Back to Journals » Infection and Drug Resistance » Volume 16
Surveillance and Outcomes of Pediatric Hematopoietic Stem Cell Transplantation Recipients During the Recent COVID-19 Outbreak in China
Authors Wang X, Yu U , Ding C , Ye H, Wang C, Yang C, Li Y, Zhou X, Zhang Q, Liu S, Wen F
Received 26 June 2023
Accepted for publication 29 November 2023
Published 7 December 2023 Volume 2023:16 Pages 7455—7464
DOI https://doi.org/10.2147/IDR.S427762
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Xiaodong Wang,1,2,* Uet Yu,2,* Chao Ding,2,3 Huiying Ye,2 Chunjing Wang,2 Chunlan Yang,2 Yue Li,2 Xiaohui Zhou,2 Qian Zhang,2 Sixi Liu,2 Feiqiu Wen1,2
1Department of Pediatrics, First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China; 2Department of Hematology and Oncology, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China; 3Discipline of Pediatrics, China Medical University, Shenyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feiqiu Wen, Department of Pediatrics, First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China, Email [email protected] Sixi Liu, Department of Hematology and Oncology, Shenzhen Children’s Hospital, 7019 Yitian Road, Futian, Shenzhen, 518038, People’s Republic of China, Email [email protected]
Background: The COVID-19 pandemic presents challenges for healthcare systems globally, especially in vulnerable populations such as pediatric hematopoietic stem cell transplant (HSCT) recipients. This study examines the clinical characteristics and outcomes of COVID-19 infection in pediatric HSCT recipients within one year post-HSCT.
Methods: Retrospective analysis was conducted on data from 247 pediatric patients. None of them had received SARS-CoV-2 vaccination or had prior infection. SARS-CoV-2 infection was confirmed using RT-PCR testing. COVID-19 disease severity was categorized according to established guidelines. Demographic, clinical, laboratory, imaging and treatment data were collected.
Results: The median age of the cohort was 7± 3.7 years, with thalassemia major as the predominant underlying disease. Allogeneic HSCT was performed in the majority of cases, with haploidentical donors being the most common source of grafts. Nearly half of the patients developed COVID-19, with significantly higher infection rates observed in recipients over 100 days compared to recipients within 100 days post-HSCT (40.1% vs 21.7%, p< 0.05, Fisher’s Exact test). Fever (n=107, 43.2%) and cough (n=88, 35.6%) were the most common symptoms. While most patients had mild disease and did not require specific anti-viral treatment, a significant proportion required hospitalization (n=34, 13.8%). Various treatments were employed hospitalized patients, including Paxlovid (n=19, 55.9%), methylprednisolone (n=7, 20.6%), IL-6 antibody (n=2, 5.9%), mesenchymal stem cells (n=3, 8.8%), and exosomes nebulization therapy (n=2, 5.9%). Despite multidisciplinary approaches, one patient died from severe respiratory failure. However, overall survival of all patients remained high (99.53%; CI 96.72– 99.93%), indicating favorable outcomes in pediatric HSCT recipients with COVID-19.
Conclusion: This study provides insights into clinical features, therapeutic measures, and outcomes of pediatric HSCT recipients following COVID-19 infection in a large HSCT center in China. These findings contribute to our understanding of COVID-19 in this population and inform strategies to mitigate the impact the pandemic’s impact on their care.
