Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Survival Time to COVID-19 Severity and Its Predictors in South Gondar Zone, North-West Ethiopia: A Prospective Cohort Study
Authors Yemata GA , Tesfaw A , Mihret G , Tiruneh M , Walle Z , Molla E, Sisay E , Admassu FT , Habtie E, Desalagn T , Shimels H , Teshome F
Received 14 February 2022
Accepted for publication 19 May 2022
Published 23 May 2022 Volume 2022:15 Pages 1187—1201
DOI https://doi.org/10.2147/JMDH.S361061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Getaneh Atikilt Yemata,1 Aragaw Tesfaw,1 Gashaw Mihret,2 Mulu Tiruneh,1 Zebader Walle,1 Eshetie Molla,1 Ermias Sisay,3 Fitalew Tadele Admassu,4 Eyaya Habtie,5 Tsion Desalagn,1 Habtamu Shimels,3 Fentaw Teshome1
1Department of Public Health, College of Health Sciences, Debre Tabor University, Debre Tabor, Amhara Region, Ethiopia; 2Department of Pediatrics, College of Health Sciences, Debre Tabor University, Debre Tabor, Amhara Region, Ethiopia; 3Department of Pediatrics and Child Health Nursing, College of Health Sciences, Debre Tabor University, Debre Tabor, Amhara Region, Ethiopia; 4Department of Biomedical Science, College of Health Sciences, Debre Tabor University, Debre Tabor, Amhara Region, Ethiopia; 5Department of Midwifery, College of Health Sciences, Debre Tabor University, Debre Tabor, Amhara Region, Ethiopia
Correspondence: Getaneh Atikilt Yemata, Email [email protected]
Background: Coronavirus disease is still a global public health emergency. Due to an inadequate healthcare system in low-income nations like Ethiopia, the pandemic has had a devastating impact. Despite this, information on the severity of COVID-19 and related difficulties in Ethiopia is sparse. Therefore, we aimed to determine the survival time to severity and predictors of COVID-19 in Northwest Ethiopia.
Methods: A prospective follow-up study was conducted among 202 adult COVID-19 patients in the South Gondar zone treatment centers. Data were entered using EpiData version 3.1 and then exported to Stata 16 for analysis. Kaplan–Meier was used to estimate mean survival time, and Log rank tests were used to compare survival time between explanatory variable groups. A cox-proportional hazards regression model with a 95% confidence interval and a p-value of 0.05 was used to identify covariates associated with the outcome variable.
Results: The patients’ average age was 41.2 years. With an IQR of 4– 7 days, the median time to COVID-19 severity was 5 days. The overall COVID-19 severity rate was 6.35 (95% CI: 5.17– 7.86) per 100 person-days observed. Senior adult age group (51– 59 years) (AHR = 3.59, 95% CI: 1.05, 12.23), elderly age group (≥ 60 years) (AHR = 2.11, 95% CI: 1.09, 12.67), comorbidity (AHR = 3.26, 95% CI: 1.48, 7.18), high blood pressure at admission (AHR = 4.36, 95% CI: 1.99, 9.54), and high temperature at admission (AHR = 5.60, 95% CI: 2.55, 12.46) were significantly associated with COVID-19 severity time.
Conclusion and Recommendation: Patients with COVID-19 had a short median severity time, and factors like older age, comorbidity, high temperature, and high blood pressure were all independent predictors of severity time. Patients with high body temperature, blood pressure, comorbidity, and advanced age should be the focus of interventions to reduce progression time and improve clinical outcomes.
