Back to Journals » Cancer Management and Research » Volume 13
Survival and Predictors of Mortality among Breast Cancer Patients in Northwest Ethiopia: A Retrospective Cohort Study
Authors Tiruneh M , Tesfaw A , Tesfa D
Received 18 September 2021
Accepted for publication 6 December 2021
Published 16 December 2021 Volume 2021:13 Pages 9225—9234
DOI https://doi.org/10.2147/CMAR.S339988
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Mulu Tiruneh, Aragaw Tesfaw, Desalegn Tesfa
Department of Public Health, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Mulu Tiruneh
Department of Public Health, College of Health Science, Debre Tabor University, PO Box 272, Debre Tabor, Ethiopia
Tel +251 913249415
Email [email protected]
Background: Breast cancer is the most common malignant tumor and the leading cause of cancer death in over 100 countries. Despite the high burden of difficulty, the survival status and the predictors for mortality are not yet determined in Ethiopia. Studies related to this area are scarce. Therefore, we aimed to estimate the survival status and predictors of mortality among breast cancer patients in Northwest Ethiopia.
Methods: A retrospective cohort study design was carried out from September 2015 to August 2020 among 482 women who had breast cancer in Northwest Ethiopia. A systematic sampling technique was employed to select the required representative sample. The Cox regression model was used to identify the predictors of mortality among breast cancer patients.
Results: For this study, 482 participants had followed for 8824 person-months total analysis time or at-risk time. In our findings, the overall survival of breast cancer patients at the end of two and five years was 54.24% and 25.8%, respectively. In the multivariable Cox regression model, age, stage of BC, menopausal status, and surgical therapy were significant predictors of death.
Conclusion: The overall survival after two years was 54.24%, and after five years was 25.8%. This result is lower than the recently published report and indicates that in LMIC, especially in rural cancer centers, the infrastructure and resources for routine screening mammography are often unavailable. Therefore, there is a need to promote early diagnosis of BC at each level of health-care delivery point.
Keywords: breast cancer, survival, predictors, Ethiopia
Background
Breast cancer is the most frequently occurring cancer in women globally approximately 1.7 million new cases and around 522,000 related deaths occurring in 2012.1 Cancer is a public health threat and a third leading cause of death in the Africa region. It is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells in the body.2 In Ethiopia, about 7% of mortality is due to cancer.3 The annual incidence of cancer is around 60,960 cases, and the annual mortality is over 44,000. The most prevalent cancers in Ethiopia among the entire adult population are breast cancer (BC) (30.2%), cervical cancer (13.4%), and colorectal cancer (5.7%). The estimated prevalence of BC cases in 2015 was 13,987 by a crude incidence rate of 28.2 per 100,000. The trend of breast cancer has significantly increased year to year among females than males.4,5
Previous studies revealed that tumor characteristics, metastasis, advanced disease stage, lymph vascular space invasion, multiple metastases, sites, maintenance, endocrine therapy are predictors of survival of breast cancer patients.6,7 Similarly, young age, late-stage at diagnosis, positive lymph node status, tumor size 3 and 4, and hormone receptor-negative status are predictors of survival.8
In Ethiopia, the Addis Ababa cancer registry reports show that breast cancer accounts for 34% of all female cancer cases, followed by cervical cancer accounting for 16% of cases.4,9 The disease remains a public health concern in developing and low-middle income countries (LMIC).10
Exposure to exogenous hormones such as oral contraceptives, hormone replacement therapy,11 and dietary fat intake12,13 increases the risk of breast cancer. Despite the perception of all these risk factors, about 70% of females who develop breast cancer do not have identifiable risk factors.14 However, the most significant risk factors for breast cancer are gender (being a woman) and age (with most cases developing in women after menopause).15–17 The above reasons and others like changes in lifestyle and lack of clinical advances to combat the disease, especially in developing countries, lead the inclination of the disease to increase from year to year.18 Studies on cancer survival are vital to formulate cancer control strategies, prioritize cancer control measures, and assess the effectiveness and cost-effectiveness of those strategies.19
Ethiopia had planned a national cancer control program (NCCP) in 2016.20 However, even though there is a strategy to expand other additional centers, there is only one radiation therapy center in Ethiopia which is found in the capital city (Tikur Anbessa Specialized Hospital), and very limited chemotherapy centers at the national level.21
There are few studies in Ethiopia, which conducted by using the five-year survival of breast cancer. Thus, still, there is a gap to know the overall survival of breast cancer patients. Hence, the purpose of this study was to estimate survival status and predictors of mortality among women diagnosed with breast cancer in Northwest Ethiopia.