Keywords: COVID-19, SARS-CoV2, hematopoietic stem cell transplantation, pediatrics
Introduction
The COVID-19 pandemic has presented significant challenges for healthcare system globally.1,2 Although children and adolescents generally exhibit milder symptoms and have a lower rate of severe disease and death from COVID-19 compared to adults,3,4 those who have undergone hematopoietic stem cell transplantation (HSCT) are more susceptible to severe COVID-19 infection due to their impaired immune response and underlying comorbidities.5,6 While there is evidence that pediatric HSCT recipients have a better prognosis than adults, they remain at risk for severe complications caused by COVID-19 infection, including severe respiratory dysfunction, multi-organ failure, and potentially fatal outcomes.7,8 In addition to the direct impact of COVID-19 infection, the pandemic may also create obstacles for the ongoing care and treatment of these susceptible patients, potentially disrupting their regular care and follow-up.9
Since 2000, China has implemented a series of strategies to control the spread of the virus, including lockdowns, travel restrictions, and widespread testing and quarantine measures.10,11 As a result of these efforts, the transmission of the virus was effectively controlled and none of pediatric HSCT patients receiving treatment in our center contracted the virus. However, with the easing of these measures, outbreaks of COVID-19 have been reported in several parts of the country, including our own, in December 2022. Unlike many other countries, there is limited data available on the effects of COVID-19 on pediatric HSCT recipients in China, raising concerns about the potential impact on their outcomes and prognosis.8 Moreover, the vaccination rate among pediatric patients undergoing HSCT remains low in China. Given that patients undergoing immune reconstitution and receiving active immunosuppressive therapy are more susceptible to COVID-19 infection, we conducted the current study to examine the clinical characteristics and outcomes of COVID-19 infection in children within one year after HSCT.
Methods
Patient Participation
Between January and December 2022, 247 pediatric patients aged between 6 months to 7 years of age underwent HSCT at Shenzhen Children’s Hospital in China and were recruited for this study. None of these patients received SARS-CoV-2 vaccine or had prior SARS-CoV-2 infection. SARS-CoV-2 infection was confirmed by RT-PCR in nasopharyngeal or pharynx swabs. The severity of COVID-19 was determined according to guidelines published by China Center for Disease Control and Prevention.12 COVID-19 treatment related data were collected from the electronic patient system for hospitalized patients. The remaining patients were followed up through outpatient clinics or phone interviews between 1st February 2023 and 15th February 2023. Demographic data including age and gender; patients’ clinical information such as primary diseases, HSCT type, COVID-19 related symptoms, and treatment before and after HSCT, and their outcomes; laboratory results including SARS-CoV-2 RT-PCR test and antigen test results; imaging findings; and information regarding COVID-19 related treatment were collected. This study was approved by the Ethics Committee of Shenzhen Children’s Hospital (No.2021102) and was conducted in compliance with the Declaration of Helsinki and its later amendments. Written consent was obtained from the parents and legal guardians of the patients for the collection and publication of their clinical data.
Statistical Analysis
All statistical analyses were performed using the GraphPad Prism software version 9. Categorical analyses were performed using the Fisher’s exact test. Survival rate was calculated using the Kaplan-Meier analysis. A p value less than 0.05 was considered statistically significant.
Results
Patient Characteristics
Table 1 displays the characteristics and outcomes of the study population, which consisted of 247 pediatric patients who underwent HSCT. The patients’ median age was 7±3.7 years (ranging from 6 months to 17 years), with 83 patients being female (33.6%) while 164 patients were male (66.4%). Thalassemia major was the primary disease for the majority of patients (n=184, 74.5%), followed by acute myeloid leukemia (n=14, 5.7%) acute lymphoblastic leukemia (n=12, 5.0%), primary immune deficiencies (n=10, 4%), neuroblastoma (n=9, 3.6%), severe aplastic anemia (n=7, 2.8%), lymphoma (n=3, 1.2%), adrenoleukodystrophy (n=3, 1.2%), brain tumors (n=2, 0.8%), pyruvate kinase deficiency (n=2, 0.8%), and yolk sac tumor (n=1, 0.4%). Allogeneic HSCT was performed in 230 patients (93.1%), and autologous HSCT in 17 patients (6.9%). The majority of allogeneic HSCT patients received grafts from haploidentical donors (n=176, 69.6%), while others received grafts from matched related (n=36, 14.6%) and unrelated (n=22, 8.9%) donors.