Keywords: COVID-19, time to severity, predictors, South Gondar zone, Ethiopia
Introduction
The world is closely watching the outbreak of respiratory illness associated with the novel beta coronavirus.1 Initially, the illness was termed 2019 – novel coronaviruses (n CoV), now officially named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).2,3 Following the SARS outbreak, five additional coronaviruses have been discovered in humans, most recently, the Corona Virus Disease-2019 (COVID-19) believed to have originated in Wuhan, China, is particularly pathogenic in humans and is associated with high mortality rates.4,5
According to the World Health Organization (WHO), Coronavirus disease continues to emerge and represents a serious issue to public health due to the severe infectivity and pathogenicity which has been classified as public health emergency of international concern.6
Globally COVID-19 is the major public health burden due to its dramatic increase in morbidity and mortality rate from time to time.7 In the WHO African region, in 2020, South Africa, Ethiopia, Algeria, Kenya, and Nigeria were the countries that reported the highest number of COVID-19 confirmed cases.8
The clinical features of patients infected with COVID-19 include fever, cough, myalgia, fatigue, and shortness of breath.1 Respiratory viruses are most contagious when a patient is symptomatic. However, there is an increasing body of evidence to suggest that human-to-human transmission may be occurring during the asymptomatic incubation period of COVID-19, which has been estimated to be between 2 and 10 days. People of all ages are susceptible to COVID-19. The elderly and those with underlying chronic diseases are more likely to become severe cases.9
The disease severity was classified into different categories (mild, moderate, severe, or critical) based on the patients’ clinical symptoms, radiologic assessment, and routine laboratory investigations to help health-care workers prioritize treatment for patients with more severe diseases and to reduce the burden on health-care systems caused by COVID-19. Mild cases are characterized by fever, malaise, cough, upper respiratory symptoms, and/or less common features of COVID-19 (headache, loss of taste or smell) and no pneumonia on a lung CT. If the patients have a fever, cough, and a lung CT with pneumonia but can maintain oxygenation on room air, they are considered moderate. Patients who presented with respiratory distress (respiratory rate >30 breaths/min), hypoxia (oxygen saturation ≤93% at rest and/or ratio of arterial oxygen partial pressure to fractional inspired oxygen ≤300 mmHg), and more than 50% involvement seen on chest imaging were classified as severe cases, whereas those who presented with the aforementioned criteria of respiratory failure receiving mechanical ventilation, shock, and/or organ failure other than lung and/or ICU involvement were classified as critical cases.10–12
In Africa, where most countries have weak health-care systems, including inadequate surveillance and laboratory capacity, scarcity of public health human resources, and limited financial means, the pandemic may have a devastating effect.13 Furthermore, multiple health challenges of the continent like; rapid population growth and increased movement of people; existing endemic diseases, such as human immunodeficiency virus, tuberculosis, malaria, and increasing incidence of non-communicable diseases might increase the spread and advancement of the disease.14
Poor prevention practices can lead to economic, social, and political crises, and an increased risk of death.15 The benefits of COVID prevention, particularly in the developing setting like Ethiopia are invaluable because of the deprived health system.15–17 Therefore, evidence-based prevention practices are critical to combat the spread and advancement of Coronavirus diseases.18
Studies showed that more than a quarter of COVID-19 patients progressed to severe illnesses. In addition, the case fatality rate of severely ill patients was reported high and reaches up to 65%.6 COVID-19 patients with severe disease exert great pressure on the shortage of intensive care resources. To combat these challenges, it is a necessary issue to identify and predict those seemingly-mild cases promisingly progressing to critical cases.1,19 Therefore, evidence on survival time to severity and predictors of COVID −19 severity is important to postulate a strategy to reduce the transition from mild to severe and critically ill conditions.
As evidence shows the incidence and severity, as well as the clinical and molecular presentation of COIVD-19, are varied from place to place and recent studies also reported as unusual features are now being observed in the patients with COVID-19 in different countries across the globe.20–22 Having those assumptions by researchers and witnesses from literature makes this study more meaningful to the study area.
There is no evidence-based information on survival time and predictors of severe COVID-19 infection in Ethiopia in general and in the South Gondar Zone in particular, though knowing these epidemiological characteristics is essential to strengthening the existing prevention and control system and for providing pieces of evidence to the scientific world. Therefore, we aimed to determine the survival time of COVID-19 severity and its predictors among confirmed cases in South Gondar zone established treatment centers.
Materials and Methods
Study Area and Period
The study was conducted in South Gondar zone treatment centers from December 2020 to April 2021. Debre Tabor comprehensive referral hospital COVID-19 treatment center and Atsie Seyfeyared health center COVID-19 treatment center was the only treatment centers in the South Gondar Administrative zone which is located in Debre Tabor town. Debre Tabor town is the capital of the South Gondar Zone located 667 km from Addis Ababa (the capital city of Ethiopia). There are seven primary and one referral hospital and more than 187 health centers in the zone. According to the 2007 E.C population census report, the total population of the zone is around 2,578,906. Currently, the zone is hardly working to control the COVID-19 global pandemic and there were five isolation centers prepared in the woredas (Debre Tabor, Lay Gayint, Addis Zemen, Woreta, and Estie) of the south Gondar zone.
