site with clinical symptoms related to inflammatory lesions associated with bacterial localization. Osteoarticular disease is the most common complication and can include peripheral arthritis, sacroilitis, and spondylitis. Even untreated, human brucellosis is generally associated with low mortality. Relapse of infection is common, but, in part due to a lack of natural plasmids in Brucella, relapses are usually not associated with emergence of antibiotic-resistant strains.124 More recently, infection with Brucella has recently been linked to the occurrence of brain neoplasms in humans. Although linked to 25% of medulloblastomas, 60% of glioblastomas, and 25% of metastatic carcinomas, DNA sequences recovered by PCR amplification from formalin-fixed tissues did not allow specific differentiation to B. abortus, B. suis, or B. melitensis species.125
Methodology used to find cited literature for this review
This paper was prepared based upon the >50-year combined experience in brucellosis research by the authors, and a systematic review of PubMed using the terms “swine brucellosis” and “Brucella suis”. The review was also augmented by relevant scientific papers on swine brucellosis identified within a file of research publications maintained within the Brucellosis Research Project at the National Animal Disease Center in Ames, IA, for over 60 years. Literature related to vaccine development was limited to data obtained on natural hosts of B. suis.
Conclusion
B. suis remains a significant threat for human zoonotic infection worldwide, although differences in risk of exposure occur geographically. Feral or wild populations of swine remain as significant reservoirs for transmission of swine brucellosis to domestic livestock and humans. There is a need for improved serologic tests as current diagnostic tests are best utilized at a herd, rather than at an individual animal level. There is also a need for safe and efficacious vaccines for preventing brucellosis in swine which could be used in intervention strategies to address this disease. Although not feasible in all situations, whole-herd depopulation is the most effective regulatory mechanism to control swine brucellosis. Novel approaches using antibiotic treatment of infected swine herds have been shown to be beneficial but alone are not sufficient for disease control. Epidemiologic data would suggest that the incidence and geographic range of swine brucellosis will continue to expand. Due to limited tools for reducing the high prevalence of disease in natural hosts and its significant virulence in humans, current knowledge suggests that B. suis will remain as one of the most important zoonotic pathogens across the world.
Disclosure
The authors report no conflicts of interest in this work.
References
Roth F, Zinsstag J, Orkhon D, et al. Human health benefits from livestock vaccination for brucellosis: case study. Bull World Health Organ. 2003;81(12):867–876. | ||
Jelastopulu E, Bikas C, Petropoulos C, Leotsinidis M. Incidence of human brucellosis in a rural area in Western Greece after the implementation of a vaccination programme against animal brucellosis. BMC Public Health. 2008;8:241. | ||
Zinsstag J, Schelling E, Roth F, Bonfoh B, de Savigny D, Tanner M. Human benefits of animal interventions for zoonosis control. Emerg Infect Dis. 2007;13(4):527–531. | ||