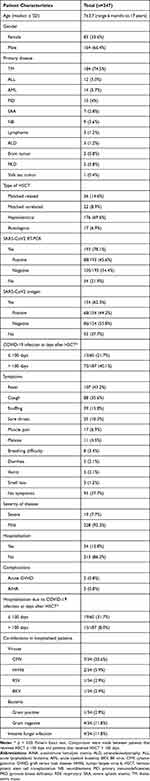 |
Table 1 Patient Characteristics |
Patients received SARS-CoV-2 testing via RT-PCR or antigen self-detection kits, but only those testing positive for SARS-CoV-2 by RT-PCR were considered to have confirmed SARS-CoV-2 infection. Almost half of the HSCT patients in 2022 developed COVID-19, but only 35% of the patients had confirmed infection. Of the 193 patients tested for SARS-CoV-2 by RT-PCR, 45.6% were positive (n=88), and 44.2% of the 154 patients tested using antigen detection were positive (n=68). Most patients developed mild diseases and 19 patients (7.7%) developed severe diseases according to published guidelines. The confirmed COVID-19 infection rate was significantly higher in patients who received HSCT over 100 days prior compared to those who received it within 100 days (40.1% vs 21.7%, p<0.05, Fisher’s Exact test). Furthermore, patients receiving HSCT within 100 days had a significantly higher percentage of hospitalizations than those who received HSCT over 100 days (31.7% vs 8.0%, p<0.01, Fisher’s Exact test).
The majority of patients (n=154, 62.3%) experienced COVID-19 related symptoms, with fever being the most common symptom (n=107, 43.2%), followed by cough (n=88, 35.6%), snuffing (n=39, 15.8%), and sore throat (n=25, 10.3%). Other symptoms were less frequent, including muscle pain, malaise, breathing difficulties, diarrhea, and vomiting. Most of the patients developed mild diseases, approximately one third of the infected patients required hospitalization (n=34, 13.8%), and 19 patients (7.7%) developed severe SARS-CoV2 pneumonia according to the China CDC guidelines. Among all patients that had confirmed SARS-CoV2 infection, two patients developed acute graft versus host disease (0.8%) and two patients (0.8%) experienced autoimmune hemolytic anemia.
Co-infections with other pathogens were studied in the 34 patients who were hospitalized. Seven patients (20.6%) had cytomegalovirus (CMV) reactivation, two patients (5.9%) were infected with human herpes virus type 6 (HHV6), one patient (2.9%) had a respiratory syncytial virus (RSV) infection, and one patient (2.9%) developed hemorrhagic cystitis associated with BK virus infection. Gram-positive bacterial infections were observed in one patient (2.9%), while gram-negative bacterial infections were observed in four patients (11.8%). Additionally, four patients (11.8%) developed invasive fungal infection at the time of COVID-19 infection.
Treatment and Outcomes of Pediatric HSCT Recipients Following COVID-19
Table 2 summarizes the treatment and outcomes of the patients. The overall survival rate from the time of confirmed SARS-CoV2 infection to the last follow-up visit was 99.53% (CI 96.72–99.93%) for all patients. Of the total patients, 19 patients received Paxlovid treatment (nirmatrelvir 150 mg/ ritonavir 100mg per day for 5 days for patients <12 years old or <40kg; nirmatrelvir 300mg/ritonavir 100mg per day for 5 days for patients ≥12 years old or ≥40kg), 7 patients received methylprednisolone, 2 patients received IL-6 antibody treatment, and 3 patients received mesenchymal stem cells infusions. Three patients required mechanical ventilation and were transferred to the intensive care unit. One patient died due to severe respiratory failure. The two surviving patients received exosome nebulisation and later recovered gradually. Of the 34 hospitalized patients, 29 patients achieved complete resolution, four patients had residual lung lesions, and one remained positive for SARS-CoV-2 by RT-PCR two months after infection.
