Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Survival and Predictors of Mortality Among HIV Positive Adult Patients on Highly Active Antiretroviral Therapy in Public Hospitals of Kambata Tambaro Zone, Southern Ethiopia: A Retrospective Cohort Study
Authors Abuto W, Abera A , Gobena T , Dingeta T, Markos M
Received 26 December 2020
Accepted for publication 26 February 2021
Published 12 March 2021 Volume 2021:13 Pages 271—281
DOI https://doi.org/10.2147/HIV.S299219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Wondimu Abuto,1 Admas Abera,2 Tesfaye Gobena,2 Tariku Dingeta,2 Melese Markos3
1Public Health Emergency Management, Kembata Tembaro Zonal Health Department, Durame, Southern Nations Nationalities Peoples’ Region, Ethiopia; 2School of Public Health, Haramaya University, Harar, Ethiopia; 3Department of Public Health, Dire Dawa University, Dire Dawa, Ethiopia
Correspondence: Admas Abera
Haramaya University, P.O. Box 235, Harar, Ethiopia
Email [email protected]
Background: Human Immune Deficiency Virus (HIV) infection remains the leading cause of morbidity and mortality. In Ethiopia, despite test and treat all HIV positives are adopted, a significant number of people eligible for Anti-Retroviral Therapy (ART) show up with advanced disease and at lower CD4 count. There is currently paucity of studies conducted that investigate predictors of mortality among adults on ART in the study area.
Objective: To explore Survival and predictors of mortality among adult HIV-positive patients on ART in Kambata Tambaro Zone, Ethiopia, from August 2013 to February 2019.
Methods: A health facility-based retrospective cohort study was conducted among records of 467 adult HIV-positive patients on ART selected using simple random sampling. Data were collected using standardized abstraction tool. Kaplan–Meier, Log rank tests and Cox regression model was applied to estimate survival status and identify predictors of mortality, respectively.
Results: Of the total 467 study subjects, 59 (12.63%) of them died in the study period. The median follow-up time of the cohort was 40.1 (IQR=13.6– 59.0) months. The mortality rate of the cohort was 4.1 per 100 PYO. The overall survival probability of the cohort was 84.38% (95 CI=80.08– 87.82) at 66 months. Bedridden function AHR=3.0 (95% CI, 1.44– 6.64), Fair-adherence AHR=3.3 (95% CI, 1.50– 7.07), Poor-adherence AHR=3.8 (95% CI, 1.88– 7.96), presence of OIs AHR=4.2 (95% CI, 1.98– 8.50), Late diagnosis (CD4 count >/=350) AHR=3.0 (95% CI, 1.91– 6.42) and Immunologic failure AHR=3.5 (95% CI, 1.41– 6.29) were independent predictors of time to death in Cox-Regression.
Conclusion: Late Diagnosis, poor adherence, being bedridden, having OI and Immunologic failure were independently associated with time to death. Early diagnosis to start treatment and emphasizing on close follow-up care to improve treatment adherence should be given special emphasis.