Study Design
An open prospective cohort study was used in Debre Tabor comprehensive referral hospital and Atsie seyfeyared health center COVID-19 treatment centers of the South Gondar Zone from December 2020 to April 2021. The investigator identified the study population who were diagnosed with COVID-19 at the beginning of the study and accompanies the subjects through time. The investigator then determined the present case status of individuals, selecting only non-severe COVID-19 cases to follow forward in time. The exposure status of the study population was determined at the beginning of the study.
Study Population
All adult individuals who were tested positive for COVID-19 by using the RT-PCR test and admitted to the South Gondar zone treatment centers from December 2020 to April 2021 were the study populations. All cases with COVID-19 enrolled in this study were diagnosed based on World Health Organization interim guidance.23
Eligibility Criteria
Inclusion Criteria
Adult individuals who fulfilled the standard case definition of COVID-19 infection and were admitted to the treatment centers of the South Gondar Zone during the follow-up period despite the disease severity, type of drugs, presence of symptoms, and co-morbidity status were included in the study.
Exclusion Criteria
Study subjects with no clear case definitions.
Sample Size and Sampling Techniques
All Covid-19 patients admitted to the Debre Tabor comprehensive referral hospital and Atsie seyfeyared health center COVID-19 treatment centers during the study period (December 2020 to April 2021) and fulfilled inclusion criteria were included in this study. A total of 202 COVID-19 cases were admitted to the treatment centers and included in this study.
Variables of the Study
Dependent variable: Survival time to COVID-19 severity.
Independent variables: Sex, age, religion, occupational status, educational status, residence, marital status, alcoholic consumption, cigarette smoking, previous history of co-morbidities (hypertension, DM, HIV, asthma, heart failure, renal and liver problems), symptoms at presentation, high temperature at admission, blood pressure at admission, co-infection, and laboratory variables such as; WBC, lymphocyte count, neutrophil count, and PLT.
Operational Definition and Term Definition
Survival time is the follow-up time in days from the patient was diagnosed positive for COVID-19 by using the RT-PCR test to the occurrence of the outcome (event/censored). The event was the patients who have developed the severity of COVID-19 infection. Censored were those individuals who have a negative test result of COVID-19, mild or moderate COVID-19 cases, lost to follow up including discharged to home, discharged against medical advice, or transfer out to other health institutions without knowing the outcome. Survival status is the outcome of a confirmed COVID-19 case either having an event (severity status) or censored (negative result, loss to follow up, mild or moderate). Follow-up time: From the time of admission of the case until discharge either an event or censorship occurs. Severity rate: the incidence of COVID-19 severity among the confirmed non-severe cases in the specified study period from December 2020 to April 2021 was measured in person-time rates.
Severe COVID-19 cases are those COVID-19 patients that meet any of the following criteria: dyspnea or respiratory rate ≥30 breaths/min, a saturation of peripheral oxygen (SPO2) ≤93% at a rest state, arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg, patients with >50% lesion progression in the lung imaging within 48 hours, presence of respiratory failure and need of mechanical ventilation, the occurrence of shock, and complicated with other organ failures that require admission to Intensive Care Unit (ICU). Moderate COVID-19 cases are those patients that had symptoms including fever, respiratory tract symptoms, and an imaging finding of pneumonia, but fails to meet the criteria for the severe group. Mild COVID-19 cases are those patients with mild symptoms and had no abnormal radiological findings, including mild pneumonia.10,24
Data Collection Tools and Procedures
The data were collected through clinical observation and face-to-face interviews using structured questionnaires which were developed after a review of relevant pieces of literature and other materials that can address the objectives of the study. The components of the questionnaires were demographic, epidemiological, laboratory, clinical, dates of important time points (eg, date of admission, date of outcome occurrence (event or censored), and outcome data. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) testing was used to confirm the diagnosis of COVID-19 (SARS COV-2) according to the recommended protocol.1 Blood tests and other examinations were performed upon admission, and the time point of re-examinations was determined by physicians according to guidance aforementioned and individual differences. The data collectors were experienced medical doctors and BSC nurses who were working in the established treatment centers. One supervisor (medical doctor) was recruited for supervising the data collectors in the treatment center.