Olsen SC, Palmer MV. Advancement of knowledge of Brucella over the past 50 years. Vet Pathol. 2014;51(6):1076–1089. | ||
Verger JM, Grimont F, Grimont PAD, Grayon M. Brucella a monospecific genus as shown by deoxyribonucleic acid hybridization. Int J Syst Bacteriol. 1985;35:292–295. | ||
Verger JM, Grayon M, Cloeckaert A, Lefevre M, Ageron E, Grimont F. Classification of Brucella strains isolated from marine mammals using DNA-DNA hybridization and ribotyping. Res Microbiol. 2000;3(9):797–799. | ||
Foster JT, Beckstrom-Sternberg SM, Pearson T, et al. Whole-genome-based phylogeny and divergence of the genus Brucella. J Bacteriol. 2009;191(8):2864–2870. | ||
Alton GG, Jones LM, Angus RD, Verger JM. Techniques for the Brucellosis Laboratory. Paris: INRA Publications; 1988. | ||
Garin-Bastuji B, Vaillant V, Albert D, et al. Is brucellosis due to biovar 2 of Brucella suis an emerging zoonosis in France? Two case reports in wild boar and hare hunters. In: Proceedings of the International Society of Chemotherapy Disease Management meeting, 1st international meeting on treatment of human brucellosis; 2006. | ||
Olsen SC, Garin-Bastuji B, Blasco JM, Nicola AM, Samartino L. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Hokoben: Wiley-Blackwell; 2012:697–708. | ||
Traum J. Annual report to the chief of the Bureau of Animal Industry, year ending June 30, 1914. USDA Bureau of Animal Industry; 1914:30. | ||
Frye GH. Swine brucellosis – a vanishing disease. Proc Annu Meet US Anim Health Assoc. 1983;87:137–146. | ||
Huddleston IF. The differentiation of the species of the genus Brucella. Bull Mich Agric Exp Sta. 1929;100:1–16. | ||
Stoffregen WC, Olsen SC, Wheeler J, et al. Diagnostic characterization of a feral swine herd enzootically infected with Brucella. J Vet Diagn Invest. 2007;19(3):227–237. | ||
Higgins J, Stuber T, Quance C, et al. Molecular epidemiology of Brucella isolates from cattle, elk, and bison in the United States, 1998 to 2011. Appl Environ Microbiol. 2012;78(10):3674–3684. | ||
Roani Z, Kreizinger Z, Dan A, et al. First isolation and characterization of Brucella microti from wild boar. BMC Vet Res. 2015;11:147. | ||
Tae H, Shallom S, Settlage R, Preston D, Adams LG, Garner HR. Revised genome sequence of Brucella suis 1330. J Bacteriol. 2011;193(22):6410. | ||
Paulen IT, Seshadri R, Nelson KE, et al. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc Natl Acad Sci U S A. 2002;99(20):13148–13153. | ||
Wattam AR, Foster JT, Mane SP, et al. Comparative phylogenomics and evolution of the Brucellae reveal a path to virulence. J Bacteriol. 2014;196(5):920–930. | ||
Schneiker S, Keller M, Droge M, Lanka E, Puhler A, Selbitschka W. The genetic organization and evolution of the broad host range mercury resistance plasmid pSB102 isolated from a microbial population residing in the rhizosphere of alfalfa. Nucleic Acids Res. 2001;29(24):5169–5181. | ||
Sankarasubramanian J, Vishnu US, Gunasekaran P, Rajendran J. A genome-wide SNP-based phylogenetic analysis distinguishes different biovars of Brucella suis. Infect Genet Evol. 2016;20(6):375–385. | ||
Lopez-Goni I, Garcia-Yoldi D, Marin CM, et al. New Bruce-ladder multiplex assay for the biovar typing of Brucella suis and the discrimination of Brucella suis and Brucella canis. Vet Microbiol. 2011;154(1–2):152–155. | ||