Keywords: survival, mortality, predictors, HIV/AIDS, HAART, low-resource setting
Introduction
Human Immune Deficiency Virus (HIV) infection remains the leading cause of morbidity and mortality, and continues to be a challenge to major global public health issues. Globally, 78 million people have been infected with this virus and 35 million people have died from AIDS-related illnesses since the start of the epidemic.1
In 2016, 36.9 million people were living with HIV of which 35.1 million were adults and 21.7 million People Living With HIV (PLWHIV) were accessing ART and about 1 million AIDS-related deaths have been registered.2,3 AIDS-related death remained static, 1 million deaths each year since 2015.1,7 Although Sub Saharan Africa (SSA) is home to only 12% of the global population, it accounts for 75% AIDS-related deaths in which 81% of deaths in SSA were recorded only in 10 countries including Ethiopia.3 Moreover, higher rate of mortality as high as 26% has been recorded particularly at early year of ART initiation in SSA including Ethiopia.8,9
Antiretroviral therapy (ART) is a great public health success that led to improved survival among HIV-infected people. The reduction in AIDS-related death is attributed to global scale-up of ART.4 By the end of 2016, 70% of PLWHIV were diagnosed, 77% of those who knew their HIV status received ART and 82% of those on treatment were virally suppressed.5 Even though none of the countries achieve the so-called 90–90-90 targets, the lowest achievement rates were in low and middle-income countries (LMICs).6
In Ethiopia, HIV infection continues to be a major public health problem and AIDS has claimed the lives of 1.3 million since the start of the epidemic.10 The prevalence of HIV among adults was 1.16%, with substantial prevalence variation between regions. An estimated number of PLWHIV in Ethiopia were 738,976 in which 426,000 were on ART.2,10–13
Ethiopia launched implementing test and treat all policy in testing and preventing target populations for strengthening prevention, monitoring and response to HIV by sustaining HIV treatment scale-up. Despite this, there are still people who present to healthcare facilities with advanced HIV disease. Nationally in 2018, 67% of PLHIV know their status, 88% of them were on ART and 86% of people on ART have viral suppression.10 Insufficient detection, late diagnosis, inadequate viral suppression; and inadequate support to sustained HIV care and treatment increase AIDS-related mortality.11
Survival is reduced among PLWHIV after 2011, even though eligibility has progressively increased and patients are starting care earlier.10,20 One systematic review estimates the mortality rate to be 40.8% with the highest mortality at early months of follow-up within 3 to 12 months of ART initiation.19 Different predictors were independently associated with mortality in different parts of Ethiopia.14–18
Understanding context-specific predictors of survival in PLWHIV are essential to monitor and improve program effectiveness which in turn improves the clinical outcome of patients on ART. However, little is known about the survival status and its predictors after ART initiation in the study area. Moreover, studies showed variations and inconsistencies from region to region21–24 which indicates predictors of time to death have not yet been well understood in resource-limited settings like Ethiopia including Kambata Tambaro Zone. Therefore, this study was aimed to assess Survival and predictors of mortality among adult HIV-positive patients on ART in Kambata Tambaro Zone, Ethiopia.
Methods and Materials
Study Design and Area
Institution-based retrospective longitudinal study was used for 5 years in public hospitals of Kambata Tambaro Zone, Southern Ethiopia from March 10–31, 2019. Kambata Tambaro zone is one of the central zone of SNNPR and has 3 administrative cities and 8 woredas. There are 7 health centers and 4 public Hospitals that provide ART services. The study was conducted in Durame General Hospital, Shinshicho Primary Hospital, Doyogena Primary Hospital and Mudula Primary Hospital. There were 1174 patients ever start ART and currently 786 patients were receiving ART during the study period.
Study Participants and Follow-Up Time
The study participants were adults aged 15 or older who started ART between August 2013 and August 2018. Additional 6 months follow-up was added to account for those patients entering the cohort late in the study period and, to account for those patients in the cohort who had to wait until their CD4 count falls below 350 which was the guideline used before 2017. Patients who started ART outside the study facilities and have incomplete baseline data (transferred in), and patients with incomplete baseline parameters (no recorded CD4 count, no recorded WHO stage and no recorded specific ART regimen type) were excluded from the study. A total of 110 records were excluded (43 of them had no recorded CD4 count and 67 were transferred in at initiation from other facilities).
The sample size was calculated based on the assumption that type I error of 5%, 80% power and considering the proportion of death among exposed patients (using CD4 cell count <350) was 11.25% and AHR= 2.3425 and the proportion of lost to follow-up was anticipated to be 15%. Based on the above assumptions the final estimated sample size of the study was 467. These participants were selected using simple random sampling from 1174 patient records obtained from the ART clinics of the health institutions.