Data Quality Assurance
To maintain data quality, structured questionnaires were prepared after an extensive search and review of relevant studies on the issue. Before undertaking the data collection, the questionnaires were submitted to experts mainly for health professionals who were working in the area to test the face and content validity and to assess whether they met the study objectives. The questionnaire was prepared in English and was translated to the local language (Amharic); then it was re-translated again to English by language experts to ensure consistency. The supervisor and data collectors were trained for two days on basic principles of data collection, study overview, the questionnaires, and how to do other related activities during data collection. The actual data collection was conducted using the local language (Amharic version). Strict daily supervision of the data collection process was maintained throughout the data collection period. The supervisor has checked the study site daily and received filled questionnaires after checking completeness. Completed questionnaires were submitted to the principal investigator for the final check.
Data Analysis
Before and during data processing, the information was checked for completeness and internal consistency. For data processing, a master sheet or template was prepared and the data was coded and entered using EpiData version 3.1 and exported to STATA version 16 for further analysis. There were special columns for no response or missing data to arrive at accurate total figures. The outcome of each participant was dichotomized into censored or event. The case severity within the follow-up period was divided by the total person-time at risk on follow-up and reported. Kaplan–Meier was used to estimate the mean survival time and cumulative probability of survival, and Log rank tests were used to compare the survival time between different categories of explanatory variables. To show the significance of survival differences among different categories of covariates for pairwise or multiple comparisons, the Log rank test with a post hoc test was used. Continuous variables with a normal distribution were represented by the mean and standard deviation (SD) while medians with interquartile range (IQR) were used for skewed data. Frequency distribution was used for the categorical data. For confirmed cases, demographic, epidemiological, laboratory, and clinical characteristics were summarized using descriptive statistics.
Before running the Cox PH regression model, multi-co-linearity was checked. Residuals also were checked using the goodness-of-fit test by Cox Snell residuals, which were satisfied with the model test. The Cox-proportional hazard regression model assumption was also checked using Schoenfeld residual test. A bi-variable Cox-proportional hazards regression model was fitted for each explanatory variable. Variables having a p-value <0.25 in the bivariate analysis were fitted to the multivariable Cox-proportional hazards regression model. Hazard ratio with a 95% confidence interval and p-value <0.05 was used to measure the strength of association and to identify statistically significant results. The result of the final model was expressed in terms of Adjusted hazard Ratio (AHR) and 95% confidence intervals (CI) and Statistical significance were declared if the P-value is less than 0.05.
Result
Socio-Demographic Characteristics of Patients
A total of 202 patients with COVID-19 were admitted to South Gondar zone COVID treatment centers from December 2020 through April 2021. The mean age of the patient was 41.2 (SD± 19) years. The majority of 90 (44.4%) patients’ age were 18–30 years. More than half, 125 (61.9%) of patients were residing in rural areas and around 163 (80.7%) of patients were male. A greater number of study subjects attended secondary education 77 (38.1%) and half of the study subjects were government employers 64 (31.7%) and farmers 43 (21.7%) (Table 1).
 |
Table 1 Sociodemographic Characteristics of Study Subjects Admitted to South Gondar Zone COVID-19 Treatment Centers |
Lifestyle Characteristics of Study Participants
Around three-fourth 141 (69.8%) study subjects had no history of alcohol consumption and almost all 200 (99%) of them had no history of cigarette smoking. Of female study subjects, the majority were given birth 27 (69.2%), not pregnant during the study 33 (84.6%), and used contraceptives 25 (64.1%) (Table 2).
 |
Table 2 Life Style Characteristics of Study Participants in South Gondar Zone Treatment Centers |
Baseline Clinical Characteristics of COVID-19 Patients
Of the total participants, 132 (65.4%) cases had a history of comorbidity. The comorbidities assessed in study subjects were HIV 15 (7.4%), hypertension 27 (13.4%), heart disease 39 (19.3%), asthma 10 (5%), and diabetes mellitus 25 (12.4%). Around one-third of the study, subjects had co-infection 60 (29.7%) and community-acquired pneumonia 44 (73.3%) were the leading type of co-infections. More than half 109 (54%) of participants had clinically presented with cough on admission to the treatment centers. Around one-fourth of participants had shown a fever of 47 (23.3%) and weakness of 56 (27.7%) during admission. Thirty-three (16.4%) and twenty-eight (11.9%) of study subjects had high blood pressure and high temperature on presentation to treatment centers, respectively (Table 3).