Tay BY, Ahmad N, Hashim R, et al. Multiple-locus variable-number tandem-repeat analysis (MLVA) genotyping of human Brucella isolates in Malaysia. BMC Infect Dis. 2015;15:220. | ||
Duvnjak S, Racic I, Spicic S, Zdelar-Tuk M, Reil I, Cvetnic Z. Characterisation of Brucella suis isolates from Southeastern Europe by multi-loci variable-number tandem repeat analysis. Vet Microbiol. 2015;180(1–2):146–150. | ||
Kamath PL, Foster JT, Drees KP, et al. Genomics reveals historic and contemporary transmission dynamics of a bacterial disease among wildlife and livestock. Nat Commun. 2016;7:11448. | ||
Quance C, Robbe-Austerman S, Stuber T, et al. Identification of source of B. suis infection in human by using whole-genome sequencing, United States and Tonga. Emerg Infect Dis. 2016;22(1):79–82. | ||
van der Leek ML, Becker HN, Humphrey P, et al. Prevalence of Brucella sp. antibodies in feral swine in Florida. J Wildlife Dis. 1993;29(3):410–415. | ||
Wood GW, Hendricks JB, Goodman DE. Brucellosis in feral swine. J Wildlife Dis. 1976;12(4):579–581. | ||
Becker HN, Belden RC, Breault T, Burridge MJ, Frankenberger WB, Nicoletti P. Brucellosis in feral swine in Florida. J Am Vet Med Assoc. 1978;173(9):1181–1182. | ||
Cook DR, Noble JW. Isolation of B. suis from cattle. Aust Vet J. 1984;61(8):263–264. | ||
Cvetnic Z, Spicic S, Jukic B, et al. Brucella suis infection in domestic pigs and wild boar in Croatia. Rev Sci Tech. 2009;28(3):1057–1067. | ||
Abril C, Thomann A, Brodard I, et al. A novel isolation method of Brucella species and molecular tracking of Brucella suis biovar 2 in domestic and wild animals. Vet Microbiol. 2011;150(3–4):405–410. | ||
Lavín S, Blasco JM, Velarde R, Mentaberre G, Casas E, Marco I. Descripción del primer caso de brucelosis en la liebre europea (Lepus europaeus) en la Península Ibérica [Description of the first case of brucellosis in the European hare (Lepus europaeus) in the Iberian Peninsula]. Información Veterinaria. 2006;10:18–21. Spanish. | ||
Godfroid J, Cloeckaert A, Liautard JP, et al. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res. 2005;36(3):313–326. | ||
Samartino LE. Brucellosis in Argentina. Vet Microbiol. 2002;90(1–4):71–80. | ||
Poester FP, Goncalves VS, Lage AP. Brucellosis in Brazil. Vet Microbiol. 2002;90(1–4):55–62. | ||
Luna-Martínez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1–4):19–30. | ||
Meirelles-Bartoli RB, Mathias LA, Samartino LE. Brucellosis due to Brucella suis in a swine herd associated with a human clinical case in the state of Sao Paulo, Brazil. Trop Anim Health Prod. 2012;44(7):1575–1579. | ||
Lucero NE, Ayala SM, Escobar GI, Jacob NR. Brucella isolated from humans and animals in Latin America from 1968 to 2006. Epidemiol Infect. 2008;136(4):496–503. | ||
Deqiu S, Donglou X, Jiming Y. Epidemiology and control of brucellosis in China. Vet Microbiol. 2002;90(1–4):165–182. | ||
Jiang H, Chen H, Tian G-Z, et al. Genetic comparison of Brucella suis biovar 3 in clinical cases in China. Vet Microbiol. 2012;160(3–4):546–548. | ||
Naha K, Dasari S, Pandit V, Shubha Seshadri. A rare case of seronegative culture-proven infection with Brucella suis. Australas Med J. 2012;5(7):340–343. | ||
Nagalingam M, Shome R, Balamurugan V, et al. Molecular typing of Brucella species isolates from livestock and human. Trop Anim Health Prod. 2012;44(1):5–9. | ||
Kutlu M, Cevahir N, Erdenlig-Gurbilek S, Akalin S, Ucar M, Sayin-Kutlu S. The first report of Brucella suis isolation in human in Turkey. J Infect Public Health. Epub 2016 Mar 3. | ||