Measurements
The dependent variable is time from ART initiation to an event, categorized into Censored (Alive, Transferred out and LTFU) and Death. The date of censoring for alive patient was January 31, 2019; for LTFU was the last visit date and for transferred out was date of transfer. The main predictor variable taken to identify the implicit group of the study was CD4 count at ART initiation. CD4 count measures the immune status of patients, clinical, risk of OI and support HIV diagnosis decision.26 CD4 count below 350 cells/mm3 suggested a late diagnosis and CD4 count >/= 350 cells/mm3 was considered as early diagnosis.7,18,47 Socio-demographic predictors (Age, sex, residence, marital status, occupational status and educational status); Baseline Clinical parameters (ART start period, baseline WHO clinical stages, functional status, TB treatment, ART regimen type, ART regimen change, CPT use, IPT use, ART Adherence status, OI other than TB, disclosure status, BMI) and Baseline Laboratory tests (viral load test, hemoglobin) were the other covariates. We have also included a time-dependent variable, immunologic failure, which is defined as patients’ CD4 count declines from the baseline, or CD4 levels are persistently below 100 cells/mm3 (more than two times after baseline).27
Operational Definitions
Good adherence: If the percentage of missed dose is >95% (<2 doses of 30 doses or <3 doses of 60 doses) as documented by ART physicians.7
Fair adherence: If the percentage of missed dose is between 85–94% (3–5 doses of 30 doses or 3–9 doses of 60 doses) as documented by ART physician.7
Poor adherence: If the percentage of missed dose is <85% (>6 doses of 30 doses or >9 doses of 60 doses) as documented by ART physician.7
Late diagnosis: HIV positive patients (who present with CD4 cell count <350 cells/mm3)5,18,47
Early diagnosis: HIV positive patients (who present with CD4 cell count ≥350 cells/mm3)5,18,47
Functional Status:
Ambulatory: able to perform routine activities of daily living.7
Bedridden: A patient unable to perform routine activities of daily living.7
Working: A patient able to perform his/her usual work.7
Lost to follow up: A patient not seen for 1–3 months after the last appointment.7
Immunologic Failure: patients’ CD4 count declines from the baseline, or CD4 levels are persistently below 100 cells/mm3 (more than two times after baseline).27
Data Extraction and Data Quality Control
A pre-prepared data retrieval (abstraction tool) form was developed from records of the Federal Ministry of Health, ART entry and follow-up forms, patient records, laboratory requests and computer data. Death information was confirmed from reviewing the death summary, medical registration in ART clinic, or registration by ART adherence supporter through calling using the registered phone number or neighborhoods of the patients. The recent data before ART initiation was taken as baseline and data before one month of ART initiation was used for patients who started ART immediately after diagnosis. Data was extracted after giving 2 days training for data collectors and supervised by the Principal investigator. The extracted data were checked for the completeness, accuracy and clarity.
Ethical Considerations
Ethical approval of the study was obtained from the Haramaya University, College of Health and Medical Sciences, Institutional Health Research Ethics Review Committee (IHRERC) with Reference Number IHRERC/039/2019. Letter of permission was obtained from Kambata Tambaro Zonal Health Department and agreement consent was obtained from each Public Hospital managers prior to the study. Due to difficulty to reach patients to obtain informed consent, data were extracted anonymously ensuring patient data confidentiality, and all data collection was conducted in compliance with the declaration of Helsinki.
Data Processing and Analysis
Data were entered into Epi-Data version 4.1 and exported to STATA version 14.1for data processing and analysis. Data were coded, labeled and categorized for analysis. The time that each participant contributed to the study was calculated by months from date of ART start to the date of censored or death. The time that the patient discontinued ART was reduced for re-entered patients after attrition. Kaplan–Meier was used to estimate the survival probability of the cohort and category of each predictor. Log rank test was employed to compare (test) equality of surviving, ie, checking whether survival probabilities are statistically significant. Semi-parametric (Cox regression) Model was applied to identify the predictors of time-to-death and to calculate the hazard ratios (HR).
Bivariate Cox-Regression analysis was performed, forward variable selection using AIC (Akaike Information Criteria) was used with a liberal p-value of 0.1 to ensure essential predictor variables are retained. The proportionality hazard assumption test was checked using Schoenfeld residuals on functions of time.
Results
A total of 467 study participants’ records were reviewed, among them 263 (56.3%) were females and the overall median age was 30 years (IQR=26-38) and 213 (54.4%) participants live in rural areas (Table 1).