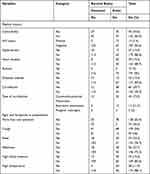 |
Table 3 Baseline Clinical Characteristics of Covid 19 Patients Admitted to South Gondar Zone Treatment Centers |
Baseline Laboratory Parameters of COVID-19 Patients
Most of the participants had a normal range of WBC 178 (88.1%), Platelet 182 (90%), and neutrophil count 189 (93.5%). While 81 (40.1%) of the study subjects had a high lymphocyte count. Furthermore, one hundred eighty-five (91.6%) and one hundred ninety-one participants had a normal range of bilirubin and creatinine, respectively (Table 4).
 |
Table 4 Baseline Laboratory Parameters of COVID-19 Patients Admitted to South Gondar Zone Treatment Centers |
Outcome Variable Among Patients with COVID-19 Admitted to South Gondar Zone Treatment Center
Among the 202 patients, 120 (59.4%) observations were censored. Among the censored observations, 80 (66.7%) recovered and discharged, 5(4.2%) were transferred to another hospital without knowing the outcome and 35 (29.1%) were on follow-up (free from the event) when the study was completed. At the end of the follow-up, 80 (39.6.%) of the patients developed the event (disease severity).
The Incidence Rate of Severity and Median Time to Develop COVID-19 Severity
A total of 202 study subjects were followed for a median time of 5 days. Eighty observations have developed an event (severity) with a median time to COVID-19 severity of 5 days with an IQR of 4–7 days. During follow-up time, a total of 1258 person day risks were observed with a minimum and maximum follow-up time of 1 and 21 days, respectively. The overall incidence rate of COVID-19 severity was 6.35 (95% CI: 5.17–7.86) per 100 person-days observations.
A Kaplan–Meier estimation technique was used to see the estimate of survival time. The overall graph of the Kaplan–Meier survival function depicted that the graph decreases rapidly during the first 7 days showing most patients developed COVID 19 severity during this time (Figure 1). A separate Kaplan–Meier survival functions curve was constructed to estimate the survival time based on different covariates to see the existence of a difference in severity rate between categories of individual covariates. From the Kaplan–Meier survival curve of individual covariates, there was no difference in severity rate among alcoholic consumers and non-alcoholics (Figure 2), and those residing in urban and rural areas (Figure 3). However, there was a difference in survival probability/disease severity rate for the covariates: comorbidity (Figure 4), sex (Figure 5), BP at admission (Figure 6), and high temperature at admission (Figure 7). To show the significance of survival difference, the Log rank test was computed at a 5% significance level. Accordingly, there was a significant difference in COVID-19 severity with age category, sex, comorbidity, body temperature at presentation, blood pressure, coinfection, and lymphocyte count.
 |
Figure 1 Overall Kaplan–Meier survival probability curve. |
 |
Figure 2 Kaplan–Meier survival curve for alcohol consumption. |
 |
Figure 3 Kaplan–Meier survival curve for residence. |
 |
Figure 4 Kaplan–Meier survival curve for comorbidity. |
 |
Figure 5 Kaplan–Meier survival curve for sex. |
 |
Figure 6 Kaplan–Meier survival curve for blood pressure. |
 |
Figure 7 Kaplan–Meier survival curve for body temperature. |
Predictors of Severity Time to COVID-19
Covariates that had P-value ≤0.25 in bivariable cox regression analysis were selected for multivariable cox regression analysis. Residence, sex, age category, presence of cough, comorbidity, co-infection, lymphocyte count, high blood pressure, and high temperature at presentation were selected for multivariable cox regression. Finally, four of the predictors (age category, comorbidity, high temperature at presentation, and high blood pressure) were found to have a statistically significant association with disease severity time during multivariable cox proportional regression analysis.