Praud A, Gimenez O, Zanella G, et al. Evaluation of five serologic tests for the diagnosis of porcine brucellosis in French Polynesia. Trop Anim Health Prod. 2013;45(4):931–933. | ||
van der Giessen JWB, Priadi A. Swine brucellosis in Indonesia. Vet Q. 1988;10(3):172–176. | ||
McDermott JJ, Arimi SM. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol. 2002;90(1–4):111–134. | ||
Menshawy AM, Perez-Sancho M, Garcia-Seco T, et al. Assessment of genetic diversity of zoonotic Brucella spp. recovered from livestock in Egypt using multiple locus VNTR analysis. Biomed Res Int. 2014;2014:353876. | ||
Ledwaba B, Mafofo J, van Heerden H. Genome sequences of Brucella abortus and Brucella suis strains isolated from bovine in Zimbabwe. Genome Announc. 2014;2(5):e01063-14. | ||
Ewalt DR, Payeur JB, Rhyan JC, Geer PL. Brucella suis biovar 1 in naturally infected cattle: a bacteriological, serological, and histological study. J Vet Diagn Invest. 1997;9(4):417–420. | ||
Kin MS, Fort M, de Echaide ST, Casanave EB. Brucella suis in armadillos (Chaetophractus villosus) from La Pampa Argentina. Vet Microbiol. 2014;170(3–4):442–445. | ||
Paolichchi FA, Terrzolo HR, Campero CM. Isolation of Brucella suis from the semen of a ram. Vet Record. 1993;132(3):67. | ||
Ramamoorthy S, Woldemeskel M, Ligett A, Snider R, Cobb R, Rajeev S. Brucella suis infection in dogs, Georgia, USA. Emerg Infect Dis. 2011;17(12):2386–2387 | ||
Sting R, Schwabe I, Oehme R, Elschner MC, Melzer F. First report of a Brucella suis infection in rose deer (Capreolus capreolus). Berl Munch Tierarzti Wochenschr. 2014;127(3–4):120–122. | ||
Cvetnic Z, Spicic S, Curic S, et al. Isolation of Brucella suis biovar 3 from horses in Croatia. Vet Rec. 2005;156(18):584–585. | ||
Ma JY, Wang H, Zhang XF, et al. MLVA and MLST typing of Brucella from Qinghai, China. Infect Dis Poverty. 2016;5:26. | ||
Cutler SJ, Whatmore AM, Commander NJ. Brucellosis - new aspects of an old disease. J Appl Microbiol. 2005;98(6):1270–1281. | ||
Fugier E, Salcedo SP, de Chastellier C, et al. The glyceraldehyde-3-phosphate dehydrogenase and the small GTPase Rab2 are crucial for Brucella replication. PLoS Pathogens. 2009;5(6):e100487. | ||
Roop RM, Gee JM, Robertson GT, Richardson JM, Ng WL, Winkler ME. Brucella stationary-phase gene expression and virulence. Annu Rev Micriobiol. 2003;57:57–76. | ||
Bellaire BH, Elzer PH, Baldwin CL, Roop RM. Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro. Infect Immun. 2003;71(5):2927–2832. | ||
Jimenez de Bagüés MP, Terraza A, Gross A, Domand J. Different responses of macrophages to smooth and rough Brucella spp.: relationship to virulence. Infect Immun. 2004;72(4):2429–2433. | ||
Gross A, Terraza A, Ouahrani-Bettache S, Liautard JP, Domand J. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect Immun. 2000;68(1):342–351. | ||
Hutchings LM, Delez AL, Donham CR. Studies on brucellosis of swine: I. Infection experiments with weanling pigs. Am J Vet Res. 1944;5:195–208. | ||
Johnson HW, Huddleson IF. Natural Brucella infection in swine. J Am Vet Med Assoc. 1931;78:849–862. | ||
Manthei CA. Aspectos clínicos y patológicos de la brucelosis suína [Clinical and pathological aspects of brucellosis suina]. Rev Nac Hig. 1970;7:105–106. Spanish. | ||