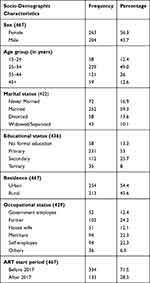 |
Table 1 Patients Baseline Sociodemographic Characteristics, Kambata Tambaro Zone, Ethiopia, August 2013 to February 2019 |
Clinical and Laboratory Characteristics
From the total 437 records reviewed, 173 (37.0%) patients had BMI <18.5 Kg/m2, 107 (22.9%) patients were in Ambulatory functional status and 60 (13%) were Bedridden. About 171 (36.6%) had OI other than TB, 183 (39.2%) were in WHO stage III or IV and 57 (12.2%) patients had not received CPT. Further, about 189 (40%) had CD4 <350 cells/mm3, 31 (11.3%) had Viral load >1000 copies, and 116 (30.8%) had Hemoglobin level below 10 (Table 2).
 |
Table 2 Clinical and Laboratory Characteristics of Patients in Kambata Tambaro Zone, Ethiopia, August 2013 to February 2019 |
Survival Status of the Cohort
In this cohort, out of 467 study subjects on ART, 408 (87.4%) patients were censored and 59 (12.6%) patients died. From 408 censored subjects, 308 (75.5%) were Alive and 69 (16.9%) were LTFU and 31 (7.6%) were transferred out (Figure 1).
 |
Figure 1 Survival status of HIV positive patients on ART in Kambata Tambaro Zone, Ethiopia from August 2013 to February 2019. |
The overall follow-up of the cohort was 1412.7 person-years of observation (PYO). The median follow-up of the overall cohort was 40.1 (IQR=13.63–59.0) months. The total mortality rate of the cohort over 66 months of follow-up was 4.1 per 100 PYO. The survival probability of the cohort at 3 months, 6 months, 1 year, 2 years, 3 years, 4 years and 5 and half years was 96.9%, 93.8%, 91.9%, 88.5%, 85.9%,85.5% and 84.4%, respectively. The overall failure probability of the cohort was 15.6% at 66 months (Figure 2).
 |
Figure 2 Kaplan–Meier survival rate estimate of HIV positive patients on ART in Kambata Tambaro Zone, Ethiopia from August 2013 to February 2019. |
The survival probability descends sharply in the first 6 months, steadily descends until 36 months and descends slowly until it reaches its minimum (84.23%) at 60 months (Figure 3A). On the other hand, the probability of survival among patients diagnosed late and early was 78.2% and 93.2%, respectively (Figure 3B). The survival rate of patients diagnosed late declines steadily after ART start in the first month until 30 months reaching the minimum of 78.2%. Fourteen (23.7%) of deaths were recorded in the first 3 months and another 14 (23.7%) deaths between 3 and 6 months of follow-up and 28 (47.4%) death happened between 6 and 36 months (Table 3).
 |
Table 3 Life Table Showing Pattern of Death with Time Intervals Among HIV Positive Patients on ART in Kambata Tambaro Zone, Ethiopia, August 2013 to February 2019 |
In the current study, sex, marital status, education level, residence, occupational status, ART start year, care category, eligibility criteria, disclosure status, ART regimen type, ART side effect, ART regimen change and Viral load (<1000 Vs >/=1000) were not significantly associated with survival of patients.
In bivariate cox regression model, variables that were significantly associated with survival of participants include; Age at ART initiation, BMI, functional status, WHO clinical stages, ART adherence, tuberculosis, IPT use, CPT use, OI other than Tb, CD4 count at ART initiation (Late diagnosis, ie, CD4 Count <350 cell/µL Vs Early Diagnosis, ie, CD4 Count >/=350cell/µL), Immunologic failure and hemoglobin level (Table 4).
 |
Table 4 Predictors of Survival Rate in Cox Regression Model Analysis Among Adult HIV Positive Patients, Kambata Tambaro Zone, from August 2013 to February 2019 |
After controlling for confounding in the multivariable analysis of Cox Regression Model, variables that are independent predictors of time to death include: being bedridden patient (AHR = 3.0, 95% CI = 1.44–6.64), Fair adherence to ART (AHR=3.26, 95% CI=1.50–7.05), Poor adherence to ART (AHR=3.87, 95% CI=1.88–7.95), presence of OIs other than TB (AHR= 4.09, 95% CI= 1.98–8.50), Late diagnosis measured by CD4 count (CD4 count >/=350) (AHR=2.99, 95% CI=1.41–6.30), and Immunologic failure (AHR=3.50, 95% CI, 1.90–6.41) (Table 4).