 |
Table 2 Treatment and Outcomes of Hospitalized HSCT Patients with COVID-19 |
Discussions
The COVID-19 pandemic has demonstrated vulnerabilities in both adult and pediatric HSCT recipients. Adult HSCT patients typically exhibit a propensity for severe COVID-19 diseases and higher mortality rates compared to their pediatric counterparts, reflecting a trend in age-related outcomes of HSCT recipients after COVID-19 infection.13–15 However, the survival rate of HSCT recipients with COVID-19 has improved in recent years.7,16,17 Due to strict COVID-19 quarantine policies in China, our patients were only exposed to the virus during the latest outbreak since December 2022. Our study found that most pediatric HSCT patients developed COVID-19 related symptoms during this period, with approximately one-third of patients confirmed positive for SARS-CoV-2. This incidence is consistent with previous studies reported by other countries, highlighting the susceptibility of pediatric HSCT patients to COVID-19.14,16,18 Nonetheless, most patients made a good recovery after COVID-19 infection, with a survival rate of 99.53% among all patients and 85.3% of the hospitalized patients achieving complete resolution of lung lesions at the last follow-up visit.
COVID-19 related symptoms were similar to those reported in existing literature,16 with fever and upper respiratory symptoms being the most prevalent manifestations among the infected patients. Most patients developed mild diseases and did not require specific treatment. In the current study, 19 patients developed severe diseases and 34 patients were hospitalized due to COVID-19 infection and received specific treatment. Our study demonstrated that patients who received HSCT more than 100 days before contracting COVID-19 had a lower rate of confirmed infection than those who received HSCT within 100 days, potentially due to that HSCT recipients may attain donors’ immunity during the early stage after HSCT.19–21 Thus, HSCT recipients should be closely monitored for a longer period. Additionally, we observed that patients who developed COVID-19 within 100 days of HSCT were more likely to require hospitalization, suggesting a potential vulnerability to severe disease. However, the higher hospitalization rate may also be due to these patients remaining in the hospital and not being discharged after HSCT. Further research is needed to confirm this finding.
The impact of COVID-19 on HSCT complications remains a significant concern for pediatric HSCT recipients. In the context of COVID-19, HSCT patients may have a predisposition to higher risks of viral, bacterial and fungal infections.22,23 However, this study did not report higher rates of co-infections with other pathogens compared to data from other studies and our own previous reports. Although an increased incidence of GVHD was not observed during the COVID-19 period in this study, COVID-19 may trigger a profound inflammatory response, which can exacerbate cytokine release, potentially leading to the onset or increased severity of GVHD.24 Additionally, “long-COVID-19” symptoms and other post-acute sequelae have been described in literature, hence longer follow-up is required to understand the long-term impact of COVID-19 on our patients.25
The high incidence of COVID-19 among pediatric HSCT patients raises the question of whether they should vaccinated against SARS-CoV-2.26–28 Studies have shown that pediatric and adolescent HSCT patients can develop protective antibodies after vaccination, indicating its potential for reducing the risk of COVID-19.29–31 However, declined vaccine efficacy have been observed in HSCT recipients.32 None of the patients in this study were vaccinated prior to the recent outbreak. This low vaccination rate highlights the need to raise awareness among patients, their families and healthcare providers about the importance of vaccination. In addition, the optimal timing of vaccination in pediatric patients post HSCT, as well as the most suitable vaccine type and dosage, require further investigation.
The treatment of COVID-19 has significantly evolved since the onset of the pandemic, with various therapeutic approaches being explored to improve patient outcomes. In this study, Paxlovid, a combination of the antiviral agents nirmatrelvir and ritonavir, has shown promising outcomes in the treatment of COVID-19.33,34 Paxlovid was used off-label for children in this study.35 A total of 19 patients received Paxlovid treatment, with doses adjusted for those under 12 years of age or weighing less than 40 kg. Paxlovid was not administered to all hospitalized patients due to limited availability during the outbreak. Nonetheless, we believe that Paxlovid may have contributed to the favorable outcomes observed, given the high survival rate among all hospitalized patients. In addition, no severe drug-related toxicities were observed in our patients. Further investigation is necessary to evaluate the efficacy and safety of Paxlovid in a larger pediatric population.