The age of the patients was one of the variables that predict the covid-19 severity rate. Severity rate of the senior adult age group (51–59 years) was 3.59 times higher as compared to patients who were aged less than 30 years (AHR = 3.59, 95% CI: 1.05, 12.23). In addition, the COVID-19 severity rate of the elderly age group (≥60 years) was 2.11 times higher as compared to patients aged less than 30 years (AHR=2.11,95% CI: 1.09,12.67). Study subjects who had comorbidity were 3.26 times more likely to develop covid-19 severity as compared to their counterparts (AHR = 3.26, 95% CI: 1.48, 7.18). Participants who had high blood pressure at presentation were 2.81 times more likely to develop COVID-19 severity as compared to those who had normal blood pressure (AHR = 4.36, 95% CI: 1.99, 9.54). Patients who were detected with high temperature at admission were at a higher rate of severity than patients who showed normal body temperature (AHR=5.60,95% CI: 2.55, 12.46) (Table 5).
 |
Table 5 Multivariable Cox Regression Analysis of Median Severity Time and Its Predictors Among Patients Admitted with COVID-19 Cases in South Gondar Zone Treatment Centers, 2021 |
Discussion
This study was aimed to determine the time to severity of novel coronavirus disease (SARS COV-2), and its predictors in South Gondar zone COVID-19 treatment centers. This study pointed out that the median time to the severity of SARS COV-2 was 5 days. This is similar to the studies done in Eka Kotebe general hospital treatment centers, Ethiopia.25 However, the median severity time was lower as compared to the previous studies done in Wuhan China,26 France, and Belgium.19 The possible reason for the observed discrepancy between the studies might be due to variation in sample size, study setting, socioeconomic characteristics, and medical services.27
The present study found older age as an independent predictor of the early development of severity time from coronavirus disease. This is consistent with previous study findings from Kuyha COVID-19 Treatment Centre, Mekelle City, Northern Ethiopia,28 Wuhan China,26 and South Korea.29 This might be because older age is associated with degeneration of pulmonary function and compromised immunity that contributes to severe COVID-19 cases and poor clinical outcomes.30
The current study has demonstrated that patients with comorbid conditions had more than three times higher risk of developing severity rates from coronavirus disease compared to their counterparts. Similarly, existing evidence is supporting the present study’s findings, for instance, the study was done in Ankara city hospital, Turkey,31 the hospital of Shenzhen China,32 Renmin Hospital of Wuhan University, China,33 showed that comorbid conditions majorly cardiovascular disease contributed to the early progression of coronavirus disease to the severity status. This study found that patients with high temperature at presentation were 5.6 times more likely to develop a severity rate from coronavirus disease compared to those who had normal body temperature at presentation. This is in line with a study conducted in Eka Kotebe General hospital treatment center of Ethiopia,25 Renmin hospital of Wuhan University, China,33 Tongji Hospital, China,26 and Henan province, China.34 This could be because the function of the respiratory system is dependent on body temperature variations. This can be explained by the fact that an increment in body temperature results in an increment in respiratory rate which increases the pulmonary workload eventually leading to pulmonary insufficiency and a higher severity rate.35
Furthermore, our study has also identified that patients with high blood pressure at presentation were around four times more likely to develop a severity rate from coronavirus disease compared to those who had normal blood pressure at presentation. This is supported by the study finding from Turkey,31 South Korea,29 and Wuhan China.33 This could be due to high blood pressure altering ACE2 expression, increasing the risk of cardiovascular and renal failure, and may cause organ damage; thus, the detrimental effect of high blood pressure on COVID-19 outcomes.36
In other studies,37–40 factors such as sex, clinical symptoms like headache or cough, co-infection, lymphocytopenia, leukopenia, and low platelet count were related to COVID-19 severity and mortality. However, in our study, these variables were not statistically associated with COVID-19 severity. This could be due to sampling size differences, low prevalence of factors in the study population, or differences in the study population characteristics.
Limitations
Some laboratory parameters, like D-dimer, that can predict the outcome variables were not measured and analyzed due to feasibility issues. Our target, on the other hand, was to find an easily accessible common laboratory parameter. As a result, further study is needed to emphasize the necessity of using such unattainable laboratory parameters to predict the severity time of the COVID-19.