Manthei CA, Deyoe BL. Brucellosis. In: Dunne HW, editor. Diseases of Swine. 3rd ed. Ames, IA: Iowa State University Press; 1970:433–456. | ||
Thomseon A. Brucella infection in swine. Studies from an epizootic in Denmark 1929-1932. Acta Pathol Microbiol Scand. 1934;Suppl 21:115–130. | ||
Hutchings LM, Delez AL, Donham CR. Studies on brucellosis in swine II: exposure and reexposure experiments with Brucella suis. Am J Vet Res. 1946;7:11. | ||
Hutchings LM, Delez AL, Donham CR. Brucellosis in swine V: reproduction studies with naturally infected sows and boars. Am J Vet Res. 1946;7(25):388–394. | ||
Manthei CA, Mingle CK, Carter RW. Brucella suis infection in suckling and weanling pigs. I. J Am Vet Med Assoc. 1952;121(908):373–382. | ||
Deyoe BL. Research finds applicable to eradication of swine brucellosis. Proc Annu Meet US Anim Health Assoc. 1972;76:108–114. | ||
Hutchings LM, Andrews FN. Studies on brucellosis in swine: III: Brucella infection in the boar. Am J Vet Res. 1946;7:379–384. | ||
Manthei CA. Brucellosis. In: Dunne HW, editor. Diseases of Swine. 2nd ed. Ames, IA: Iowa State University Press; 1964:338–340. | ||
Olsen SC. Recent developments in livestock and wildlife brucellosis vaccination. Rev Sci Tech. 2013;32(1):207–217. | ||
Kagan JC, Iwasaki A. Phagosome as the organelle linking innate and adaptive immunity. Traffic. 2012;13(8):1053–1061. | ||
Barquero-Calvo E, Chaves-Olarte E, Weiss DS, et al. Brucella abortus use a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS One. 2007;2(7):e631. | ||
Douglas JT, Palmer DA. Use of monoclonal antibodies to identify the distribution of A and M epitopes on smooth Brucella species. J Clin Microbiol. 1988;26(7):1353–1356. | ||
Pedersen K, Quance CR, Robbe-Austerman S, et al. Identification of Brucella suis from feral swine in selected states in the USA. J Wildlife Dis. 2014;50(2):171–179. | ||
Ferris RA, Schoenbaum MA, Crawford RP. Comparison of serologic tests and bacteriologic culture for detection of brucellosis in swine from naturally infected herds. J Am Vet Med Assoc. 1995;207(10):1332–1333. | ||
Bricker BJ, Halling SM. Differentiation of Brucella abortus bv 1, 2, and 4 and Brucella melitensis, Brucella ovis, and Brucells suis bv1 by PCR. J Clin Microbiol. 1994;32(11):2660–2666. | ||
Ratushna VG, Sturgill DM, Ramamoorthy S, et al. Molecular targets for rapid identification of Brucella spp. BMC Microbiol. 2006;6:13. | ||
Nan W, Tan P, Wang Y, et al. Duplex PCR for differentiation of the vaccine strain Brucella suis S2 and B. suis biovar 1 from other strains of Brucella spp. Vet J. 2014;201(3):427–428. | ||
Huber B, Scholz HC, Lucero N, Busse HJ. Development of a PCR assay for typing and subtyping of Brucella species. Int J Med Microbiol. 1009;299(8):563–573. | ||
Ferrao-Beck L, Cardoso R, Munoz PM, et al. Development of a multiplex PCR assay for polymorphism analysis of Brucella suis biovars causing brucellosis in swine. Vet Microbiol. 2006;115(1–3):269–277. | ||
Redkar R, Rose S, Bricker B, DelVecchio V. Real-time detection of Brucella abortus, Brucella melitensis, and Brucella suis. Mol Cell Probes. 2011;15(1):43–52. | ||
Hansel C, Mertens K, Eischener MC, Melzer F. Novel real-time PCR detection assay for Brucella suis. Vet Rec Open. 2015;2(1):e00084. | ||
Fretin D, Whatmore AM, Al Dahouk S, et al. Brucella suis identification and biovar typing by real-time PCR. Vet Microbiol. 2008;131(3–4):376–385. | ||