The pathogenesis of COVID-19 is characterized by dysregulated immune responses and excessive inflammation.36,37 Consequently, various approaches aim to modulate the immune response and mitigate inflammation, particular in patients with severe diseases. Steroids, due to their anti-inflammatory properties, are recommended for managing severe COVID-19.38 The use of IL-6 antibodies has also been supported by evidence pointing to their role in reducing cytokine release syndrome and hyperinflammation.39 In this study, seven patients received methylprednisolone and two of the three patients admitted to the ICU were treated with the IL-6 antibody tocilizumab. Additionally, MSC treatment, known for its immune modulation and potential regenerative capacity, was administered to patients in the ICU.40,41 It is noteworthy that the two ICU survivors were treated with exosome nebulization, which delivers therapeutic agents directly to the respiratory system, the primary site of SARS-CoV-2 infection.42 Nebulization facilitates the distribution of exosomes throughout the respiratory tract, allowing for interaction infected cells and modulation of the local immune response.42 However, the precise mechanisms by which exosome therapy exserts its effects in COVID-19 have yet to be fully understood. Furthermore, the safety profile of exosome nebulization must be thoroughly evaluated to ascertain and address any potential adverse effects.
This study has several limitations. Firstly, it included patients only from a single centre in China hence the findings may not be generalizable to other regions or countries. Another limitation is that most HSCT patients had non-malignant diseases, which might underrepresent the post-COVID-19 comorbidities and mortalities associated with malignant conditions. Additionally, this study did not investigate the long-term effects of COVID-19 infection in pediatric HSCT patients. Further research is needed to explore the long-term impact of COVID-19 infection on the immune system and the prognosis of pediatric HSCT recipients.
In conclusion, this study highlights the high incidence of COVID-19 in pediatric HSCT patients who have neither received the SARS-CoV-2 vaccination nor been previously infected with SARS-CoV-2. These findings underscore the importance of preventive and control measures for COVID-19 in this vulnerable group of patients, including vaccination, vigilant monitoring, and timely diagnosis and treatment. Further research is necessary to determine the benefits of SARS-CoV-2 vaccination in pediatric HSCT patients and to explore the long-term effects of COVID-19 infection on this population.
Ethical Approval
This study was approved by the Ethics Committee of Shenzhen Children’s Hospital (approval number 2021102).
Consent for Publication
Written consent was obtained from the parents of the patient for the participation of this study.
Acknowledgments
Xiaodong Wang and Uet Yu are co-first authors for this study. Sixi Liu and Feiqiu Wen are co-correspondence authors for this study. We thank the patients and families for participation in this study. We thank Dr. Kongpeng Lyu from Shenzhen Archean Biotechnology for the manufacturing and providing exosomes for the patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Shenzhen Science and Technology Innovation Commission (RCBS20200714114858018), Shenzhen Key Medical Discipline Construction Fund (SZXK034), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP012), Shenzhen Children’s Hospital Research fund (ynkt2021-zz26, ynkt2020-zz01), and Guangdong and Shenzhen High-level Hospital Construction Fund.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi:10.1016/s0140-6736(22)00327-0
2. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Eng J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