Materials and Methods
Study Setting and Design
This retrospective cohort study had conducted at the oncology units of the University of Gondar comprehensive specialized hospital, Northwest Ethiopia. The hospital currently provides diagnostic, surgical, and chemotherapy treatment services for cancer patients with breast cancer. Medical reviews of BC patients who had been diagnosed from September 2015 to August 2020 were reviewed retrospectively.
Sample Size Calculation and Sampling Method
The representative sample for this study had calculated by applying Stat-Calc Epi info version 7.2 by considering 34% of the proportion among outcomes from the previous study with a 95% two-sided confidence level.8 Thus, the required representative sample was 482.
Sampling Methods and Procedures
We had applied the systematic sampling method to select the required representative sample from a given population according to a random starting point. Typically, every nth member is selected from the total population for inclusion in the sample population.
A total of 482 breast cancer patient cards had revised from September 2015 to August 2020. From the total samples, their phone number was not found on 102 patient charts. The remaining 380 cards have telephone numbers, and a telephone interview was made with 300 patients or their relatives whose age is greater than 18 years. The 80 phone calls are not successful due to different reasons.
Inclusion and Exclusion Criteria
Patients confirmed with breast cancer and who have clear stage information on their medical charts were included in the study. However, patients with incomplete follow-up (lost follow-up for more than six months) were excluded.
Operational Definition
Lost to follow-up (LTF): Patients that are lost to follow-up for >6 months.
Worst-case analysis: All patients within LTF developed distant metastasis three months after the last date of the visit.8
Censored: Patients who had been alive at the end of the study were right-censored, and those who developed the event or LTF were left censored.
Metastasis: Is a pathogenic agent distributed from a starting point to a different site on the host body.
Survival time: The total time the patient had survived without developing the outcome after diagnosis.
Measurement
According to the International Coding standards for cancer registries,22 the date of incidence had defined as the first consultation at the hospital for cancer in question. The stage of BC was determined by the American Joint Committee on Cancer staging system AJCC (seventh edition) using the information on tumor size (T) and nodal status (N).23 Two observations had used in this regard; TNM staging at the time of diagnosis and the last follow-up to confirm the progression of the disease. Tumor size is primarily ascertained by clinical examination of the oncologist, if not available when it had obtained from a biopsy. Histological type and nuclear grading had taken from biopsy results.
Data Collection and Quality Control Procedures
The outcome variable for this study was time to death. Breast cancer patients’ medical records had reviewed based on the eligibility criteria in the selected hospital during the study period. After this, a phone call to patients with BC had made to confirm their current status, whether alive or dead. Data were collected on the socio-demographic characteristics, clinical and pathological characteristics, and type of BC therapy. A three-day training was given to data collectors (five BSc nurses) on the overall data collection procedure by the principal investigators to ensure data quality and to keep its consistency. Strict supervision and monitoring was done during data collection.
Data Processing and Analysis
Demographic and clinical data at baseline were collected from medical records and the telephone interview based on a structured questionnaire. Data cross-checking was done before analysis, and data were coded and entered in STATA statistical software and analyzed using STATA 16.0. The overall survival was estimated by the Kaplan–Meier curve. A Log rank test had used to compare survival among groups with a confidence interval of 95%. A P-value of 0.25 and less from the univariate analysis were potential candidate variables for the multivariate model (Weibull regression model), and variables with a p-value <0.05 were considered statistically significant. Multi-collinearity and interaction for the main effect model have been checked, and the variance inflation factor of more than ten has been considered denoting its existence. The assumptions of the Cox Proportional Hazard regression model are checked by using the global test and Schoenfeld residual plots. Finally, the goodness of fit of the model was assessed by the Cox-Snell residual plot.