Conclusion and Recommendation
In general, this study found a short severity time of COVID-19. The study revealed that older age, high temperature at admission, high blood pressure at admission, and having at least one comorbid condition was independent predictors of the COVID-19 severity time. Thus, older ages and individuals with comorbidity have to get due attention to prevent the early development of severe COVID-19 infection. In addition to body temperature, blood pressure screening during admission should get attention to prevent the early development of advanced coronavirus disease. Moreover, interventions to delay the progression time and enhance good clinical outcome at the treatment center have to focus on patients with high body temperature, blood pressure, comorbidity, and being older.
Declarations
Ethical Consideration
The study protocol was developed by the study team, reviewed, and approved by the Debre Tabor University research ethics committee. Following the authorization by the research ethics committee, the treatment center was informed about the study through a support letter. Concerned bodies in the treatment center were briefed about the study before the start of data collection. Written informed consent was taken from all study participants. Data security and participants’ confidentiality were maintained at all levels of data management. This study was carried out in such a way that the potential harm to the study participant was kept to a minimum. Prior to the study, a comprehensive assessment of the expected risks and burdens to the individuals participating in the study were made, as well as the expected benefits to them and other persons affected by the disease under investigation. In general, this research adheres to the Helsinki Declaration. All methods were performed following the relevant guidelines and regulations.
Abbreviations
AIDS, Acquired immunodeficiency disease; ARDS, Acute Respiratory Distress Syndrome; DM, Diabetes Maleates; COV, Coronaviruses; COVID-19, Coronavirus disease 2019; DM, Diabetes Maleates; FiO2, Fraction of Inspired Oxygen; HIV, Human immunodeficiency virus; PaO2, Arterial Partial pressure of Oxygen; SARS-Cov-1, Severe Acute Respiratory Syndrome Coronavirus 1; SARS-Cov-2, Severe Acute Respiratory Syndrome Coronavirus 2; SPO2, Saturation of Peripheral Oxygen; WHO, World Health Organization.
Data Sharing Statement
The data sets in this study are accessible from the corresponding author on reasonable request.
Acknowledgments
The authors would like to acknowledge Debre Tabor University, College of Health Sciences for approving the ethical review process. The authors are also wanted to express thankfulness to data collectors, Debre Tabor’s comprehensive specialized hospital staff, Atsie Seyfeyared health center staff, supervisors, and study participants.
Author Contributions
G.A.Y, A.T., and G.M conceived the original idea and were involved in proposal development, design, execution, acquisition of data, analysis, and interpretation and in all stages of the research project. M.T, Z.W, E.M, E.C, F.T, E.H, T.D, H.S, and F.T made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation. All authors contributed to the article’s drafting, revision, or critical review; gave final approval of the version to be published; agreed on the journal to which the article was submitted; and agreed to be responsible for all parts of the work.
Disclosure
The authors declare no competing interests in this work.
References
1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
2. Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi:10.1016/S0140-6736(20)30154-9
3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi:10.1016/S0140-6736(20)30251-8
4. Yang Y, Peng F, Wang R, et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. doi:10.1016/j.jaut.2020.102434
5. International CSG. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(1):1. doi:10.1038/s41564-019-0652-x
6. Lai -C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi:10.1016/j.ijantimicag.2020.105924
7. Gebru AA, Birhanu T, Wendimu E, et al. Global burden of COVID-19: situational analysis and review. Hum Antibodies. 2021;29(2):139–148. doi:10.3233/HAB-200420
8. Mboussou F, Impouma B, Farham B, et al. Analysing the reported incidence of COVID-19 and factors associated in the World Health Organization African region as of 31 December 2020. Epidemiol Infect. 2021;149:1–21.
9. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi:10.1056/NEJMc2001468
10. McFee DRB. COVID-19 medical management including World Health Organization (WHO) suggested management strategies. Dis Mon. 2020;66(9):101068. doi:10.1016/j.disamonth.2020.101068
11. World Health Organization. Clinical management of severe acute respiratory infection ( SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization; 2020.