3. Howard-Jones AR, Bowen AC, Danchin M, et al. COVID-19 in children: i. Epidemiology, prevention and indirect impacts. J Paediatr Child Health. 2022;58(1):39–45. doi:10.1111/jpc.15791
4. Howard-Jones AR, Burgner DP, Crawford NW, et al. COVID-19 in children. II: pathogenesis, disease spectrum and management. J Paediatr Child Health. 2022;58(1):46–53. doi:10.1111/jpc.15811
5. Vicent MG, Martinez AP, Trabazo Del Castillo M, et al. COVID-19 in pediatric hematopoietic stem cell transplantation: the experience of Spanish Group of Transplant (GETMON/GETH). Pediatr Blood Cancer. 2020;67(9):e28514. doi:10.1002/pbc.28514
6. Mobile RZ, Warnawin S, Kojo TK, et al. SARS-CoV-2 in saliva, viremia and seroprevalence for COVID-19 surveillance at a single hematopoietic stem cell transplantation center: a prospective cohort study. Rev Inst Med Trop Sao Paulo. 2022:
7. Bhatt NS, Sharma A, St Martin A, et al. Clinical Characteristics and Outcomes of COVID-19 in Pediatric and Early Adolescent and Young Adult Hematopoietic Stem Cell Transplant Recipients: a Cohort Study. Transplant Cell Ther. 2022;28(10):696.e1–696.e7. doi:10.1016/j.jtct.2022.06.026
8. Dallas RH. #46 A Multicenter Study Exploring the Epidemiology and Outcomes of COVID-19 Among Pediatric Hematopoietic Cell Transplant, Cellular Therapy and Solid Organ Transplant Recipients: preliminary Results from the Pediatric COVID-19 U.S. Registry. J Pediatric Infectious Dis Society. 2022;11(Supplement_1):S2–S3. doi:10.1093/jpids/piac041.007
9. Cesaro S, Ljungman P, Mikulska M, et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia. 2022;36(6):1467–1480. doi:10.1038/s41375-022-01578-1
10. Liu Y, Zheng F, Du Z, et al. Evaluation of China’s Hubei control strategy for COVID-19 epidemic: an observational study. BMC Infect Dis. 2021;21(1):820. doi:10.1186/s12879-021-06502-z
11. Zheng Z, Lu Y, Wang M, et al. Low COVID-19 vaccine coverage and guardian acceptance among pediatric transplant recipients. J med virol. 2023;95(1):e28377. doi:10.1002/jmv.28377
12. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. doi:10.1001/jama.2020.2648
13. Merli M, Perricone G, Lauterio A, et al. Coronaviruses and Immunosuppressed Patients: the Facts During the Third Epidemic. Liver Transpl. 2020;26(11):1543–1544. doi:10.1002/lt.25806
14. Averbuch D, de la Camara R, Tridello G, et al. Risk factors for a severe disease course in children with SARS-COV-2 infection following hematopoietic cell transplantation in the pre-Omicron period: a prospective multinational Infectious Disease Working Party from the European Society for Blood and Marrow Transplantation group (EBMT) and the Spanish Group of Hematopoietic Stem Cell Transplantation (GETH) study. Bone Marrow Transplant. 2023;58(5):558–566. doi:10.1038/s41409-023-01941-5
15. Balduzzi A, Brivio E, Rovelli A, et al. Lessons after the early management of the COVID-19 outbreak in a pediatric transplant and hemato-oncology center embedded within a COVID-19 dedicated hospital in Lombardia, Italy. Estote parati. Bone Marrow Transplant. 2020;55(10):1900–1905. doi:10.1038/s41409-020-0895-4
16. Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185–e193. doi:10.1016/s2352-3026(20)30429-4
17. Ljungman P, Tridello G, Piñana JL, et al. Improved outcomes over time and higher mortality in CMV seropositive allogeneic stem cell transplantation patients with COVID-19; An infectious disease working party study from the European Society for Blood and Marrow Transplantation registry. Front Immunol. 2023;14:1125824. doi:10.3389/fimmu.2023.1125824
18. Kebudi R, Kurucu N, Tuğcu D, et al. COVID-19 infection in children with cancer and stem cell transplant recipients in Turkey: a nationwide study. Pediatr Blood Cancer. 2021;68(6):e28915. doi:10.1002/pbc.28915
19. Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2021;21(5):277–291. doi:10.1038/s41577-020-00457-z
20. Bhatt ST, Bednarski JJ. Immune Reconstitution in Pediatric Patients Following Hematopoietic Cell Transplant for Non-malignant Disorders. Front Immunol. 2020;11:1988. doi:10.3389/fimmu.2020.01988