Discussion
This retrospective cohort study shows early diagnosis before starting ART treatment regardless of CD4 count and WHO staging improves survival of patients on ART. Further, CD4 count <350 (Late diagnosis), poor adherence, being bedridden functional status, having OI and immunologic failure on ART follow-up patients have higher risk of mortality among adults on ART.
The probability of survival for 66 months was 84.4%, which is consistent with the study done in Nepal,32 India33 and elsewhere in Ethiopia.14,15,25 This finding, however, is higher than the study done in Cameroon28 where 1167 aged 15 years and above patients were followed from 2001 to 2006, and in Ethiopia,24,29 where records of 930 adults were studied between 2005 and 2010. The discrepancy can be attributed to the difference in study time. The finding of the present study was lower than study done in Southern Ethiopia,30 Aksum,31 Nekemte17 in which the studies recruited relatively higher number of participants. These differences can be attributed to the difference in study time and sampling as well as the difference in length of follow-up.
The mortality rate of the cohort in the current study was 4.1 per 100 PYO. This finding is lower than the study done in Cameroon,34 India,33 Nepal32 and Ethiopia,30 but higher than the study done in Jinka;29 Debre Markose;24 Harar.25 This might be probably due to the difference in diagnosis and ART initiation time and the difference in proportion of advanced WHO stages in the studies.
Late HIV diagnosis was a strong predictor of mortality in this study. This was in line with the study done in Tigray.18 Other studies reported in agreement with this study, thus an increase in baseline CD4 count reduce AIDS-related mortality35 and the lower CD4 count at ART initiation have higher risk of mortality, Hind,33 Uganda,36 Kenya,37 Ethiopia.15,23,25,38–40 This is because high CD4 count reduces the occurrence of opportunistic infection and reduces AIDS-related mortality.7
This study revealed that the presence of opportunistic infections is a strong predictor of time to death. Accordingly, patients with OI are four folds at increased risk of mortality compared to patients without OI. The finding is supported by various other studies elsewhere.35,41,42 The occurrence of Opportunistic infection determines the level of immunity and is the predominant cause of morbidity and mortality among HIV-infected people.7,47
This study also revealed that adherence level to ART is a strong predictor of survival. Patients with poor adherence are almost four times at increased risk of mortality compared to patients with good adherence. Different studies in India43 and Ethiopia16,24,41 reported in agreement with the present study. This might be due to the fact that low adherence for treatment decreases the effectiveness and benefits of the ART treatment.48
Functional status was the indicator for poor performance scale for capability of self-care and able to perform routine daily activities by themselves to improve quality of life and survival. This study revealed that bedridden functional status was a significant predictor of reducing survival of patients on ART. This is supported by a study done in India,33 in Kenya37 and elsewhere in Ethiopia.14,29,38,39,44
Moreover, the study revealed that immunologic failure was strong predictors of reducing survival in patients on ART which is in line with previous studies.21,45 A successful ART regimen offers the best opportunity for effective immune recovery and prevent OIs. Continuous monitoring of CD4 count alerts to start and continue prophylaxis for OI to recover immunologic failure. Immune suppression may persist in patients who do not experience a significant increase in CD4 count, failure to achieve CD4 recovery and presence of CD4 decline that reduce length of survival.
WHO stage was not a predictor of time to death in the current study, while the WHO Stage was a significant predictor of mortality elsewhere.24,25 This is indeed a surprising finding that the advanced stage of HIV has no association with mortality. This might be due to compared to the proportion of people in WHO stage I and II, the low proportion of patients in stages III and IV in our study sample. Further, the presence of TB was not independently associated with mortality in this study whereas other studies indicate that TB was a significant predictor of mortality.25,46 This can be attributed to the early initiation of ART after Tuberculosis infection with high CD4 count does not increase the risk of death.