Ethical Approval and Informed Consent
Ethical clearance was obtained from the Research and Publication directorate office of Debre Tabor University. Approval and permission were obtained from the administrative officer of the specialized hospital to review patient cards and contact patients on a phone call. Verbal consent had been obtained by telephone from patients, and patients who were dying of it had obtained from their relatives who were more than 18 years. Confidentiality of patient information was kept and the research was conducted based on the declaration of Helsinki.
Results
Patient’s Socio-Demographic and Clinical Characteristics
A total of 482 patients, with BC, were diagnosed from September 1st, 2015 to August 30th, 2020, in the University of Gondar Comprehensive Specialised Hospital. From the total sample, we obtained 380 patient charts with telephone addresses. Of these, the outcome of 300 (62.2%) patients had confirmed (135 were alive, 165 have died), 182 (37.8%) patients were lost to follow-up. The breast cancer patient’s years ranged from 21 to 77 (median age 61 years), and in this study, the women age group 40–65 was the largest compared to other age groups (55%). Out of the total patients, 276 (57.26%) of them were from urban areas. Of the whole women, the majority thirty hundred sixty-nine were married. Among the samples, 174 (36.1%) were illiterate, and 203 (42.12%) were housewives. In this retrospective cohort study, almost half of the women were postmenopausal 295 (61.2%). Of the total patients, the majority of the women had pathology reports showing grade III, the BC patients had the most frequent ductal carcinoma, and 317 (65.77%) had positive lymph nodes. Out of the total patients, most women were presented with stages three and four at the first hospital visit, 119 (24.69%) were stage III, and 195 (40.46%) were stage IV. About 89 (18.5%) of the women had a family history of breast cancer.
Of the total patients, 235 (48.76%) with breast cancer had been treated with breast surgery. Chemotherapy was administered to 382 breast cancer patients. Of these, 91 (18.9%) had completed the treatment (good adherence), on the other hand, 291 (60.4%) had discontinued (poor adherence), and 100 (20.8%) were on schedule at the time of data collection (Table 1).
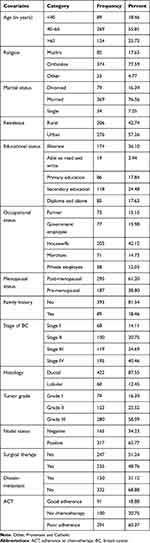 |
Table 1 Socio-Demographic Characteristics of Breast Cancer Patients in Northwest Ethiopia, 2015–2020 |
Survival Status of the Patients
For this study, 482 participants were followed for 8824 person-months total analysis time or at-risk time. From the whole patient’s 150 (31.12%) of their results showed that distant metastasis occurred during the follow-up time. There was a minimum of 8 months and a maximum of 60 months follow-up time with a median follow-up time of 15 months. Fifty-three patients have visited the hospital within ten months before the end of the study. The overall survival of BC patients at the end of two and five years was 54.24% and 25.8%, respectively. The overall survival for stage I, stage II, stage III, and stage IV of BC at the end of 2 years was 54.54%, 53%, 50.9%, and 46.4%, while at the end of 5 years, 23.5%, 22.5%, 20.9%, and 20.6%, respectively (Table 2).
 |
Table 2 Log Rank Test for Equality of Survival Function of Patients with Breast Cancer Diagnosed in Northwest Ethiopia, 2015–2020 |
We have used the Kaplan–Meier survival curves to see whether there is a difference in breast cancer survival between different groups of covariates. The Kaplan–Meier survival curves for each study variable provide an initial insight into the shape of the survival function. We observed in Figure 1, the BC patients at the beginning months of follow-up had a better probability of overall survival (OS), then it becomes decreased in the next consecutive follow-up months (Figure 1).