Lõpez-Goñi I, Garcia-Yoldi D, Marin CM, et al. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella strains including the vaccine strains. J Clin Microbiol. 2008;46(10):3484–3487. | ||
Garcia-Yoldi D, Le Fleche P, De Miguel MJ, et al. Comparison of multiple-locus variable-number tandem-repeat analysis with other PCR-based methods for typing Brucella suis isolates. J Clin Microbiol. 2007;45(12):4070–4072. | ||
Bricker BJ, Ewalt DR, Halling SM. Brucella ‘HOOF-Prints’ strain typing by multi-locus analysis of variable number tandem repeats (VNTRs). BMC Microbiol. 2003;3:15. | ||
Le Fleche P, Jacques I, Grayon M, et al. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 1006;6:9. | ||
Al Dahouk S, Scholz HC, Tomaso H, et al. Differential phenotyping of Brucella species using a newly developed semi-automated metabolic system. BMC Microbiol. 2010;10:269. | ||
García-Carillo C, Cedro VCF, de Benedettí LME. Evaluación de técnicas serológicas en cerdos con infección reciente de Brucella suis [Evaluation of Serological Techniques in Recent Infection of Pigs with Brucella suis]. Buenos Aires: Revista de Investigaciones Agropecuarías, INTA; 1971:99–107. Spanish. | ||
Rogers RJ, Cook DR, Ketterer PJ. An evaluation of three serological tests for antibody to Brucella suis in pigs. Aust Vet J. 1989;66(3):77–80. | ||
Lord VR, Cherwonogrodzky JW, Melendez G. Serological and bacteriological study of swine brucellosis. J Clin Microbiol. 1997;35(1):295–297. | ||
Nielsen K, Gall D, Smith P, et al. Validation of the fluorescence polarization assay as a serological test for the presumptive diagnosis of porcine brucellosis. Vet Microbiol. 1999;68(3–4):245–253. | ||
Paulo PS, Vigliocco AM, Ramondina RF, et al. Evaluation of primary binding assays for presumptive serodiagnosis of swine brucellosis in Argentina. Clin Diagn Lab Immunol. 2000;7(5):828–831. | ||
Praud A, Gimenez O, Zanella G, et al. Estimation of the sensitivity and specificity of five serological tests for the diagnosis of porcine brucellosis. Prev Vet Med. 2012;104(1–2):94–100. | ||
Munoz PM, Blasco JM, Engel B, et al. Assessment of performance of selected serological tests for diagnosing brucellosis in pigs. Vet Immunol Immunopathol. 2012;146(2):150–158. | ||
Di Febo T, Luciani M, Portanti O, Bonfini B, Lelli R, Tittarelli M. Development and evaluation of diagnostic tests for the serologic diagnosis of brucellosis in swine. Vet Ital. 2012;48(2):133–156. | ||
Cortina ME, Balzano RE, Serantes DAR, et al. A bacterial glycoengineered antigen for improved serodiagnosis of porcine brucellosis. J Clin Microbiol. 2016;54(6):1448–1455. | ||
McGiven JA, Nicola A, Commander NJ, et al. An evaluation of the capability of existing and novel serodiagnostic methods for porcine brucellosis to reduce false positive serological reactions. Vet Microbiol. 2012;160(3–4):378–386. | ||
Dieste-Perez L, Blasco JM, de Miquel MJ, Moriyon I, Munoz PM. Diagnostic performance of serological tests for swine brucellosis in the presence of false positive serological reactions. J Microbiol Methods. 2015;111:57–63. | ||
Bhongbhibhat N, Elberg S, Chen TH. Characterization of Brucella skin-test antigens. J Infect Dis. 1970;122(1):70–81. | ||
EFSA (European Food Safety Authority). AHAW Panel (ESA Panel on Animal Health and Welfare). Porcine brucellosis (Brucella suis). EFSA J. 2009;1144:1–112. | ||