Limitations of the Study
Selection bias might have been introduced since records with incomplete information or charts that were lost for some patients were excluded. All-cause death might overestimate the mortality since the cause of death in this study was not identified. On the other hand, participants who were lost to follow up and those who do not have phone numbers on their folder were not traced back which might underestimate the mortality rate of the cohort.
Conclusion
Our study found that the proportion of death late diagnosis independently predicts time to death among people with HIV/AIDS on ART. The present study also revealed that bedridden patients, poor adherence to ART, presence of OIs and immunologic failure were positively associated with increased risk of death. The findings indicate that local health bureaus in collaboration with NGOs and other stakeholders need to continually work to raise awareness that HIV/AIDS is still a major public health issue so as to diagnose patients early and start them on ART. In longitudinal HIV care, patients with OI and patients with poor adherence to ART ought to be highlighted for close follow-up care. Last but not least, health institutions need to strengthen their data recording practices including baseline information (phone number, address of patients and family) which are essential in tracking of LTFU patients.
Data Sharing Statement
All data are available upon request of the authors of the study.
Acknowledgments
Authors are thankful to data collectors, ART focal persons and adherence supporters in the study area for their assistance during data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No organization funded this study.
Disclosure
The authors declare that they have no conflicts of interests for this work.
References
1. UNAIDS. Fact sheet—latest global and regional statistics on the status of the AIDS epidemic. Geneva: UNAIDS. 2017. Available from: http://www.unaids.org/sites/default/files/media asset/UNAIDS FactSheet en.pdf
2. UNAIDS. Global AIDS Monitoring 2018: Indicators for Monitoring the 2016 United Nations Political Declaration on Ending AIDS. UNAIDS; 2017. Available from: https://reliefweb.int/sites/reliefweb.int/files/resources/2017-Global-AIDS-Monitoring_en.pdf.
3. UNAIDS. UNAIDS DATA 2017. UNAIDS; 2017. Available from: https://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf.
4. Noemi. A, Franco M. Single-Tablet Regimens in HIV Therapy. Infect Dis Ther. 2014;3:1–17. doi:10.1007/s40121-014-0024-z
5. WHO. HIV Drug Resistance Report 2017. Geneva: WHO; 2017. Available from: https://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf.
6. Levi J, Alice R, Anton P, Pietro V, Philipp K, Andrew H. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Health. 2016;1(2):e000010. doi:10.1136/bmjgh-2015-000010
7. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection 2016 Recommendations for a Public Health Approach Second Edition. WHO; 2016.
8. Stephen D, Anthony D, Xavier A, Landon M, Robin W. Early mortality among adults accessing antiretroviral treatment program in sub-Saharan Africa. NIH AIDS. 2008;22:15.
9. Degu J, Endale A, Hailu Y, Lindtjorn B. Predictors of early death in a cohort of Ethiopian patients treated with HAART. BMC Infect Dis. 2006;6:136. doi:10.1186/1471-2334-6-136
10. Tadele. G, Abebaw. W, Abdulsemed. W. Trend of HIV/AIDS for the last 26 years and Predicting achievement t of the 90–90-90 HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18(320):1–10.
11. FMOH. Ethiopia Country/ Regional Operational Plan (COP/ROP) 2017: Strategic Direction Summary. FMOH; 2017. Available from: https://www.state.gov/wp-content/uploads/2019/08/Ethiopia-18.pdf.
12. EPHI. HIV Related Estimates and Projections for Ethiopia–2017. EPHI; 2017. Available from: https://www.ephi.gov.et/images/pictures/download2009/HIV_estimation_and_projection_for_Ethiopia_2017.pdf.
13. Deribew. A, Sibhatu. B, Kebede. D, Tariku. D, Gizachew A. The Burden of HIV/AIDS in Ethiopia from 1990 to 2016: evidence from the Global Burden of Diseases 2016 Study. Ethiop J Health Sci. 2019;29:1.
14. Bereket D, Bezatu M, Tadesse A. Survival and determinants of mortality in adult HIV/Aids patients initiating antiretroviral therapy in Somali Region, Eastern Ethiopia. Pan African Med J. 2015;22:138.