21. Naik S, Vasileiou S, Aguayo-Hiraldo P, et al. Toward Functional Immune Monitoring in Allogeneic Stem Cell Transplant Recipients. Biol Blood Marrow Transplantation. 2020;26(5):911–919. doi:10.1016/j.bbmt.2020.01.005
22. Piñana JL, Pérez A, Chorão P, et al. Respiratory virus infections after allogeneic stem cell transplantation: current understanding, knowledge gaps, and recent advances. Transplant Infectious Dis. 2023:e14117. doi:10.1111/tid.14117
23. Al-Ramahi JS, Shahzad M, Li K, et al. Lessons learned from COVID-19 pandemic: outcomes after SARS-CoV-2 infection in hematopoietic cell transplant and cell therapy recipients. Leukemia Lymphoma. 2023:1–11. doi:10.1080/10428194.2023.2243355
24. Lin CY, Chien HJ. Acute exacerbation of ocular graft-versus-host disease and anterior uveitis after COVID-19 vaccination. BMC Ophthalmol. 2023;23(1):360. doi:10.1186/s12886-023-03103-z
25. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi:10.1136/bmj.m3026
26. Zimmermann P, Pittet LF, Finn A, Pollard AJ, Curtis N. Should children be vaccinated against COVID-19? Arch Dis Child. 2022;107(3):e1. doi:10.1136/archdischild-2021-323040
27. Altmann DM, Boyton RJ. COVID-19 vaccination: the road ahead. Science. 2022;375(6585):1127–1132. doi:10.1126/science.abn1755
28. Mohamed K, Rzymski P, Islam MS, et al. COVID-19 vaccinations: the unknowns, challenges, and hopes. J med virol. 2022;94(4):1336–1349. doi:10.1002/jmv.27487
29. Áñez G, Dunkle LM, Gay CL, et al. Safety, Immunogenicity, and Efficacy of the NVX-CoV2373 COVID-19 Vaccine in Adolescents: a Randomized Clinical Trial. JAMA Netw Open. 2023;6(4):e239135. doi:10.1001/jamanetworkopen.2023.9135
30. Doucette EJ, Gray J, Fonseca K, et al. A longitudinal seroepidemiology study to evaluate antibody response to SARS-CoV-2 virus infection and vaccination in children in Calgary, Canada from July 2020 to April 2022: Alberta COVID-19 Childhood Cohort (AB3C) Study. PLoS One. 2023;18(4):e0284046. doi:10.1371/journal.pone.0284046
31. Sadeghi S, Kalantari Y, Shokri S, et al. Immunologic response, Efficacy, and Safety of Vaccines against COVID-19 Infection in Healthy and immunosuppressed Children and Adolescents Aged 2-21 years old: a Systematic Review and Meta-analysis. J clin virol. 2022;153:105196. doi:10.1016/j.jcv.2022.105196
32. Lee A, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. doi:10.1136/bmj-2021-068632
33. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54(1):516–523. doi:10.1080/07853890.2022.2034936
34. Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and Nirmatrelvir-Ritonavir: oral Coronavirus Disease 2019 Antiviral Drugs. Clin Infect Dis. 2023;76(1):165–171. doi:10.1093/cid/ciac180
35. Yan G, Zhou J, Zhu H, et al. The feasibility, safety, and efficacy of Paxlovid treatment in SARS-CoV-2-infected children aged 6-14 years: a cohort study. Ann Transl Med. 2022;10(11):619. doi:10.21037/atm-22-2791
36. Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436. doi:10.1136/bmj.n436
37. Gusev E, Sarapultsev A, Solomatina L, Chereshnev V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int J Mol Sci. 2022;23(3). doi:10.3390/ijms23031716
38. Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease 2019 (COVID-19; Metcovid): a Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin Infect Dis. 2021;72(9):e373–e381. doi:10.1093/cid/ciaa1177
39. Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi:10.1002/rmv.2141
40. Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, Phase 1/2a, randomized controlled trial. Stem Cells Translational Med. 2021;10(5):660–673. doi:10.1002/sctm.20-0472
41. Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. doi:10.1186/s13287-020-01875-5
42. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi:10.1089/scd.2020.0080
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