 |
Figure 1 Plot of the overall estimate of Kaplan–Meier survivor function of breast cancer patients in Northwest Ethiopia, 2015–2020. |
The Log rank test indicates that statistically, there is a significant difference in survival experience among different predictors. The overall survival at the beginning of follow-up was better in the women aged group of below 40 years and had lower in age groups of greater than 65 years, but, after 30 months of follow-up, the age group 40–65 years had better overall survival (Figure 2).
 |
Figure 2 Kaplan–Meier survival estimates of patients with breast cancer diagnosed by age groups in Northwest Ethiopia, 2015–2020. |
Factors Influencing the Overall Survival of Patients
Variables that are significant at a p-value less than 0.25 in the Log rank test procedure could be a potential candidate variable for multivariable statistical analysis. Age, marital status, menopausal status, stage of BC, tumor grade, histology, nodal status, surgical therapy, and adherence to chemotherapy were potential candidate variables for the multivariable model.
Since the PH assumptions satisfy, we can apply the Cox Proportional Hazards Model to determine the factors that influence the overall survival of breast cancer patients. Thus, to obtain factors that influenced overall survival, the Cox Proportional Hazards model was employed.
The hazards of death in the age group 65 years and above patients with breast cancer were 2.04 times higher when compared with that of women age group less than 40 years (AHR 2.04, 95% CI 1.36–3.05). In patients that presented to the oncology unit, the age group of 40–65 had 1.7 times increased risk of death when compared with that of the women age group of below 40 years (AHR 1.7, 95% CI 1.19–2.43). The hazard of death for premenopausal patients was 1.33 times higher as compared to postmenopausal patients (AHR 1.33, 95% CI 1.03–1.72). Those stage II BC patients had 1.56 times the risk of death compared to stage I BC, after keeping all other covariates at some constant level (AHR 1.56, 95% CI 1.09–3.42). Similarly, patients who presented in the oncology unit with stage III BC had 1.75 times the risk of death compared to stage I BC (AHR 1.75, 95% CI 1.24–3.45). The hazard of death for women presenting with stage IV BC patients was 1.82 times (AHR 1.82, 95% CI 1.52–3.62) higher than with stage I BC. The hazard of death for patients who took surgical therapy has 31% less risk of death than their counterparts who did not take surgical therapy (AHR 0.69, 95% CI 0.54–0.89) (Table 3).
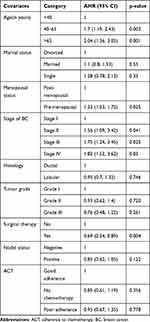 |
Table 3 Multivariable Cox Regression Analysis Model for Survival of Breast Cancer Patients Diagnosed in Northwest Ethiopia, 2015–2020 |
Discussion
This retrospective cohort study was the first detailed study on BC survival in Northwest Ethiopia. Overall survival probability in our study of Ethiopian women with breast cancer was 54.24% after two years and 25.8% after five years with a median follow-up 15 months.
Age, stage of BC, surgical therapy, and menopausal status were significant predictors of survival. Patient characteristics in our study tended to be unfavorable compared with other studies; more than 60% were postmenopausal, aged 40–65 years, and stage of BC (stage III and stage IV disease). The histological type was more favorable; the majority was ductal with tumor grade III.