15. Daniel F, Teklu W, Alula MT, et al. Predictors of Survival among Adult Ethiopian Patients in the National ART Program at Seven University Teaching Hospitals: a Prospective Cohort Study. Ethiop J Health Sci. 2017;27(1):63–71. doi:10.4314/ejhs.v27i1.7S
16. Nurilign A, Kassahun A, Tadese A, Amanuel AA. Survival status of HIV positive adults on antiretroviral treatment in Debre Markos Referral Hospital, Northwest Ethiopia: retrospective cohort study. Pain African Med J. 2014;17:88.
17. Mitiku TH, Ahmed A, Yadeta D. Determinants of Mortality among HIV Positives after Initiating Antiretroviral Therapy in Western Ethiopia: a Hospital-Based Retrospective Cohort Study Hindawi Publishing Corporation ISRN AIDS. Int Schol Res Notices. 2013.
18. Hadera. B, Fessahaye. A, Angesom T, Hintsa S, Abay M. Effect of late HIV diagnosis on HIV-related mortality among adults in general hospitals of Central Zone Tigray, northern Ethiopia: a retrospective cohort study. HIV/AIDS Res Palliative Care. 2017;9:187–192. doi:10.2147/HIV.S141895
19. Mohammed BA. Mortality and Its Predictors among HIV Infected Patients Taking Antiretroviral Treatment in Ethiopia: a Systematic Review Hindawi. AIDS Res Treat. 2017.
20. Alula M, Kesetebirhan D, Mulu A, Bekele B, Esayas K, Abiy N. Exploratory Analysis of Time from HIV Diagnosis to ART Start, Factors and effect on survival: a longitudinal follow up study at seven teaching hospitals in Ethiopia. Ethiop J Health Sci. 2017;27:1.
21. Ravimohan. S, Neo. T, Andrew. PS. Early Immunologic Failure is Associated With Early Mortality Among Advanced HIV–Infected Adults Initiating Antiretroviral Therapy with Active Tuberculosis. J Infect Dis. 2013;208(11):1784–1793. doi:10.1093/infdis/jit368
22. Endalkachew M, Ashenafi B. Predictors of mortality among adult patients enrolled on Antiretroviral Therapy in Hiwot Fana Specialized University Hospital, Eastern Ethiopia: retrospective Cohort study. J HIV Clin Sci Res. 2018;5:1.
23. Kidane T, Fisaha H, Neway H. Predictors of mortality among patients enrolled on antiretroviral Therapy in Aksum Hospital, Northern Ethiopia: a Retrospective Cohort Study. PLoS One. 2015;9:1.
24. Ashenif T, Ashenafi S, Neway H. Survival and predictors of mortality among adult patients on highly active antiretroviral therapy at debre-markos referral hospital, North West Ethiopia; a retrospective cohort study. J AIDS Clin Res. 2014;5:2.
25. Tesfaye D, Berhanu S, Lamessa O. Survival and Predictors of Mortality among Adults on Antiretroviral Therapy in Selected Public Hospitals in Harar, Eastern Ethiopia. J Trop Dis. 2014;2:5.
26. Nathan. F, Greame. M, Marco. V. The evolving role of CD4 cell counts in HIV care; Current Opinion HIV AIDS. HIV Diagnostics. 2017;12:123–128.
27. WHO. WHO, Consolidated Guidelines on the Use of Antiretroviral Drugs for Preventing and Treating HIV Infection, 2013. WHO; 2013. Available from: https://www.who.int/hiv/pub/guidelines/arv2013/en/.
28. Isidore S, Mohamadou S, Anne-Marie S, Joris M, Marleen B. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009;14:36–43.
29. Erdaw T, Gobena A. Survival and predictors of mortality among human immunodeficiency virus patients on anti-retroviral treatment at Jinka Hospital, South Omo, Ethiopia: a six years retrospective cohort study. Epidemiol Health. 2016;38.
30. Eyuel T, Alemayehu W. Assessment of antiretroviral treatment outcome in public hospitals, South Nations Nationalities and Peoples Region, Ethiopia. Ethiop J Health Dev. 2011;25:2.