Edmonds MD, Samartino LE, Hoyt PG, et al. Oral vaccination of sexually mature pigs with Brucella abortus strain RB51. Am J Vet Res. 2001;62(8):1328–1331. | ||
Stoffregen WC, Johnson CS, Olsen SC. Immunogenicity and safety of a natural rough mutant of Brucella suis as a vaccine for swine. Res Vet Sci. 2013;95(2):451–458. | ||
Cameron HS, Hughes EH, Gregory PW. Studies on genetic resistance in swine to Brucella infection. Cornell Vet. 1940;30:218–222. | ||
Bunnell DE, Hutching LM, Donham CR. Effects of penicillin on Brucella suis in vitro and in vivo. Am J Vet Res. 1947;8(29):367–373. | ||
Radwan AI, Bekairi SI, al-Bokmy AM, Prasad PV, Mohamed OM, Hussain ST. Successful therapeutic regimens for treating Brucella melitensis and Brucella abortus infections in cows. Rev Sci Tech. 1993;12(3):909–922. | ||
Radwan AI, Bekairi SI, Mukayel AA. Treatment of Brucella melitensis infection in sheep and goats with oxytetracycline combined with streptomycin. Rev Sci Tech. 1992;11(3):845–857. | ||
Nicoletti P, Milward FW, Hoffmann E, Alvater L. Efficacy of long-acting oxytetracycline alone or combined with streptomycin in the treatment of bovine brucellosis. J Am Vet Med Assoc. 1985;187(5):493–495. | ||
Dieste-Pérez L, Fraile L, de Miguel MJ, Barberan M, Blasco JM, Munoz PM. Studies on a suitable antibiotic therapy for treating swine brucellosis. J Vet Pharm Ther. 2015;38(4):357–364. | ||
Diete-Pérez L, Frankena K, Blasco JM, Munoz PM, de Jong MC. Efficacy of antibiotic treatment and test-based culling strategies for eradicating brucellosis in commercial swine herds. Prev Vet Med. 2016;126:105–110. | ||
Rubson JM, Harrison MW, Wood RN, Tilse MH, McKay AB, Brodribb TR. Brucellosis: re-emergence and changing epidemiology in Queensland. Med J Aust. 1993;159(3):153–158. | ||
Centers for Disease Control and Prevention. Brucella suis infection associated with feral swine hunting – three states, 2007-2008. Morb Mortal Wkly Rep. 2009;58(22):618–621. | ||
Huddleson F, Johnson HW, Hamann EE. A study of Brucella infection in swine and employees of packing-houses. J Am Vet Med Assoc. 1933;83:16–30. | ||
Hendricks SL, Borts IH, Heren RH, Hausler WJ, Held JR. Brucellosis outbreak in an Iowa packing house. Am J Public Health. 1962;52(7):1166–1178. | ||
Heineman HW, Dziamski IM. Brucella suis infection in Philadelphia. Am J Epidemiol. 1976;103(1):88–100. | ||
Trout D, Gomez TM, Bernard BP, et al. Outbreak of brucellosis at a United States pork packing plant. J Occup Environ Med. 1995;37(6):697–703. | ||
Beattie CP, Rice RM. Undulant fever due to Brucella of the porcine type—Brucella suis. Report of a milk-borne epidemic. JAMA. 1934;102(20):1670–1674. | ||
Borts IH, Harris DM, Joynt MF, Jennings JR, Jordan CF. A milk-borne epidemic of brucellosis caused by the porcine type of Brucella (Brucella suis) in a raw milk supply. JAMA. 1943;121(5):319–325. | ||
Jordan CF, Borts IH, Harris DM, Jennings JR. Brucellosis: consideration of its epidemiology, diagnosis and control. Am J Public Health Nations Health. 1943;33(7):773–779. | ||
Pappas G, Akritidis N, Bosikovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352(22):2325–2336. | ||
Zhang B, Izadjoo M, Horkayne-Szakaly I, Morrison A, Wear DJ. Medulloblastoma and brucellosis – molecular evidence of Brucella sp in association with central nervous system cancer. J Cancer. 2011;2:136–141. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.