When we were observed 5-year overall survival probabilities worldwide, the percentages ranged from 89% in developed countries and decreased to 12% in the developing country especially in Gambia.24,25 A study done in Uganda showed that the 5-year overall survival probability for stage I and II cancer of 74% and stage III–IV cancers of 39%.26 In our study, stage I patients showed 23.5%, stage II patients 22.5%, stage III patients 20.9%, and stage IV patients 20.6% overall survival probability, and overall survival, expected to be lower. This is due to the fact that in low- and middle-income countries, the infrastructure, and resources for routine screening mammography are often unavailable. As a result, of such lower resource settings, breast cancers are commonly diagnosed at late stages (stage III and stage IV), and women may receive inadequate treatment, pain relief, or palliative care. In the present study, the median age of 61 years (21–77) was high compared to the study done in Ethiopia.8 Previous studies revealed that the median age of 46 years in Mali, 49 years in Ghana, and 48 years in Nigeria.27–29 The contradiction of the result could be due to the use of different source populations and the use of different age ranges. In our study, differences in survival in the age groups less than 40 years show a better prognosis in terms of survival of BC. This finding had confirmed by a study conducted in Iran.30 However, our finding contradicts Western studies that younger women have the worse outcome.31 Most likely in our groups of women aged greater than 65 years showed a poorer prognostic survival rate of breast cancer. This difference may be due to the younger women age group’s earlier presentation to cancer treatment, adequate screening services, and quality of care when relatively compared with the older age group. In addition to this, different categorizations and settings might be an explanation for this difference.
In our cohort retrospective study, most patients with breast cancer (65% with stage III and stage IV) presented with late stages of the disease. This finding is in line with the previous studies.26,32 This is due to LMIC’s long delay in consultation, access barriers, negative symptom interpretation, fear, belief in alternative medicine, social relations, and networks.33,34
We found that the HR of BC patients greater than 65 years of age was higher (AHR 2.04, 95% CI 1.36–3.05) compared with that of women age group less than 40 years. Similarly, the patient age group 40–65 was 1.7 times increased risk of death compared with age group less than 40 years. Hence, this indicates that age was the prognostic factor for BC survival. The same result was shown in previous studies.35,36 On the other hand, other studies suggested that the survival among women below 40 years was as poor as that of women of older ages.37,38 This discrepancy may result from the use of different age ranges and study settings.
In our results, stage IV breast cancer patients have 1.82 times increased risk of death when compared with stage I patients. Similarly, BC patients with stage III disease have 1.75 times increased risk of death compared to early stages. This is in line with a study conducted in Ethiopia,8,39 which indicates that earlier detection would improve the outcome in breast cancer patients in Ethiopia. Therefore, advanced stage (stage III and stage IV) at diagnosis is a common problem in cancer care and has been widely reported, which significantly affects breast cancer survival.
The result of this analysis shows that surgery received compared to no surgery significantly impacted survival. When compared with no surgery and mastectomy, breast conservative surgery increases the survival of BC. This result is in line with the previous findings.40,41
In this study, there is a higher proportion of postmenopausal compared to a pre-menopausal group of women when they were first admitted to the hospital (at the time of diagnosis), which contradicts the previous study conducted in Malaysia.42 This discrepancy may become the difference in population structure, which had included the population age at the time of diagnosis.
Strength and Limitations of the Study
Our study has some strengths and limitations: We used all available data which minimizes the sampling error, and it is the most up-to-date and the first for the study area. This is a retrospective study and, missing data was a challenge such as socio-economic variables and other tumor characteristics. As a result, these are not well addressed, some missing information is associated with study participants who might have died from causes other than BC, and these rates do not take that into account.
Conclusion
The overall survival after two years and after five years is below 54.24% and 25.8%, respectively, this result is lower than the recently published report. This indicates that in LMIC, especially in rural cancer centers, the infrastructure and resources for routine screening mammography are often unavailable. Thus, the study could fill the information gap by estimating overall survival and identifying its determinants in settings that have adjuvant therapies and use. Stage of BC, age of participants, surgical therapy, and menopausal status are independent predictors of poor survival. The majority of the patients treated in the University of Gondar Comprehensive Specialised Hospital had mainly presented with stage III and stage IV BC with the median survival time of 15 (8–60) months. Therefore, there is a need to promote early diagnosis of breast cancer at each level of health-care delivery point.
Abbreviations
BC, breast cancer; HR, hazard ratio; OS, overall survival; PH, proportional hazard.