31. Kidane T, Fisaha H, Neway H. Predictors of Mortality among Patients Enrolled on Antiretroviral Therapy in Aksum Hospital, Northern Ethiopia: a Retrospective Cohort Study. PLoS One. 2015;9:1.
32. Laxmi B, Elise K, Keshab D, Rachana S. Survival on antiretroviral treatment among adult HIV-infected patients in Nepal: a retrospective cohort study in far-western Region, 2006–2011. BMC Infect Dis. 2013;13:604. doi:10.1186/1471-2334-13-604
33. Jaya C, Narendra KT, Shashi RP, Saurabh S. Determinants of survival in adult HIV patients on antiretroviral therapy in Eastern Uttar Pradesh: a prospective study. Indian J Med Res. 2014;140:491–500.
34. Isidore S, Mohamadou S, Anne-Marie S, Joris M, Marleen B. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009;14:36–43. doi:10.1111/j.1365-3156.2008.02183.x
35. Ketema K, Eshetu W. Survival analysis of HIV-infected patients under antiretroviral treatment at Teaching Hospital, Addis Ababa, Ethiopia. Ethiop J Health Dev. 2012;26:3.
36. Andrew GF, Godwin A, Frank M, Agnes NK, Moses K. Socioeconomic position and ten-year survival and virologic outcomes in a Ugandan HIV cohort receiving antiretroviral therapy. PLoS One. 2017;12:12.
37. Samuel O, Memiah P, Biadgilign S, Ndirangu M, Kyomuhangi L. Effects of highly active antiretroviral therapy on the survival of HIV-infected adult patients in urban slums of Kenya. Pan African Med J. 2015;20:63.
38. Jemal A, Helen M. Identifying Factors Related to the Survival of AIDS Patients under the Follow-up of Antiretroviral Therapy (ART): the Case of South Wollo. Int J Data Envelop Anal Operations Res. 2014;1(2):21–27.
39. Sibhatu B, Ayalu AR, Tesfaye D. Predictors of mortality among HIV infected patients taking antiretroviral treatment in Ethiopia: a retrospective cohort study. AIDS Res Ther. 2012;9(1):15. doi:10.1186/1742-6405-9-15
40. Wolde BS, Nahom TN, Almaz HC. Survival Pattern and Its Determinants among Adult HIV-Infected Patients after Initiation of HAART in Dilla Hospital Ethiopia. J Clin Exp Immunol. 2016;1:1.
41. Wondimu A, Afework M, Alem D, Felicia A. Treatment outcomes and their determinants in HIV patients on Anti-retroviral Treatment Program in selected health facilities of Kembata and Hadiya zones, Southern Nations, Nationalities and Peoples Region, Ethiopia. BMC Public Health. 2015;15:826. doi:10.1186/s12889-015-2176-5
42. Tesfaye S, Abulie T, Tesfaye G, Dabere N, Demewoz H. Predictors of Mortality among Adult Antiretroviral Therapy Users in Southeastern Ethiopia: retrospective Cohort Study Hindawi Publishing Corporation. AIDS Res Treat. 2015;8.
43. Sandeep. R, Bidhubhusan. M, Subhashish S, et al. Adherence to Antiretroviral Therapy and Its Effect on Survival of HIV-Infected Individuals in Jharkhand, India. PLoS One. 2013;8:6.
44. Solomon H, Girma T, Henok T, Prabhanjan KV. Determinants of Survival in HIV Patients: a Retrospective Study of Dilla University Hospital HIV Cohort. Int J Virol AIDS. 2016;3:2.
45. Hant. PW, Martin. JN, Sinclair. E, Hagos. E. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–1543. doi:10.1086/374786
46. Shibre M, Bekele B, Abera K. Predictors of Survival in HIV-Infected Patient after Initiation of HAART in Zewditu Memorial Hospital, Addis Ababa, Ethiopia. Int Schol Res Notices. 2014;2014.
47. Monitoring UG. Indicators for Monitoring the 2016 United Nations Political Declaration on Ending AIDS. Geneva: UNAIDS; 2018. Available from: https://www.unaids.org/en/resources/documents/2020/global-aids-monitoring-guidelines.
48. Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med. 2016;13(11):e1002183. doi:10.1371/journal.pmed.1002183
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