12. Son KB, Lee TJ, Hwang SS. Disease severity classification and COVID-19 outcomes, Republic Of Korea. Bull World Health Organ. 2021;99(1):62–66. doi:10.2471/BLT.20.257758
13. Nkengasong JN, Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet. 2020;395(10227):841–842. doi:10.1016/S0140-6736(20)30464-5
14. Iroulo LC, Boateng O. African States Must Localise Coronavirus Response. 2020.
15. Dahab M, van Zandvoort K, Flasche S, et al. COVID-19 control in low-income settings and displaced populations: what can realistically be done? Health in Humanitarian Crises Centre; 2020. Available from: https://www.lshtmacUK/research/centers/health-humanitarian-crises-centre/news/102976.
16. Tesfaye M. Federal democratic republic of Ethiopia ministry of science and higher education; 2020.
17. Ayenew B. Challenges and opportunities to tackle COVID-19 spread in Ethiopia. J PeerScientist. 2020;2(2):e1000014.
18. Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi:10.1016/j.ijid.2020.03.004
19. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
20. Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13(5):667–673. doi:10.1016/j.jiph.2020.03.019
21. Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation, and treatment. Postgrad Med J. 2021;97(1147):312–320. doi:10.1136/postgradmedj-2020-138577
22. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi:10.1001/jama.2020.12839
23. World Helath Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected Interim guidance; 2020.
24. Liu D, Cui P, Zeng S, et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: a multicenter, retrospective, cohort study. EClinicalMedicine. 2020;25:100471. doi:10.1016/j.eclinm.2020.100471
25. Abrahim SA, Tessema M, Ejeta E, et al. Median duration and factors that influence the duration of symptom resolution in COVID‐19 patients in Ethiopia: a follow‐up study involving symptomatic cases. Lifestyle Med. 2021;2:4. doi:10.1002/lim2.46
26. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi:10.1016/j.jaci.2020.04.006
27. Alene KAGY, Fetene DM. COVID-19 in Ethiopia: a geospatial analysis of vulnerability to infection, case.severity, and death. BMJ Open. 2021;11(2):e044606. doi:10.1136/bmjopen-2020-044606
28. Abraha HE, Gessesse Z, Gebrecherkos T, et al. Clinical features and risk factors associated with morbidity and mortality among patients with COVID-19 in northern Ethiopia. Int J Infect Dis. 2021;105:776–783. doi:10.1016/j.ijid.2021.03.037
29. Huh K, Lee R, Ji W, et al. Impact of obesity, fasting plasma glucose level, blood pressure, and renal function on the severity of COVID-19: a matter of sexual dimorphism? Diabetes Res Clin Pract. 2020;170:108515. doi:10.1016/j.diabres.2020.108515
30. Rod JE, Oviedo-Trespalacios O, Cortes-Ramirez J. A brief-review of the risk factors for covid-19 severity. Rev Saude Publica. 2020;54:60. doi:10.11606/s1518-8787.2020054002481
31. Bastug A, Bodur H, Erdogan S, et al. Clinical and laboratory features of COVID-19: predictors of severe prognosis. Int Immunopharmacol. 2020;88:106950. doi:10.1016/j.intimp.2020.106950
32. Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi:10.2337/dc20-0576
33. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi:10.1016/j.jinf.2020.03.019
34. Li J, Chen Z, Nie Y, Ma Y, Guo Q, Dai X. Identification of symptoms prognostic of COVID-19 severity: multivariate data analysis of a case series in Henan Province. J Med Internet Res. 2020;22(6):e19636. doi:10.2196/19636
35. Gallo marin B, Aghagoli G, Lavine K, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31(1):1–10. doi:10.1002/rmv.2146
36. Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304–309. doi:10.20452/pamw.15272
37. Figliozzi S, Masci PG, Ahmadi N, et al. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50(10):e13362. doi:10.1111/eci.13362
38. Leulseged TW, Abebe KG, Hassen IS, et al. COVID-19 disease severity and associated factors among Ethiopian patients: a study of the millennium COVID-19 care center. PLoS One. 2022;17(1):e0262896. doi:10.1371/journal.pone.0262896
39. Li J, He X, Yuan Y, et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Infect Control. 2021;49(1):82–89. doi:10.1016/j.ajic.2020.06.008
40. Sharma J, Rajput R, Bhatia M, Arora P, Sood V. Clinical predictors of COVID-19 severity and mortality: a perspective. Front Cell Infect Microbiol. 2021;11:674277. doi:10.3389/fcimb.2021.674277
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