Data Sharing Statement
The data for this study were obtained from the corresponding author on a reasonable request.
Acknowledgment
We thank the University of Gondar comprehensive specialized hospitals, especially the Oncology unit staff and data collectors for their valuable collaboration.
Author Contributions
All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article was submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declared no conflicts of interest for this work.
References
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Ferlay JSI, Ervik M, Dikshit R, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide. France: International Agency for Research on Cancer IARC Cancer Base No. 11 Lyon; 2013.
3. World Health Organization. Non-communicable disease country profile; 2018.
4. Memirie ST, Habtemariam M, Asefa M, et al. Estimates of cancer incidence in Ethiopia in 2015 using population-based registry data. J Global Oncol. 2018;4(4):1–11. doi:10.1200/JGO.17.00175
5. Abate S, Assefa M, Tigeneh W. Trends of breast cancer in Ethiopia. Int J Cancer Res Mol Mech. 2016;2(1):1.
6. Arora DHS, Hasan S, Male E, Pruszynski J, Ord C, Rao A. Prognostic factors affecting outcomes in triple-negative breast cancer. Int J Radiat Oncol Biol Phys. 2015;93(3):E33. doi:10.1016/j.ijrobp.2015.07.626
7. Leivonen MK, Kalima TV. Prognostic factors associated with survival after breast cancer recurrence. Acta Oncol. 1991;30:583–586. doi:10.3109/02841869109092422
8. Kantelhardt EJ, Zerche P, Mathewos A, et al. Breast cancer survival in Ethiopia: a cohort study of 1070 women. Int J Cancer. 2014;135(3):702–709. doi:10.1002/ijc.28691
9. Cancer RoAA. Top 10 female cancer in Addis Ababa; 2015.
10. Al-Foheidi M, Al-Mansour MM, Ibrahim EM. Breast cancer screening: a review of benefits and harms, and recommendations for developing and low-income countries. Med Oncol. 2013;30:471. doi:10.1007/s12032-013-0471-5
11. Urban M, Canfell K, O’Connell D, et al. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black South African women: a case-control study. PLoS Med. 2012;9:e1001182. doi:10.1371/journal.pmed.1001182
12. Kotepui M, Chupeerach C. Age distribution of breast cancer from a Thailand population-based cancer registry. Asian Pac J Cancer Prev. 2013;14(6):3815–3817. doi:10.7314/APJCP.2013.14.6.3815
13. Velie EM, Schairer C, Flood A, et al. Empirically derived dietary patterns and risk of postmenopausal breast cancer in a large prospective cohort study. Am J Clin Nutr. 2005;82(6):1308–1319. doi:10.1093/ajcn/82.6.1308
14. WCRF International. Breast cancer statistics. WCRF International; 2012.
15. Beiki OHP, Hall P, Ekbom A, Moradi T. Breast cancer incidence and case fatality among 4.7 million women with social and ethnic background: a population-based cohort study. Breast Cancer Res. 2012;14:R5. doi:10.1186/bcr3086
16. Harhra NA, Basaleem HO. Trends of breast cancer and its management in the last twenty years in Aden and adjacent governorates, Yemen. Asian Pac J Cancer Prev. 2012;13(9):4347–4351. doi:10.7314/APJCP.2012.13.8.4247
17. Morris CR, Nasser K, Hofer BM, et al. (2010) Trends in cancer incidence, mortality, risk factors, and health behaviors in California. Sacramento, CA: California Department of Public Health, Cancer Surveillance Section.
18. International Agency for Research on Cancer. Latest world cancer statistics Global cancer burden rise to 14.1 million new cases in 2012: marked increases in breast cancer must be addressed. WHO: Press release 223; 2013.
19. Allemani C, Weir H, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (Concord-2). Lancet. 2015;385(9972):977–1010. doi:10.1016/S0140-6736(14)62038-9
20. Ethiopian Federal Ministry of Health. Ethiopian National Cancer Control Plan, 2016–2020; 2015.
21. Eber-Schulz P, Tariku W, Reibold C, et al. Survival of breast cancer patients in rural Ethiopia. Breast Cancer Res Treat. 2018;170(1):111–118. doi:10.1007/s10549-018-4724-z
22. Jensen OM. Cancer Registration: Principles and Methods. France: IARC Scientific Publication No 95 Lyon, WHO; 1991.
23. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
24. Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11(2):165–173. doi:10.1016/S1470-2045(09)70335-3
25. Youlden DR, Cramb SM, Dunn NAM, et al. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival, and mortality.. Cancer Epidemiol. 2012;36:237–248. doi:10.1016/j.canep.2012.02.007
26. Gakwaya AK, Kigula-Mugambe JB, Kavuma A, et al. Cancer of the breast: 5-year survival in a tertiary hospital in Uganda. Br J Cancer. 2008;99(1):63–67. doi:10.1038/sj.bjc.6604435
27. Ly M, Antoine M, Dembélé AK, et al. High incidence of triple-negative tumors in sub-Saharan Africa: a prospective study of breast cancer characteristics and risk factors in Malian women seen in a Bamako University Hospital. Oncology. 2012;83(5):257–263. doi:10.1159/000341541
28. Ohene-Yeboah M, Adjei E. Breast cancer in Kumasi, Ghana. Ghana Med J. 2012;46:8–13.
29. Adebamowo CA, Famooto A, Ogundiran,TO, et al. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat. 2008;110:183–188. doi:10.1007/s10549-007-9694-5
30. Movahedi M, Haghighat S, Khayamzadeh M, et al. Survival rate of breast cancer based on geographical variation in Iran, a National Study. Iran Red Crescent Med J. 2012;14(12):798. doi:10.5812/ircmj.3631
31. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2012;62:220–241. doi:10.3322/caac.21149
32. Mousa SM, Seifeldin A, Hablas A, et al. Patterns of seeking medical care among Egyptian breast cancer patients: relationship to late-stage presentation. Breast. 2011;20(6):555–561. doi:10.1016/j.breast.2011.07.001
33. Unger-Saldaña K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol. 2014;5(3):465. doi:10.5306/wjco.v5.i3.465
34. Donkor A, Wiafe S, Vanderpuye V, et al. Factors contributing to late presentation of breast cancer in Africa: a systematic literature review. Arch Med. 2015;8(2.2):1–10.
35. Ribeiro GG, Swindell R. prognosis of breast carcinoma in women aged less than 40 years. Clin Radiol. 1981;32:231–236. doi:10.1016/S0009-9260(81)80168-7
36. Mueller CB, Ames F, Anderson GD. Breast cancer in 3558 women: age as a significant determinant in the rate of deaths and causes of death. Surgery. 1978;83:123–132.
37. Lees AW, Jenkins HJ, May CL, et al. Risk factors and 10-year breast cancer survival in northern Alberta. Breast Cancer Res Treat. 1989;13:143–151. doi:10.1007/BF01806526
38. Levi F, Randimbison L, Vecchia CL. Breast cancer survival with sex and age. Oncology. 1992;49:41. doi:10.1159/000227083
39. Shita A, Yalew AW, Tesfaw A, et al. Survival and predictors of mortality among breast cancer patients diagnosed at Hawassa comprehensive specialized and teaching hospital and private oncology clinic in Southern Ethiopia: a retrospective cohort study; 2020.
40. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi:10.1001/jama.295.21.2492
41. Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88(1):114–123. doi:10.1002/(SICI)1097-0142(20000101)88:1<114::AID-CNCR16>3.0.CO;2-J
42. Abdullah NA, Mahiyuddin WRW, Muhammad NA, et al. Survival rate of breast cancer patients in Malaysia: a population-based study. Asian Pac J Cancer Prev. 2013;14(8):4591–4594. doi:10.7314/APJCP.2013.14.8.4591
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
